Abstract
Nanotechnology, a fascinating and interesting field of science has attracted researchers to explore its various applications in medicine, biology, environment, electronics, medical devices, food and agriculture. The engineered metallic nanoparticles (NPs) exhibit unique physicochemical and biological properties owing to their small size, varying shapes and surface plasmon dynamics. Metallic NPs have gained exceptional importance and are continuously explored for new opportunities. NPs display superior chemical stability, conductivity, catalytic activity, and possess superior pharmacological properties such as antimicrobial, antioxidant, anti-inflammatory activities and many more. In general, NPs are conventionally synthesized by physical or chemical methods that employ expensive and hazardous chemicals. The health risks associated with these toxic NPs cannot be ignored and hence, not preferred for many biological applications. Therefore, research is progressing towards the development of an eco-friendly and reliable biological approaches of nanoparticle synthesis. In this regard, biosynthesis of NPs using either plants, phytocompounds or microbes is gaining more importance in recent times among researchers owing to their potential pharmacological benefits. Microbes, such as fungi, bacteria, yeasts, and viruses have intrinsic bulks to reduce silver metal through their metabolic pathways. Microbe-assisted NPs synthesis avoids elaborate cell culture maintenance and yields in diverse size range, and morphologies that imparts unique biological properties. However, various factors such as bioresources, biomolecules, pH, temperatures and exposure time plays a significant role in the biosynthesis of crystalline microbe-assisted NPs. In this chapter, a comprehensive information about the biosynthesis of NPs using microbes including bacteria, fungi, and yeast is described. In addition, a view on the mechanism of action and their potential pharmacological benefits are discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanotechnology has emerged as a new and promising discipline of modern science that encompasses interdisciplinary topics such as material science, physics, chemistry and biology. Thus, nanotechnology has become more fascinating field of study by several group of scientific community including physicists, chemists, material scientists, electronics and mechanical engineers, biomedical researchers, and biologists. The advancement of nanoparticle synthesis and characterization has witnessed novel applications of nanoparticles (NPs) in various fields and it is evolving very rapidly in recent times (Rudramurthy et al. 2016). Typically, NPs are structural materials that possess a specific dimension within the nanoscale range (1–100 nm) (Li et al. 2011; Kato 2011). NPs are composed of three layers namely, the surface layer, shell layer and the core. Mostly, the surface layer is fabricated with different macromolecules, surfactants, metal ions or polymers to impart novel and unique properties (Khan et al. 2017).
These nanosized particles have greatly attracted because of their uncommon, but attractive physicochemical, electronic, dielectric, electrical, magnetic, mechanical, and biological properties (Narayanan and Sakthivel 2010). In general, NPs exhibit relatively a greater surface to volume ratio, higher Raman scattering and Rayleigh scattering effect in metallic nanomaterials that imparts diverse properties to NPs making them more advantageous as compared to their bulk material counterparts (Narayanan and Sakthivel 2010; Swamy et al. 2015a, b; Akthar et al. 2015). Overall, unique and enhanced properties of nanomaterial are mostly due to their altered shape, size, distribution pattern and other morphological features compared to their bulk particles from which they are synthesized (Swamy et al. 2015a; Raza et al. 2016; Khan et al. 2017). Therefore, a precise control over these factors will govern the characteristic features of NPs and their applications . The controlled synthesis of metallic nanostructures with a definite dimension and organized monodispersity is one of the greatest challenge in nanotechnology research (Maliszewska 2011). In addition, some of the other issues such as easy recovery and purification of the synthesized NPs for their effective uses are equally important. The synthesized nanomaterials with a definite shape, size, and composition are widely explored in numerous fields such as biomedicine , food, cosmetics, energy science, electronics, optoelectronics, agriculture , chemical industries, textiles, optical devices, electrochemical applications , and environment (Iravani et al. 2014; Rudramurthy et al. 2016).
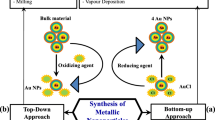
NPs with a size ranging between 0.1 and 1000 nm are frequently synthesized via bottom-up and top-down approaches (Narayanan and Sakthivel 2010; Rudramurthy et al. 2016). In bottom-up approach of nanofabrication, the substrates are added with a building blocks to form nanostructure. Otherwise, molecular nanostructures are formed by the assembly of atoms or molecules that give rise to crystal planes which further stack onto each other to form nanostructures. Usually, bottom-up approach is employed for biological or chemical synthesis of nanostructures. On the other hand, top-down approach employs a gradual degradation or breakdown of larger bulk materials into nano-sized structures. In this method, the nanomaterials are produced by incising out crystals planes that are previously coexistent on the bulk substrate.
Various physical and chemical procedures are broadly utilized for the synthesis of unique monodispersed NPs. The major concern is that both employs toxic chemicals such as such as surfactants, strong reducing agents, polymer capping agents, organic solvents for the synthesis of nanostructures. Also, the nanomaterials synthesized from these methods are unstable for long time and the cost of the chemicals are very expensive. Hence, the adsorption of toxic chemicals on NPs surface and organic solvents used during the chemical synthesis greatly limit their uses in biomedical fields (Narayanan and Sakthivel 2010; Maliszewska 2011; Rudramurthy et al. 2016). Consequently, developing an ecofriendly, cost effective, clean, non-toxic and biologically compatible NPs synthesis procedures are more appreciated. Accordingly, recent times have witnessed the development of biological approaches for nanoparticle synthesis. Biological or green synthesis approaches comprise mainly polysaccharides, mixed-valence polyoxometalates, biosynthetic and irradiation methods. A careful selection of an appropriate green route synthesis methods must be considered with two important facts such as the right selection of solvent system and toxic free reducing or stabilizing agents (Iravani et al. 2014).
Biosynthesis methods employs an eco-friendly unicellular and multicellular biological entities including plants, fungi, yeast, bacteria, actinomycetes and viruses (Kowshik et al. 2003; Kuber and Souza 2006; Merzlyak and Lee 2006; Swamy et al. 2015a, b; Chengzheng et al. 2018; Akhtar et al. 2015; Rudramurthy et al. 2016; Shah et al. 2015). Among these, plant based NPs synthesis approach is relatively more advantageous and can be converted into a resourceful bio factories (Thakkar et al. 2010; Iravani et al. 2014; Shah et al. 2015). Nevertheless, microbe based approaches have few disadvantages such as microbes isolation, culturing and maintenance that requires more time, difficulty in obtaining better control over NPs size, shape, dispersion, crystallinity, and slow production rate while, phytosynthesis of NPs is a straight forward process which do not require any complex or multi-step processes and is rapid. Phytosynthesis approaches are simpler, economical and can be easily scaled up for producing NPs in bulk quantities (Swamy et al. 2015a, b; Iravani et al. 2014; Shah et al. 2015). Nevertheless, the understandings on microbial strain selection, manipulation of culture conditions such as pH, temperature, time, and metal ions concentrations has given some hope on the possible implementation of microbe based approaches for large scale production of NPs. A possible use of genetically manipulated microbes to overproduce definite reducing agents can further simplify the process in obtaining NPs with a definite sizes and shapes (Narayanan and Sakthivel 2010; Musarrat et al. 2011a, b). In addition, the NPs synthesized through biogenic route possess specific surface area with a greater catalytic activity and an enhanced interaction between the enzymes and metal ions (Bhattacharya and Mukherjee 2008; Li et al. 2011; Shah et al. 2015).
It is well established fact that microorganisms adsorb and accumulate metals from their environment and thus, they are widely used in various biological applications such as biomineralization, bioremediation , and bioleaching. Also, microbes are effective in reducing environmental pollution (Darnall et al. 1986; Huang et al. 1990; Kapoor et al. 1999; Lloyd and Lovley 2000; Klaus-Joerger et al. 2001). Microbes has the potential to reduce metals to form metallic NPs and therefore, microbes including bacteria, yeast and fungi are considered as best suitable source for synthesizing environmental friendly, cost-effective nanomaterials in recent times (Ahmad et al. 2002; Musarrat et al. 2011a, b). Therefore, this chapter highlights the recent research outcomes on NPs synthesized through microbial routes and their possible applications in various biomedical fields.
2 Biosynthesis of Metallic NPs Using Bacteria
Among natural sources, bacteria are expansively utilized for the synthesis of various metallic NPs. This is due to the fact that bacterial cells can be easily manipulated and control over their culture conditions. At present, research focus is towards understanding microbes interacting with inorganic molecules. It is reported that microorganisms can secrete inorganic constituents having nanoscale dimensions either within or outside their cells. Microbes resist heavy metal compounds mainly due to several mechanisms. These include, permeability barrier exclusion, chemical detoxification using enzymes , intra- and extra-cellular sequestration, reduction reactions, and electrochemical potential change caused by metal ion efflux between the cell membrane mediated by membrane proteins or proton anti-transporter proteins (Bruins et al. 2000; Narayanan and Sakthivel 2010). Thus, bacteria can effectively detoxify heavy metal ions through reduction reactions or by precipitating soluble inorganic ions into insoluble and non-toxic metallic nanostructures. The bacterial detoxification process could be both extracellular or intracellular way and thus, biosynthesis of several metallic NPs could be achieved through both the ways i.e., intracellularly and extracellularly. In this section, biosynthesis of different metallic NPs using actinomycetes, cyanobacteria and bacteria are discussed.
2.1 Silver Nanoparticles (AgNPs)
The most common bacterial species that are utilized for the biosynthesis of metal NPs include Bacillus cereus, Escherichia coli, Klebsiella pneumonia, Actinobacter sp., Corynebacterium sp., Lactobacillus sp., and Pseudomonas sp. (Tollamadugu et al. 2011; Iravani et al. 2014; Shah et al. 2015). Different bacterial strains utilized for synthesizing AgNPs are listed in the Table 19.1. The biosynthesis of AgNPs using a bacterial strain, Pseudomonas stutzeri AG259 isolated from a silver mine was achieved (Klaus et al. 1999). These AgNPs were synthesized in the periplasmic region of the bacterial cell. The particles had a pyramidal and hexagonal shapes and their size ranged up to 200 nm. The occurrence of the Ag-binding proteins act as a source of amino acid moieties for initiating the nucleation reaction during the biosynthesis of AgNPs. It is reported that small silver-binding proteins are found in the periplasmic layer of the bacterial cell bind to silver ions and through ion efflux, prevents the further entry of metals and thus, protect the cell from metal toxicity (Li et al. 1997; Gupta and Silver 1998). The silver ions in the aqueous solution of silver nitrate were shown to get precipitated by the specific silver precipitating peptides namely, AG3 and AG4 to produce silver crystals with a face centered cubic structure (Naik et al. 2002). According to them, these peptides contains specific amino acids mainly, methionine, arginine, lysine, and cysteine which recognize and reduce the silver ions. The correct mechanism of AgNPs biosynthesis from bacteria is still not clear. However, a study by Parikh et al. (2008) has highlighted the possible molecular mechanism involved in the extracellular biosynthesis of AgNPs by Morganella sp. RP-42, isolated from the insect midgut. They observed that Morganella sp. produced crystals of spherical AgNPs with an approximate size of 20 nm. The molecular identification revealed the existence of three silver resistant genes namely, silE, silP and silS in Morganella sp. The nucleotide sequence of the gene silE obtained from Morganella sp. Showed 99% similarity match with known gene, silE known to express periplasmic silver-binding proteins. The study clearly indicated that Morganella sp. secretes silver-binding proteins to their extracellular environment during their growth and these proteins probably involve in the reduction of silver metal ions into AgNPs formation and provides stability to the formed nonocrystals (Parikh et al. 2008). Using Corynebacterium sp. SH09 cells isolated from a silver mine, Zhang et al. (2005) showed the biosorption and bioreduction of diamine silver complex. The Corynebacterium sp. SH09 showed a stronger biosorption capability for [Ag(NH3)2]+. According to them, ionized carboxyl of amino acid moieties involves in trapping [Ag(NH3)2]+ onto the cell walls. The presence of certain reducing agents for example, aldehydes and ketones involve in bioreduction of [Ag(NH3)2]+ to elemental Ag(0). The biosynthesized AgNPs on the cell walls of this bacterium were having the size ranged between 10 and 15 nm. Likewise, Sneha et al. (2010) synthesized irregular shaped AgNPs from Corynebacterium glutamicum. The bioreduction of Ag+ to Ago was achieved through an airborne Bacillus sp. and importantly, the synthesized AgNPs were localized in the periplasmic region of the cells. Further characterization using Transmission Electron Microscopy (TEM) and Eenergy Dispersive X-ray (EDX) analysis established the synthesized AgNPs with a size of 5–15 nm. A monodispersed uniform sized AgNPs were synthesized from the dried cells of Aeromonas sp. SH10. The bioreduction of [Ag(NH3)2]+ to elemental Ag0 took place rapidly in the solution and on the cells (Mouxing et al. 2006). A new isolate, Bacillus cereus PGN1 resistant to metal was used to form intracellular AgNPs (Babu and Gunasekaran 2009). The characterization of these particles revealed that AgNPs had a spherical shape with 4–5 nm of size. Likewise, a spherical shaped AgNPs with a size range of 3–170 nm were produced from B. licheniformis (an isolate from Al Thwara hot spring, Oman) (Sarangadharan and Nallusamy 2015). Similarly, B. licheniformis culture supernatant was used to synthesize AgNPs with an approximate size of 40–50 nm (Kalishwaralal et al. 2008; Kalimuthu et al. 2008). The biosynthesis of silver chloride NPs having the size range of 20–60 nm and spherical shape was prepared from Bacillus subtilis (Paulkumar et al. 2013). In another study, a rapid synthesis of AgNPs using B. subtilis culture supernatant with microwave irradiation was achieved (Saifuddin et al. 2009). The NPs were having spherical and occasionally triangular shapes. Using B. stratosphericus, Hosseini-Abari et al. (2014) synthesized AgNPs having various shapes (spherical, triangular, cubic, and hexagonal) and a size of 2–20 nm. Likewise, Das et al. (2014) synthesized BACILLUS sp. mediated AgNPs with size ranging 42–92 nm and the particles were having spherical shape. Likewise, a thermophile, Bacillus sp. AZ1, Bacillus megaterium and Bacillus flexus were also capable of synthesizing AgNPs extracellularly (Saravanan et al. 2011; Priyadarshini et al. 2013; Deljou and Goudarzi 2016). Bacillus thuringiensis and Bacillus megaterium mediated AgNPs were also synthesized (Banu and Balasubramanian 2015; Banu et al. 2014). More recently, used Bacillus amyloliquefaciens and Bacillus subtilis to produce spherical shaped AgNPs (Fouad et al. 2016). A rapid biosynthesis of AgNPs from the culture supernatant of Klebsiella pneumonia was achieved. AgNPs sizes ranged between 28.2 and 122 nm. When piperitone was added, reduction process was inhibited and thus, suggesting the role of an enzyme , nitroreductase involvement in the process (Shahverdi et al. 2007a, b). Escherichia coli was used to biosynthesize well dispersed AgNPs with a size ranging between 40 and 90 nm (Gurunathan et al. 2009; Kannan et al. 2010). Likewise, green synthesis of AgNPs using E. coli has been achieved by Divya et al. (2016). A thermophilic bacteria, Ureibacillus thermosphaericus was capable to biosynthesize polydispersed spherical 10–100 nm sized AgNPs through extracellular mechanism (Juibari et al. 2011). Extracellular synthesis of AgNPs was reported by Shivaji et al. (2011) by using culture supernatants of Arthrobacter kerguelensis, Bacillus cecembensis, Bacillus indicus, Pseudomonas meridiana, Pseudomonas antarctica, Pseudomonas proteolytica, Arthrobacter gangotriensis. Bioreduction of silver ions to AgNPs using Staphylococcus aureus was shown by Nanda and Saravanan (2009). Pseudomonas mandelii SR1 assisted in synthesizing AgNPs having an average diameter of 1.9–10 nm. These AgNPs were highly stable even after 19 months of storage period (Mageswari et al. 2015). Several Streptomyces sp. isolates were used to biofabricate spherical shaped AgNPs with the size in the range of 20–60 nm (Thenmozhi et al. 2013; Sanjenbam et al. 2014; El-Naggar et al. 2016). Likewise, Streptomyces ghanaensis VITHM1 strain was used to produce highly stable spherical shaped AgNPs with an average size between 30 and 50 nm range (Abirami and Kannabiran 2016).
2.2 Gold Nanoparticles (AuNPs)
A variety of AuNPs have been successfully synthesized by using various bacterial strains (Table 19.2). These AuNPs with different shapes have a unique properties to be considered for biomedical applications . The application of AuNPs for curing numerous diseases has been practiced ever since from 2500 BC to till date. The importance of AuNPs was recognized over 150 year back when Michael Faraday observed different properties of colloidal gold solution differing from their bulk material, gold (Li et al. 2011). In recent times, biosynthesis and applications of AuNPs has been highly acknowledged. Microbe assisted synthesis of AuNPs is an eco-friendly approach thus, gained more attention compared to other methods as synthesis occurs immediately in the surrounding temperature and pressure (Das et al. 2014; Srinath and Rai 2015). Lactobacillus strains, commonly occurring in buttermilk were used to synthesize AuNPs crystalline structures with submicron dimensions (Nair and Pradeep 2002). Shewanella algae and Shewanella oneidensis were also successfully employed to biosynthesize monodispersal AuNPs in nano regime (Konishi et al. 2004; Suresh et al. 2011). AuNPs formed extra-cellularly from Pseudomonas aeruginosa possessed size in the range of 15–30 nm (Husseiny et al. 2007). The bacteria, Rhodopseudomonas capsulata was successfully used to produce AuNPs of varied size and shape (He et al. 2007). An average size of AuNPs formed on the bacterial cells (E. coli DH5α) surface was found to be 25 ± 8 nm (Du et al. 2007). For the first time, Sharma et al. (2012) employed the marine bacteria, Marinobacter pelagius to biosynthesize AuNPs Du et al. (2007). They obtained spherical AuNPs in the range of 10–20 nm. Likewise, AuNPs from Stenotrophomonas maltophilia were having an approximate size of ~40 with oval shape (Nangia et al. 2009). A successful biosynthesis of AuNPs from Rhodopseudomonas capsulata and Pseudomonas aeruginosa was achieved by Singh and Kundu (2014). Rhodobacter capsulatus, one of the photosynthetic bacteria was shown to have a strong biosorption capability for the AuCl4 − The presence of carotenoids and certain enzymes on the cell membrane and/or found in their surroundings are believed to involve in the bioreduction of Au3+ to Au0. Further, they speculated that due to this phenomenon the bacterium can exhibit metal tolerance capability (Feng et al. 2007). Likewise, B. licheniformis, B. niabensis 45 and B. clausii were also used for extracellular biosynthesis of AuNPs (Kalishwaralal et al. 2009; Singh et al. 2014; Zhang et al. 2016; Li et al. 2016a). Further, Li et al. (2016a) predicted the role of a cyclic peptide (P2) with a molecular weight of about 1122 Da involvement in the stabilization of nanocrystals. AuNPs had a spherical shape with an average diameter of 38 nm. Similarly, Klebsiella pneumoniae was employed to synthesize spherical shaped AuNPs having monodispersal nature with a spherical shape and their size ranged between 4 and 65 nm (Malarkodi et al. 2013; Srinath and Rai 2015). Deinococcus radiodurans, known for its extreme radiations and oxidant stress resistance was used to reduce Au (III) and AuNPs synthesis (Li et al. 2016b). The particles showed polydisparity with spherical, triangular and irregular shapes and had an average size of 43.75 nm. Nadaf and Kanase (2016) used Bacillus marisflavi to produce spherical shaped AuNPs with an average size of ∼14 nm.
2.3 Magnetic Nanoparticles
Biomineralization is a naturally occurring process mainly mediated by certain microbes commonly called as magnetotactic bacteria. These bacteria use biomineralization proteins to form a well-organized magnetite nanocrystal structures (Bazylinski and Frankel 2004; Arakaki et al. 2008). Most of these magnetotactic bacteria have a diverse morphological features and exist mainly in the sediments of freshwater as well as marine region (Narayanan and Sakthivel 2010). Many kinds of magnetic NPs are currently produced due to their potential nanobiotechnological applications including cancer cure through magnetic hyperthermia. The bacterial nanoparticles are synthesized under stringent biological conditions with bacterial cell membrane lipid layer and definite membrane proteins (Chen et al. 2016). More recently, nanoscience research is focused on magnetic NPs due to the fact that they possess a unique nanoconfiguration with superior properties including high coercive force and super paramagnetic, thus broadly applied in various biomedical fields (Li et al. 2014; Rudramurthy et al. 2016; Chen et al. 2016). The isolated magnetotactic bacteria from marine sulfide-rich water and sediments produced intracellular nanocrystals of ferromagnetic iron sulfide and greigite (Fe3S4). They were aligned in chains in association with iron pyrite (FeS2) and each chain contained about ten NPs with irregular shape and 75 nm in size. Most of the particles have irregular shape, whereas some exhibit octahedral and cubo-octahedral symmetry with strong diffraction contrast (Mann et al. 1990; Narayanan and Sakthivel 2010). Magnetospirillum magneticum has the capability to form 50–100 nm sized ferromagnetic particles (Fe3O4 or Fe3S4). These NPs were surrounded by magnetosomes, an intracellular phospholipid membrane (Schuler and Frankel 1999). Magnetosomes from Desulfovibrio magneticus RS-1 were irregular/bullet shaped with ∼35 nm sized were produced by (Posfai et al. 2006). Each magnetosome contained with 1–18 NPs per chain. Likewise, Magnetobacterium bavaricum, another magnetic bacterium had a more than 100 NPs per chain of one magnetosome measuring 110–150 nm. The magnetosome had a irregular shape (Hanzlik et al. 1996). Magnetite crystals with a uniform size were produced by magnetotactic bacteria. M. magnetotacticum produced ~50 nm sized Fe3O4 NPs (Lang and Schuler 2006). Likewise, bacterial magnetic NPs (Fe3O4) with an average diameter of 70 nm were synthesized from M. gryphiswaldense MSR-1 cells (Guo et al. 2008). Magnetic iron sulfide (FeS) NPs measuring 2 nm in size were produced on the cell surface of Desulfovibrio vulgaris, a sulfate-reducing bacteria (Watson et al. 1999). A study by Bose et al. (2009) reports the biosynthesis of Fe2O3 by Shewanella oneidensis MR-1 with an average size of 30–43 nm. An iron reducing bacterium, Shewanella oneidensis was used by Perez-Gonzalez et al. (2010) to produce Fe3O4 NPs with 40–50 nm size. Bharde et al. (2005) synthesized iron oxide NPs by using aqueous iron complexes with Actinobacter spp. bacterium. Ferromagnetic particles in the size range of 10–50 nn were produced from Geobacter metallireducens GS-15 and Magnetospirillum strain AMB-1 and (Vali et al. 2004; Elblbesy et al. (2014). Different bacterial strains utilized for synthesizing magnetic NPs are listed in the Table 19.3.
2.4 Sulfide Nanoparticles
Sulfide NPs, being nano-sized possess a unique novel optical and electronic properties and thus, widely applied as used as quantum dots fluorescent biomarker, for cancer diagnosis, radio/chemosensitizing, drug delivery and labeling of cells (Juzenas et al. 2008; Smith et al. 2008; Li et al. 2011). Clostridium thermoaceticum precipitated CdS from 1 mM CdCl2 on its cell surface and to the surrounding medium when cultured in the growth media containing cysteine hydrochloride. It was proclaimed that cysteine acted as the sulfide source (Cunningham and Lundie 1993). Klebsiella aerogenes and Klebsiella pneumoniae formed CdS on their cell surface measuring 5–200 nm when exposed to cadmium ions in the culture medium (Holmes et al. 1995; Smith et al. 1998). These NPs demonstrated optical as well as photoactive features comparable to inorganically synthesized CdS systems (Smith et al. 1998). Likewise, zinc sulfide (ZnS) NPs synthesis has been reported by a sulfate-reducing bacteria (Desulfobacteriaceae). In another study by Gong et al. (2007), PbS NPs of 13 nm were synthesized from Desulfotomaculum sp. under mild conditions. In this biosynthetic process, Desulfotomaculum sp. used sulfate as electron acceptor to form sulfide which acts as sulfur source for forming PbS nanocrystals. The particles were found to have a spherical shape with 2–5 nm diameter (Labrenz et al. 2000). Similarly, Bai et al. (2006) reported a novel synthesis of ZnS NPs of 8 nm by an immobilized Rhodobacter sphaeroides. In another study, E. coli was shown to synthesize intracellular CdS crystals in nanoregime when cultured along with sodium sulfide and cadmium chloride. These nanocrystals showed the wurtzite crystal structure and their size was about 2–5 nm. Further, biosynthesis of CdS nanocrystals was found to increase up to 20-fold when cells were in the stationary phase as compared to their log phase (Sweeney et al. 2004). Lactobacillus sp. mediated biosynthesis of CdS NPs has been reported by Prasad and Jha (2010). According to them Lactobacilli sp. with a negative electro-kinetic potential readily attracted cations during the process of biosynthesis. CdS NPs had an average size of 4.93 ± 0.23 nm. Li et al. (2009) reported the CdS nanofibres formation by using the bacterial cellulose obtained from Gluconoacetobacter xylinus strains. Similarly, Rhodopseudomonas palustris assisted extracellular CdS nanocrystals formation was reported by Bai et al. (2009). According to them, the lyase activity of an enzyme (cysteine desulfhydrase) was responsible for the synthesis of these spherical shaped NPs with an average diameter of 8.01 ± 0.25 nm. Similarly, Bai and Zhang (2009) demonstrated a reliable method of biosynthesizing extracellular lead sulfide (PbS) NPs of 10.5 ± 0.15 nm size by using immobilized R. sphaeroides. Likewise, B. anthracis PS2010 was demonstrated to biosynthesize PbS NPs (El-Shanshoury et al. 2012). Interestingly, CdS quantum dots in genetically engineered E. coli by overexpressing CdS binding peptide (Kang et al. 2008; Chen et al. 2009; Mi et al. 2011). Using Antarctic psychrotolerant bacteria (Pseudomonas spp.) quantum dots were biosynthesized at low temperature (Gallardo et al. 2014). The hypothesized mechanism of PbS nanocrystals biosynthesis could be because of Pb precipitation. Lately, Plaza et al. (2016) explored the biosynthesis of CdS and CdTe quantum dots by using Antarctic bacteria (Pseudomonas strains) resistant to cadmium and tellurite. Different bacterial strains utilized for synthesizing sulphide NPs are listed in the Table 19.4.
2.5 Selenium Nanoparticles
Selenium NPs possess optical, photochemical, and semiconducting properties. Thus, they are widely used in electronic circuit devices and photocopiers (Narayanan and Sakthivel 2010). Different bacterial strains utilized for synthesizing selenium NPs are listed in the Table 19.5. A rhizobacterial strain (Stenotrophomonas maltophilia SELTE02) from the soil of selenium successfully transformed selenite to elemental selenium (Se0). They found that selenium granules accumulated in the cell cytoplasm and extracellular space (Gregorio et al. 2005). In a study by Bajaj et al. (2012) produced spherical shaped selenium NPs from Duganella sp. and Agrobacterium sp. with an approximate diameter of 140–200 and 185–190 nm respectively. Also, P. aeruginosa SNT1 from seleniferous soil was found to biosynthesize spherical amorphous selenium nanostructure both intracellularly and extracellularly (Yadav et al. 2008). Selenium NPs were also synthesized from Enterobacter cloacae SLD1a-1 (Yee et al. 2007), Bacillus cereus (Dhanjal and Cameotra 2010), Thauera selenatis (Debieux et al. 2011), Pantoea agglomerans (Torres et al. 2012) and Bacillus mycoides SeITE01 (Lampis et al. 2014). The size of these NPs ranged from 20 to 400 nm. Srivastava et al. (2014) synthesized selenium NPs from Halococcus salifodinae BK18. More recently, Pseudomonas putida KT2440 was employed to biosynthesize selenium NPs. Measurements by the transmission electron microscope showed that selenium NPs had a size between 100 and 500 nm. These particles were found either attached to the cell membrane or in the surrounding medium (Avendaño et al. 2016). Similarly, Kora and Rastogi (2016) reported the biosynthesis of selenium NPs with a size range of 47–165 nm from a Gram-negative bacterial strain, Pseudomonas aeruginosa. Lately, Azoarcus sp. CIB, a facultative anaerobic β-Proteobacterium mediated synthesis of selenium NPs was reported by Fernández-Llamosas et al. (2016). The NPs had a spherical shape and possessed an average size of 123 ± 35 nm.
2.6 Other Nanoparticles (Palladium, Uranium, Lead, Cobalt etc.)
Various other types of NPs including Pd, Ti, Ba, Co, Pb, Zn etc. NPs synthesized from different bacteria are shown in Table 19.6. Soluble palladium (Pd) salts like Na2PdCl4 was reduced to produce palladium Pd (0) metallic NPs on the cell walls and within the periplasmic space of the bacterium, Shewanella oneidensis MR-1 cells (Windt et al. 2005). Pd NPs were also synthesized form Citrobacter braakii (Hennebel et al. 2011). The presence of H2, lactate, formate, ethanol and pyruvate were reported to function in the process of biosorption and succeeding bioreduction of Pd (II). Likewise, bioreduction of uranium (VI) by S. oneidensis MR-1 and Desulfovibrio desulfuricans was also reported (Burgos et al. 2008; Bargar et al. 2008). The average size of uranite NPs was 3 nm as determined by high-resolution transmission electron microscopy and X-ray absorption spectroscopy. Also, scanning electron microscopy revealed that uraninite (UO2) NPs were observed to be formed and accumulated on exopolymeric substances of the cell surface (Marshall et al. 2006; Wall and Krumholz 2006; Bargar et al. 2008; Burgos et al. 2008). Likewise, the bioreduced UO2 NPs from S. putrefaciens were found deposited extracellularly or in the periplasmic space (Baranska and Sadowski 2013). Similarly, S. oneidensis MR-1 synthesized a long, unique uranium (VI) nanowires that differed from other biogenic uraninite NPs (Jiang et al. 2011). Kumar et al. (2008) synthesized cubic shaped Co3O4 NPs attached with proteins by using Brevibacterium casei, a metal-tolerant bacterium and aqueous cobalt acetate. These extracellularly synthesized particles had a size in the range of 5–7 nm. Both Enterobacter sp. and Bacillus anthracis when treated with dried Pb(NO3)2- produced lead oxide (PbO) NPs (El-Shanshoury et al. 2012). Enterobacter sp. showed intracellular synthesis within the periplasmic space and later exported exterior to the cell wall. While, B. anthracis showed extracellular route of biosynthesis. Lactobacillus sp. assisted titanium dioxide (TiO2) NPs were produced at ambient room temperature by Prasad et al. (2007). The NPs were in spherical form and their size ranged from 40 to 60 nm. Similarly, the biogenic synthesis of TiO2 NPs from Aeromonas hydrophila and Propionibacterium jensenii was described by Jayaseelan et al. (2013) and Babitha and Korrapati (2013), respectively. Likewise, a cost-effective and reproducible green synthesis of BaTiO3 NPs was reported by using Lactobacillus sp. (Jha and Prasad 2010). NPs were having crystalline structure of tetragonal shape with the size of 20–80 nm. According to them, reactive oxygen species and hydrogen (rH2) gas of the culture solution possibly play a role in the process of biosynthesizing nano-BaTiO3. Similar mechanistic view was proposed by Jha et al. (2009a) for the biosynthesis of TiO2 NPs by using Lactobacillus sp. TiO2 NPs had a spherical shape and the size ranged from 8 to 35 nm. Further, cubic shaped antimony trioxide (Sb2O3) NPs of 3–12 nm size were also synthesized from Lactobacillus sp. (Jha et al. 2009b). Zinc oxide (ZnO) NPs of 5–15 nm size were biosynthesized from Lactobacillus sporoge (Prasad and Jha 2009). Biosynthesis of silicon/silica nano-composites by Actinobacter sp. was demonstrated by Singh et al. (2008). These extracellularly produced quasi-spherical particles were having a size of about 10 nm. Using E. coli, extracellular biosynthesis of copper oxide (CuO) NPs was reported by Singh et al. (2010). The spectral characterization revealed the occurrence of a quasi-spherical shaped and 10–40 nm sized CuO NPs.
3 Different Nanoparticles from Cyanobacteria
A filamentous cyanobacteria (Plectonema boryanum UTEX 485) when interacted with Au(S2O3)2 3− solution promoted the biosynthesis of cubic AuNPs of size <10–25 nm (Lengke et al. 2006a). A research work on the mechanistic approach revealed that P. boryanum UTEX 485 interacts with aqueous AuCl3 and initially promote gold(I)−sulfide precipitation on the cell walls, followed by depositing octahedral metallic gold of nanosize range between ∼10 nm and 6 μm near the cell surface and in the solution (Lengke et al. 2006b). Further, the same strain was used for the extracellular biosynthesis of platinum (Pt) NPs of size ranging between 30 and 300 nm (Lengke et al. 2006c). Using some cyanobacterial strains (Limnothrix sp. 37-2-1, Anabaena sp. 66-2 and Synechocystis sp. 48-3), irregular and elongated shaped AgNPs measuring between 14 and 31 nm were synthesized by Patel et al. (2015). Likewise, the extracts of 30 Cyanobacterial strains were used to synthesize AgNPs (Husain et al. 2015). Among them, Cylindrospermum stagnale NCCU-104 was observed to produce smallest sized (38–40 nm) extracellular AgNPs with different shapes (Table 19.7). More recently, Nostoc sp. strain HKAR-2 mediated AgNPs synthesis has been reported with particle size of 51–100 nm (Sonker et al. 2017). Various NPs synthesized from cyanobacteria are shown in Table 19.7.
4 Different Nanoparticles from Actinomycetes
The secondary metabolites produced from Actinomycetes serve as a source of medicaments including antibiotics. Besides, easy genetic manipulation of actinomycetes allow to better control over size (Bhosale et al. 2015; Abd-Elnaby et al. 2016). Exploration of Actinomycetes have proved to be suitable for potential application of NPs synthesis. Actinomycetes assisted NPs exhibit relatively good stability and polydispersity (Bhosale et al. 2015; Abd-Elnaby et al. 2016). Using a Rhodococcus sp. (alkalotolerant actinomycete), AuNPs were synthesized intracellularly with the dimension of 5–15 nm (Ahmad et al. 2003a). Electron microscopic data revealed that AuNPs had a monodispersity and were produced on both cell walls and cytospasmic membranes. They also, predicted the possible role of the cell wall and cytoplasmic membrane specific enzymes in carrying out reduction of metal ions. Likewise, by using an extremophile, Thermomonospora sp. Ahmad et al. (2003b) have proved the biosynthesis of monodispersed, and spherical AuNPs with an average size of 8 nm. Further, characterization data of Fourier transform infrared spectroscopy confirmed the possible role of amide (I) and (II) bands of protein acting as capping and stabilizing agents on NPs surface. Likewise, Actinomycetes sps. mediated synthesis of AgNPs was reported by many researchers (Chauhan et al. 2013; Abdeen et al. 2014; Bhosale et al. 2015; Mohamedin et al. 2015; Składanowski et al. 2016). Most of these particles were in the nanoregime (<100 nm) and possessed spherical or oval shapes. The presence of reductase enzymes are believed to possibly involve in reducing metal ions. An industrially reliable green approach of synthesizing extracellular AgNPs using Streptomyces hygroscopicus has been proposed by Sadhasivam et al. (2010). AgNPs (20–30 nm) were observed to possess a spherical shape. Lately, AgNPs and AuNPs biosynthesized from Streptomyces sp. possessed spherical and oval shapes. The size of AgNPs remained in the nanoregime of about 8–44 nm while, AuNPs had 10 nm size (Składanowski et al. 2016). Using Streptomyces hygroscopicus (Waghmare et al. 2014), synthesized spherical AuNPs spherical in the range of 10–20 nm. Lately, Abd-Elnaby et al. (2016) biosynthesized AgNPs of 22–85 nm from S. rochei MHM13. Extracellular synthesis of CuO NPs with 100–150 nm size was recorded using Streptomyces sp., an isolate of Pichavaram mangrove , India (Usha et al. 2010). Various NPs synthesized from cyanobacteria are shown in Table 19.8.
5 Different Nanoparticles from Yeast
AgNPs with a size range of 2–5 nm were biosynthesized extracellularly by Kowshik et al. (2002a) using a silver tolerant yeast strain, MKY3. Likewise, Korbekandi et al. (2016) biologically synthesized spherical AgNPs with 2–20 nm in diameter by using dried yeast cells. Lately, Abdehgah et al. (2017) used Candida albicans to biologically synthesize crystalline AgNPS ranging 20–80 nm in size with spherical and oval morphologies. Agnihotri et al. (2009) reported the biosynthesis of hexagonal and triangular shaped AuNPs with a mean size of 15 nm from the non-conventional yeast Yarrowia lipolytica. Dameron et al. (1989) reported the biosynthesis of quantum crystallites from C. glabrata and Schizosaccharomyces pombe when grown in the presence of cadmium salts. CdS NPs synthesis from Sachharomyces cerevisiae has been reported by Prasad and Jha (2010). These CdS NPs had an average size of 3.57 ± 0.21 nm. Likewise, intracellular precipitation of CdS NPs was observed when a strain, S. pombe was challenged with cadmium (1 mM) solution (Kowshik et al. 2002b). Jha et al. (2009b) biosynthesized TiO2 NPs using Lactobacillus sp. and the nanocrystals were having a spherical shape with 8–35 nm diameter. Sb2O3 NPs were synthesized from S. cerevisiae. These nanostructures were spherical in shape with 2–10 nm size and possessed a face centered cubic unit cell structure (Jha et al. 2009b). Intracellular biosynthesis of copper NPs using Rhodotorula mucilaginosa showed the occurrence of spherically shaped particles with an average size of 10.5 nm (Salvadori et al. 2014). An efficient biosynthesis and easy harvesting method was proposed by Bao et al. (2010) to obtain biocompatible cadmium telluride (CdTe) quantum dots by using yeast cells. Fakhrullin et al. (2010) reported the possible preparation of viable yeast cells deposited with Fe3O4 NPs on the exterior of the cell’s wall. Similarly, yeast cells modified with magnetic NPs have been achieved lately (Gorobets et al. 2011, 2013; Safarik et al. 2015). AuNPs of size 13.0 ± 0.9 nm were biologically synthesized using an S. cerevisiae cells extract. The presence of yeast cell metabolites including glucose, trimethylsilyl derivatives of butan-2,3-diol, undecanoic acid and indole-3-acetic acid acted as a capping/reducing agents during AuNPs formation (Attia et al. 2016). Various NPs synthesized from cyanobacteria are shown in Table 19.8.
6 Fungal Based Nanoparticles
Fungi commonly called as decomposing organisms are considered as eukaryotic organisms which reside in almost every corner of the earth. Fungi are capable of performing several tasks which include digestion of extracellular food, conversion of complicated molecules into simpler one by enzymatic hydrolysis, and utilize the energy (Blackwell 2011). Fungi are also causative agents of many medical complications in humans as well as animals. However, apart from being pathogenic, fungi play significant role in medicine field for instance, production of antibiotics, and their role in the synthesis of NPs which are playing a key role in biomedical field and many more. NPs have been synthesized through many different techniques; one among these techniques is the biological synthesis, which includes plants, yeast, bacteria and fungi. The application of fungi in biosynthesis NPs was first reported in the production of CdSe NPs by Candida albicans (Dameron et al. 1989; Xue et al. 2016). Fungi are regarded as more advantageous compared to any other microorganisms in the biosynthesis of NPs due to several characteristic features, which include, fast growth and easy to handle (many species), fungal mycelia can better withstand bioreactor conditions such as, high pressure and agitation, secretion of more extracellular enzymes , large biomass capacity, economic livability, high metal accumulation capacity, and many more (Castro-Longoria et al. 2011; Musarrat et al. 2011a; Moghaddam et al. 2015). The tolerance and metal bioaccumulation capability of fungi has also attracted and put them to research in the biological production of metallic NPs (Sastry et al. 2003). Moreover, the scale-up of fungi is easy and is an additional advantage in utilizing them in biosynthesis of NPs, the effective secretion of enzymes (extracellular enzymes), also helps in achieving the vast production of enzymes a feasibility (Castro-Longoria et al. 2012). Most of the fungi have intracellular metal uptake and high wall-binding capacities (Volesky and Holan 1995; Moghaddam et al. 2015), which are significant in the nanoparticle synthesis. The metabolic diversity of fungi attracted them as potential candidates in biosynthesis of NPs. Fungi synthesize NPs either by intracellular or extracellular process and reduction is the key process involved in the synthesis of metallic NPs (Sadowski et al. 2008). Intracellular process involves attachment of metal ions on to the fungal cell surface, through electrostatic interaction. The opposite charges on the surface of metal ion and fungal cell surface generate electrostatic force of attraction. After the absorption of metal ions, enzymes of fungal cell wall which contain positively charged groups reduce metal ions leading to aggregation of nano structures and finally metal NPs. However, In case of extracellular synthesis the enzymes secreted by fungi (Eg. nitrate reductase) reduce metal ions, which finally leads to the formation of stable NPs (Khandel and Shahi 2016). Several different NPs such as gold, silver, zinc oxide and many more have been synthesized using different species of fungi including Fusarium oxyporum, Aspergillus niger, Aspergillus fumigatus etc. Various fungi employed in the synthesis of different NPs is shown in Table 19.9.
6.1 Silver Nanoparticles (AgNPs)
Silver and its compounds are known to have potential applications in biomedicine field. Several different techniques are available for the synthesis and characterization of AgNPs. Fungi play significant role in synthesis of AgNPs; several different species of fungi have been explored in the biosynthesis . Fusarium oxysporum has been widely employed in NP synthesis and the secretion of proteins in to the aqueous solution helps in the development of sulfate diminishing enzyme -based protocol for NPs production. AgNPs with varying morphology with a size ranging from 5 to 50 nm (Ahmad et al. 2003c) and spherical structures with size range 20–50 nm were synthesized from F. oxysporum (Durán et al. 2005). The variation in morphology and size could be due to variations in temperature, and different structures can be obtained based on the metallic ion solution and incubation parameters (Riddin et al. 2006; Moghaddam et al. 2015). Earlier studies have reported that F. oxysporum synthesizes various sizes and forms of NPs by extracellular synthesis mechanism (Moghaddam et al. 2015). Mukherjee et al. (2001) reported that F. oxysporum decreases the metal ions through NADH-based reductases and also by shuttle Quinone extracellular procedure (Mukherjee et al. 2001).
Several groups have synthesized and characterized AgNPs from different species of fungi. AgNPs with size range of 10–25 nm and Quasi-spherical were successfully produced using Rhizopus stolonifer by extracellular process exploring purified α-NADPH-dependent nitrate reductase which acts as an electron carrier (Binupriya et al. 2010). Furthermore, AgNPs synthesized using Arthroderma fulvum range in size from 15.5 ± 2.5 nm, and are spherical in shape (Xue et al. 2016). Earlier study by Gade et al. reported that AgNPs with a definite size range of 5–25 nm were synthesized within 72 h by Aspergillus niger when incubated with silver nitrate solution. The synthesized NPs were observed as aggregates on the surface of cell wall, which were then reduced to stabilize using reducing enzymes and proteins produced by the fungus A. niger (Khandel and Shahi 2016). The NPs synthesized by exposing aqueous silver nitrate solution to Fusarium acuminatum cell extract were found to be spherical in shape with a size range of 5–40 nm with an average diameter of 13 nm. The nitrate-dependent reductase could be responsible for reduction of silver ions which is found in the extra-cellular medium (Ingle et al. 2008). Further, a study by Magdi et al. (2014), reports the reduction of silver salt into AgNPs by Aspergillus fumigatus, Candida albicans, Penicillium italicum, Syncephalastrum racemosum, Fusarium oxysporum and Aspergillus ochraceus. These AgNPs were spherical in shape, with a mean size of 13.88 ± 4.11 nm (Magdi et al. 2014). Furthermore, AgNPs synthesized using Aspergillus fumigatus by extracellular process were mostly spherical with a size range of 15–45 nm (Alani et al. 2012). However, AgNPs obtained from Trichoderma viride were intracellular and mostly spherical with size range of 2–4 nm (Fayaz et al. 2010).
6.2 Gold Nanoparticles (AuNPs)
The characteristic features of AuNPs such as biocompatibility, stability and resistant to oxidation, bio-available surface, allowed them gain significant importance in medicine and biology. The chemical methods of AuNPs synthesis which raise the environmental concerns due to release of toxic residues may be replaced by low-cost and environment friendly process such as, biological synthesis. Several viable microorganisms and their cell-free extracts have been employed by different groups in the synthesis of AuNPs. The biosynthetic process of AuNPs involves two main precursors such as, gold chloride (HAuCl4) and AuCl. HAuCl4 dissociates to Au3+ ions (Khan et al. 2013) and AuCl dissociates to Au+ (Zeng et al. 2010). Like AgNPs, AuNPs can also be synthesized by either intracellular or extracellular process. In extracellular process Au3+ ions are reduced through proteins by trapping them in the cell wall. However, in the case of intracellular process, cytosolic redox mediators reduces Au3+ ions upon diffusion through the cell membrane (Das et al. 2012; Kitching et al. 2015). Nicotinamide adenine dinucleotide (NADH)/nicotinamide adenine dinucleotide phosphate (NADPH) oxidoreductases reduce Au ions either in the cytoplasm or in the cell surface. However, the mechanism of enzymatic reduction of Au3+ remains the same in both intra and extracellular process (Gupta and Bector 2013).
AuNPs synthesized by fungi vary in their shape and size, a study by Gupta and Bector (2013) reports the intracellular production of AuNPs by Aspergillus fumigatus and Aspergillus flavus, with an average diameter of 22 ± 2 nm. Spherical shaped AuNPs with varying size were synthesized using different fungi including Aspergillus niger (12.79 ± 5.61 nm), Aureobasidium pullulans (29 ± 6 nm), Fusarium semitectum (25 nm), Neurospora crassa (32 nm) and Volvariella volvacea (20–150 nm) (Sawle et al. 2008; Bhambure et al. 2009; Philip 2009; Zhang et al. 2011; Castro-Longoria et al. 2011). Furthermore, AuNPs produced from Colletotrichum sp. were decahedral and icosahedral in shape with a size range of 20–40 nm, while AuNPs from Rhizopus stolonifer were reported as irregular (uniform) with size ranging from 1 to 5 nm (Shankar et al. 2003; Sarkar et al. 2012). Gold NPs with a diameter range of 6–18 nm and spherical shape were synthesized by treating gold chloride solution with culture filtrate of the Nigrospora oryzae (Kar et al. 2014). On the other hand, Balakumarana et al. (2016) reported the synthesis of AuNPs from mycelial free filtrate of Aspergillus terreus which were spherical shape and 10–20 nm in size with; however some particles were observed in anisotropic morphology with a size of 10–50 nm. Several other fungi species have been explored in the successful synthesis of AuNPs which include Volvariella volvacea, Phanerochaete chrysosporium, Fusarium oxyporum, Rhizopus stolonifer, Cylindrocladium floridanu and many more through extracellular process. However, the species including Fusarium oxysporum, Verticillium luteoalbum, produces AuNPs through intracellular process. Some species are capable producing AuNPs by both extracellular and intracellular processes which include Coriolis versicolor, Rhizopus oryzae, Aspergillus niger, Candida albicans. Organisms such as Verticillium sp. and Rhizopus oryzae produce NPs on cell wall and cell surface respectively (Moghaddam et al. 2015).
6.3 Magnetic Nanoparticles
Magnetic NPs have broad and potential applications in biological and biomedicine fields due to their super-paramagnetic, high coercive force and unique micro-configuration properties. Magnetic NPs exhibit remarkable characteristic features such as super-paramagnetism, high saturation field, high field irreversibility, extra anisotropy contributions/shifted loops after field cooling. For biomedical applications , iron oxide NPs such as magnetite (Fe3O4) or its oxidized form maghemite (γ-Fe2O3) are most commonly employed. Even though, cobalt and nickel exhibit higher magnetic property, they are of less importance in biomedical science, due to their toxicity and susceptibility to oxidation (Akbarzadeh et al. 2012). The well-known biocompatible magnetic NPs such as Fe3O4 (magnetite) and Fe2O3 (maghemite) have been employed various biological and medicine field. Magnetite (Fe3O4) NPs have been synthesized extracellularly with the aqueous mixture of ferricyanide/ferrocyanide using fungi F. oxysporum and Verticillium sp. The proteins secreted by these fungi hydrolyze iron precursors to form magnetite (Fe3O4) at room temperature. The particles obtained from Fusarium oxysporum were found to be irregular in shape with an overall quasi-spherical morphology and size range from 20 to 50 nm; however, particles from Verticillium sp. were cubo-octahedral and quasi-spherical in shape (Bharde et al. 2006). However, iron oxide NPs synthesized by extracellular process using Aspergillus japonicus varied in size from 60 to 70 nm having cubical shape with crystal structure which corresponds to magnetite (Bhargava et al. 2013). Further, FeCl3 NPs synthesized extracellularly from Aspergillus oryzae were 10–24.6 nm in size with spherical shape (Tarafdar and Raliya 2013).
6.4 Fluorescent/Luminescent NPs
The unique size-dependent properties of fluorescent/luminescent colloidal NPs or quantum dots (QDs) which are in the size range of 1–20 nm and composed group II–IV, III–V or IV–VI elements such as, Cd, Te, Se, Zn, In, As), have attracted the researchers in both basic and applied field (Kovalenko et al. 2015; Plaza et al. 2016). Several different groups have synthesized these fluorescent/luminescent NPs and characterized for their applications in biology and medicine. Cadmium sulfide (CdS) QDs were synthesized extracellularly from the white rot fungus Phanerochaete chrysosporium by incubating in cadmium nitrate tetrahydrate solution. X-ray analysis of the CdS NPs showed face-centered cubic crystal structure with an average size of approximately 2.56 nm. Furthermore, the formation and stabilization of CdS QDs may be attributed to the secretion of cysteine and proteins from Phanerochaete chrysosporium (Chen et al. 2014). Zinc sulphide (ZnS) and CdS NPs have also been synthesized using Fusarium sp. by exposing CdSO4 and ZnSO4 solutions having sizes from 20 to 150 nm. Cadmium NPs Fusarium oxyporum produced extracellular process were found to be spherical with the size range of 9–15 nm (Kumar et al. 2007). Moreover, ZnNPs produced by an intracellular process using Fusarium spp.were irregular in shape and 100–200 nm in size range (Velmurugan et al. 2010). In another study CdS NPs have been produced extracellularly from CdSO4 solution using Fusarium oxysporum and it has been reported that CdS particles were in the size range 5–20 nm with hexagonal shape. The possibility of synthesizing PbS, ZnS, and MoS2 NPs by enzymatic reduction of sulfate ions by Fusarium oxysporum was also tested and preliminary investigations indicated the possible realization of such chemical biotransformations using fungal extracts (Ahmad et al. 2002). Several reports have described synthesis of selenium NPs (SeNPs) using fungi (Gharieb et al. 1995; Sarkar et al. 2011; Vetchinkina et al. 2013; Zare et al. 2012). Mono-dispersed spherical SeNPs have been extracellularly produced using Aspergillus terreus and Alternaria alternate and intracellularly by Lentinula edodes (Sarkar et al. 2011; Vetchinkina et al. 2013; Zare et al. 2012). Fusarium sp. and Trichoderma reesei have also been reported in the production of SeNPs (Gharieb et al. 1995).
6.5 Other Nanoparticles
Apart from the above discussed NPs several other NPs having potential biomedical applications have been mycosynthesized and characterized by several groups. Platinum NPs (PtNPs), unlike the widely researched AgNPs and AuNPs, have been synthesized from the fungus Neurospora crassa. It has been reported that intracellular single PtNPs are in the size range of 4–35 nm in diameter, while nano-agglomerates which are spherical in shape are of 20–110 nm in diameter (Castro-Longoria et al. 2012). A study by Riddin et al. (2006) also reports the production of PtNPs by another fungus F. oxysporum, which were formed both intracellularly and extracellularly (Riddin et al. 2006). A novel eco-friendly approach of producing CdS NPs was achieved under ambient conditions by immobilizing the fungus, Coriolus versicolor in a continuous column (Sanghi and Verma 2009). The CdS nanocrystals had a spherical shape with size ranging 100–200 nm. Aspergillus flavus has been employed in the synthesis of TiO2NPs which range in size from 62 to 74 nm having spherical shape (Rajakumar et al. 2012). Furthermore, Hg NPs have been synthesized using Aspergillus versicolor mycelia, the synthesized NPs accumulated on the surface of mycelia which are 20.5 ± 1.82 nm in size (Das et al. 2008).
7 Applications of Microbial Nanoparticles
Different types of NPs synthesized using fungi have found potential applications in various fields of medicine and biology including cancer treatment, treatment of bacterial infections (antibacterial activity), acts as biosensors and bioimaging agents. Antibiotics such as amoxicillin, erythromycin, clindamycin, penicillin G, and vancomycin when used along with AgNPs enhanced their antimicrobial effect against Staphylococcus aureus and Escherichia coli (Shahverdi et al. 2007b). The AgNPs synthesized from bacterial strains have shown superior antimicrobial activity against several pathogens including methicillin-resistant S. aureus, vancomycin-resistant Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis and Streptococcus pyogenes, E. coli, B. subtilis, B. cereus S. pyogenes and P. aeruginosa, Salmonella typhi, S. typhimurium, Haemophilus influenzae and Klebsiella pneumonia (Nanda and Saravan 2009; Priyadarshini et al. 2013; Abirami and Kannabiran 2016). BioNPs synthesized from B. megaterium were effective against multi-drug resistant pathogens Streptococcus pneumoniae and Salmonella typhi (Saravanan et al. 2011). The biosynthesized AgNPs from Streptomyces sp. exhibited antifungal activity against Aspergillus niger, A. flavus and A. fumigatus (Thenmozhi et al. 2013). Likewise, anticandidal properties of AgNPs from Streptomyces sp. VITPK1 was shown against Candida albicans, C. tropicalis and C. krusei (Sanjenbam et al. 2014). Biosynthesized AgNPs from S. hygroscopicus significantly inhibited several microbial pathogens such as B. subtilis, E. faecalis, E. coli, S. typhimurium and and C. albicans (Sadhasivam et al. 2010; Chauhan et al. 2013; Abdeen et al. 2014; Mohamedin et al. 2015; Składanowski et al. 2016). CuO NPs from a actinomycete showed greater activity against E. coli, S. aureus, and A. niger (Usha et al. 2010). Likewise, the microbes , E. coli, and S. typhi were greatly inhibited by AgNPs synthesized from Bacillus sp. AZ1 (Deljou and Goudarzi 2016). The AgNPs fabricated to cotton cloth significantly showed antimicrobial activity against E. coli, S. aureus, and C. albicans (El-Naggar et al. 2016). E. coli assisted AgNPs exhibited inhibitory activity against pathogens namely, Salmonella typhi, Bacillus subtilis, Vibrio cholerae, and Klebsiella pneumoniae (Divya et al. 2016). AgNPs synthesized from yeast cells effectively inhibited a haploid pathogenic yeast, Candida glabrata (Abdehgah et al. 2017). Further, microbially synthesized AgNPs function as an anti-biofouling agents (Abd-Elnaby et al. 2016). Likewise, cyanobacterial assisted AgNPs against B. megaterium, E. coli, B. subtilis, M. luteus and S. aureus have shown a significant antibacterial effect (Patel et al. 2015). Likewise, Aspergillus niger, Trichoderma harzianum, Ralstonia solanacearum and Xanthomonas campestris were inhibited by Nostoc sp. strain HKAR-2 mediated AgNPs (Sonker et al. 2017). Several groups have reported the potential applications of mycosynthesized NPs. Silver NPs produced from Arthroderma fulvum had shown considerable antifungal activity against Candida spp., Aspergillus spp., and Fusarium spp. (Xue et al. 2016). The effective antibacterial activity of AgNPs synthesized from F. acuminatum was observed against several pathogenic bacteria, including multidrug resistant Staphylococcus aureus, Staphylococcus epidermidis, Salmonella typhi, and Escherichia coli (Ingle et al. 2008). Gold NPs derived from Trichoderma viride exhibited potential antimicrobial activity (Mishra et al. (2014). Mycosynthesized AuNPs have potential applications in various fields such as in agriculture (pesticidal agents), in optro-electronics, in cancer treatment, and as antibacterial agents (Das et al. 2009; Sawle et al. 2008; Mishra et al. 2011; Moghaddam et al. 2015). B. megaterium mediated biosynthesized AgNPs exhibited a potent larvicidal activity against Culex quinquefasciatus and Aedes aegypti (Banu and Balasubramanian 2015; Banu et al. 2014). Anopheles subpictus and Culex tritaeniorhynchus larvae were inhibited by AgNPs synthesized from psychrotolerant Pseudomonas mandelii (Mageswari et al. 2015). Likewise, AgNPs synthesized using B. amyloliquefaciens and B. subtilis were effective in controlling the growth of mosquito colonies of Culex pipiens pallens (Fouad et al. 2016). The biosynthesized AuNPs from E. coli DH5a are demonstrated to be beneficial for understand the electrochemistry of hemoglobin (Du et al. 2007). The immobilized doxorubicin on bacterial magnetic NPs easily dissolved in an aqueous solution and possessed very high magnetic and ferromagnetic properties. Also, these particles exhibited strong cytotoxicity against cancer cells namely, HePG2 and MCF-7 (Guo et al. 2008). Selenium NPs by Halococcus salifodinae BK18 exhibited potent anti-proliferative activity against HeLa cell lines (Srivastava et al. 2014). AuNPs from yeast cells shows potent anticancer activity against Ehrlich ascites carcinoma cells. The cell toxicity is mainly due to the photothermal properties of AuNPs (Attia et al. 2016). More recently, Nostoc sp. strain HKAR-2 mediated AgNPs showed potent anticancerous activities against MCF-7 cells (Sonker et al. 2017). The AgNPs synthesized using fungi had shown anticancer activity against several human cancers such as, colon carcinoma, breast cancer, and hepato-cellular carcinoma cells (Magdi et al. 2014). The AgNPs synthesized from Aspergillus fumigatus exhibited antiviral against HIV-1(Alani et al. 2012); while AgNPs obtained from Trichoderma viride finds potential applications in biosensor and bio-imaging (Fayaz et al. 2010). Further, AuNPs obtained from Penicillium brevicompactum showed in vitro cytotoxic activity against mouse mayo blast cancer cells (C2C12) (Mishra et al. 2011). AuNPs produced using mycelia-free culture filtrate of Nigrospora oryzae exhibited detrimental effect on the ultra-structure of parasite belonging to Raillietina sp.; (Kar et al. 2014). Antibody-conjugated AuNPs/gold nanoparticle-based probe can serve as a simple diagnostic tool in the diagnosis of cancer that is they can differentiate between cancer and normal cells (Chuhan et al. 2011). Yeast cells with magnetic NPs can be used for biotechnological applications such as bioreactors, biosensors, and bioseparations (Fakhrullin et al. 2009). NPs that possess super paramagnetic behavior at room temperature and magnetic particles which are stable in water at pH 7 have significant and potential biomedical applications such as in therapy, biology and medical diagnosis (Cabuil 2004; Morcos 2007; Ersoy and Rybicki 2007; Akbarzadeh et al. 2012). Magnetic NPs such as Fe3O4 (magnetite) and Fe2O3 (maghemite) have been employed in magnetic resonance imaging (MRI), categorization and manipulation of stem cell, magnetic hyperthermia (cancer therapy ), gene therapy, examination of DNA and trained drug delivery (Xiang et al. 2007). Magnetic iron oxide NPs coated on viral nanofibers can be explored as biomarkers in the detection of human serum antibody (Wang et al. 2015). QDs are more photostable, brighter, and display narrower fluorescence emission spectra which upon excitation by a single wavelength allow multiplexing applications (Liu et al. 2005). SeNPs have exhibit several activities including anticancer activity against breast, lung, kidney, and osteosarcoma, antibacterial, and antioxidant activity. Moreover, they have potential environmental applications also (Sweety et al. 2016).
8 Conclusion and Future Directions
Overall, there is an increased awareness towards the better utilization of cost-effective, economically feasible, biological approaches for biosynthesizing eco-friendly biosynthesis of toxic-free NPs. Over the past few decades, various metal based functional nanomaterials from microorganisms including bacteria, fungi, yeast and cyanobacteria have been successfully synthesized. These nanostructures have been shown to have monodisparity and occur in different sizes, shapes and morphology. Importantly, the microbe assisted nanoparticle biosynthesis do not involves any toxic chemicals or cumbersome processes. Further, NPs from microbe origin have shown to exhibit an excellent physic-chemical or material characteristics with an improved biological properties. Therefore, microbes have been widely used as a new source for synthesizing several metallic NPs with different crystalline structures and properties in the current era of nanobiotechnology. Microbes have high diversity and adopt metal tolerance capabilities and thus, can innately bioreduce metal ions to form metallic NPs which is also sometimes due to a detoxification process in the harsh environment. However, there may occurs many more possible mechanisms during the biological process of nanoparticle biosynthesis which yet to be completely elucidated in detail. Some of the studies have highlighted that metal ions are trapped on the cell surface due to ionic interaction between hydroxyl, carboxyl and other chemical groups occurring on the cells. In some studies, it is reported that metal ions are bioreduced due to enzymatic reactions involving NADPH as a reducing agents. However, Further, high diversity, easy growth, maintenance and genetic manipulation of microbial cells are quite easier, they could be considered as probable biofactories to synthesize NPs in large scale. However, controlled synthesis of a nanostructures with a definite shape, size depends on various factors including the concentration of metal ions, reaction time, temperature, pH and the type of organism used in the bioreduction process. In specific, the microbially produced NPs should possess high stability which is proposed to occur due to proteins expressed by the microbes . Hence, future research should focus on developing a simple, reliable microbe based biological protocol for synthesizing different metallic NPs with uniform shape, size, monodisperity and high stability. In addition, manipulation of genes at the genetic level to overexpress specific enzymes or proteins that involve in reduction reaction or acts as capping agents during the NPs synthesis is another trust area of future discovery. Likewise, the understanding of NPs synthesis by microbes will certainly benefit in mediating the controlled synthesis of functional nanostructures. Thus, microbes based biosynthesis of NPs can be more advantageous for various biomedical applications due to their incredible physic-chemical, optoelectrical, electronic and biological properties.
References
Abdeen S, Geo S, Praseetha PK, Dhanya RP (2014) Biosynthesis of silver nanoparticles from Actinomycetes for therapeutic applications. Int J Nano Dimens 5(2):155
Abdehgah IB, Khodav A, Shamsazar A, Negahdary M, Jafarzadeh M, Rahimi G (2017) In vitro antifungal effects of biosynthesized silver nanoparticle by Candida albicans against Candida glabrata. Biomed Res 28(7):2870–2876
Abd-Elnaby HM, Abo-Elala GM, Abdel-Raouf UM, Hamed MM (2016) Antibacterial and anticancer activity of extracellular synthesized silver nanoparticles from marine Streptomyces rochei MHM13. Egypt J Aquat Res 42(3):301–312
Abirami M, Kannabiran K (2016) Streptomyces ghanaensis VITHM1 mediated green synthesis of silver nanoparticles: mechanism and biological applications. Front Chem Sci Eng 10(4):542–551
Agnihotri M, Joshi S, Kumar AR, Zinjarde S, Kulkarni S (2009) Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater Lett 63(15):1231–1234
Ahmad A, Mukherjee P, Mandal D, Senapati S, Khan MI, Kumar R, Sastry M (2002) Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J Am Chem Soc 124:12108–12109
Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, Srinivas V, Sastry M (2003a) Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 14:824. https://doi.org/10.1088/0957-4484/14/7/323
Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M (2003b) Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete Thermomonospora sp. Langmuir 19:3550–3553
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003c) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B Biointerf 28(4):313–318
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144
Akhtar MS, Swamy MK, Umar A, Sahli A, Abdullah A (2015) Biosynthesis and characterization of silver nanoparticles from methanol leaf extract of Cassia didymobotyra and assessment of their antioxidant and antibacterial activities. J Nanosci Nanotechnol 15:9818–9823
Alani F, Moo-Young M, Anderson W (2012) Biosynthesis of silver nanoparticles by a new strain of Streptomyces sp. compared with Aspergillus fumigatus. World J Microbiol Biotechnol 28:1081–1086
Arakaki A, Nakazawa H, Nemoto M, Mori T, Matsunaga T (2008) Formation of magnetite by bacteria and its application. J R Soc Interface 5:977–999
Attia YA, Farag YE, Mohamed YM, Hussien AT, Youssef T (2016) Photo-extracellular synthesis of gold nanoparticles using Baker’s yeast and their anticancer evaluation against Ehrlich ascites carcinoma cells. New J Chem 40(11):9395–9402
Avendaño R, Chaves N, Fuentes P, Sánchez E, Jiménez JI, Chavarría M (2016) Production of selenium nanoparticles in Pseudomonas putida KT2440. Sci Rep 6:37155. https://doi.org/10.1038/srep37155
Babitha S, Korrapati PS (2013) Biosynthesis of titanium dioxide nanoparticles using a probiotic from coal fly ash effluent. Mater Res Bull 48(11):4738–4742
Babu MMG, Gunasekaran P (2009) Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloid Surf B 74(1):191–195
Bai HJ, Zhang ZM (2009) Microbial synthesis of semiconductor lead sulfide nanoparticles using immobilized Rhodobacter sphaeroides. Mater Lett 63(9):764–766
Bai HJ, Zhang ZM, Gong J (2006) Biological synthesis of semiconductor zinc sulfide nanoparticles by immobilized Rhodobacter sphaeroides. Biotechnol Lett 28(14):1135–1139
Bai HJ, Zhang ZM, Guo Y, Yang GE (2009) Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris. Colloid Surf B 70:142–146
Bajaj M, Schmidt S, Winter J (2012) Formation of Se (0) nanoparticles by Duganella sp. and Agrobacterium sp. isolated from Se-laden soil of North-East Punjab, India. Microb Cell Fact 11:1–14
Balakumarana MD, Ramachandrana R, Balashanmugama P, Mukeshkumarb DJ, Kalaichelvana PT (2016) Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol Res 182:8–20
Banu AN, Balasubramanian C (2015) Extracellular synthesis of silver nanoparticles using Bacillus megaterium against malarial and dengue vector (Diptera: Culicidae). Parasitol Res 114(11):4069–4079
Banu AN, Balasubramanian C, Moorthi PV (2014) Biosynthesis of silver nanoparticles using Bacillus thuringiensis against dengue vector, Aedesaegypti (Diptera: Culicidae). Parasitol Res 113(1):311–316
Bao H, Hao N, Yang Y, Zhao D (2010) Biosynthesis of biocompatible cadmium telluride quantum dots using yeast cells. Nano Res 3(7):481–489
Baranska JA, Sadowski Z (2013) Bioleaching of uranium minerals and biosynthesis of UO2 nanoparticles. Physicochem Probl Miner Process 49(1):71–79
Bargar JR, Bernier-Latmani R, Giammar DE, Tebo BM (2008) Biogenic uraninite nanoparticles and their importance for uranium remediation. Elements 4(6):407–412
Bazylinski DA, Frankel RB (2004) Magnetosome formation in prokaryotes. Nat Rev Microbiol 2(3):217–230
Bhambure R, Bule M, Shaligram N, Kamat M, Singhal R (2009) Extracellular biosynthesis of gold nanoparticles using Aspergillus niger—its characterization and stability. Chem Eng Technol 32:1036–1041
Bharde A, Wani A, Shouche Y, Joy PA, Prasad BL, Sastry M (2005) Bacterial aerobic synthesis of nanocrystalline magnetite. J Am Chem Soc 127(26):9326–9327
Bharde A, Rautaray D, Bansal V, Ahmad A, Sarkar I, Yusuf SM, Sanyal M, Sastry M (2006) Extracellular biosynthesis of magnetite using fungi. Small 2:135–141
Bhargava A, Jain N, Manju Barathi L, Akhtar MS, Yun Y, Panwar J (2013) Synthesis, characterization and mechanistic insights of mycogenic iron oxide nanoparticles. J Nanopart Res 15:337–348
Bhattacharya R, Mukherjee P (2008) Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev 60(11):1289–1306
Bhosale RS, Hajare KY, Mulay B, Mujumdar S, Kothawade M (2015) Biosynthesis, characterization and study of antimicrobial effect of silver nanoparticles by Actinomycetes spp. Int J Curr Microbiol App Sci 2:144–151
Binupriya AR, Sathishkumar M, Yun SI (2010) Biocrystallization of silver and gold ions by inactive cell filtrate of Rhizopus stolonifer. Colloids Surf B: Biointerfaces 79:531–534
Blackwell M (2011) The fungi: 1, 2, 3 ... 5.1 million species? Am J Bot 98:426–438
Bose S, Hochella MF, Gorby YA, Kennedy DW, Mc Cready DE, Madden AS, Lower BH (2009) Bioreduction of hematite nanoparticles by the dissimilatory iron reducing bacterium Shewanella oneidensis MR-1. Geochim Cosmochim Acta 73(4):962–976
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45(3):198–207
Burgos WD, McDonough JT, Senko JM, Zhang G, Dohnalkova AC, Kelly SD, Kemner KM (2008) Characterization of uraninite nanoparticles produced by Shewanella oneidensis MR-1. Geochim Cosmochim Acta 72(20):4901–4915
Cabuil V (2004) Dekker encyclopedia of nanoscience and nanotechnology, chapter 119 magnetic nanoparticles: preparation and properties. Roldan Group Publications
Castro-Longoria E, Vilchis-Nestor AR, Avalos-Borja M (2011) Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf B: Biointerfaces 83:42–48
Castro-Longoria E, Moreno-Velásquez SD, Vilchis-Nestor AR, Arenas-Berumen E, Avalos-Borja M (2012) Production of platinum nanoparticles and nanoaggregates using Neurosporacrassa. J Microbiol Biotechnol 22:1000–1004
Chauhan R, Kumar A, Abraham J (2013) A biological approach to the synthesis of silver nanoparticles with Streptomycessp JAR1 and its antimicrobial activity. Sci Pharm 81(2):607–624
Chen YL, Tuan HY, Tien CW, Lo WH, Liang HC, Hu YC (2009) Augmentedbiosynthesis of cadmium sulfide nanoparticles by genetically engineered Escherichia coli. Biotechnol Prog 25(5):1260–1266
Chen G, Yi B, Zeng G, Niu Q, Yan M, Chen A, Du J, Huang J, Zhang Q (2014) Facile green extracellular biosynthesis of CdS quantum dots by white rot fungus Phanerochaete chrysosporium. Colloids Surf B Biointerfaces 117:199–205
Chen C, Wang P, Li L (2016) Applications of bacterial magnetic nanoparticles in nanobiotechnology. J Nanosci Nanotechnol 16(3):2164–2171
Chengzheng W, Jiazhi W, Shuangjiang C, Swamy MK, Sinniah UR, Akhtar MS, Umar A (2018) Biogenic synthesis, characterization and evaluation of silver nanoparticles from Aspergillus niger JX556221 against human colon cancer cell line HT-29. J Nanosci Nanotechnol 18(5):3673–3681. https://doi.org/10.1166/jnn.2018.15364
Chuhan A, Zubair S, Tufail S, Sherwani A, Sajid M, Raman SC, Azam A, Owais M (2011) Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. Int J Nanomedicine 6:2305–2319
Cunningham DP, Lundie LL (1993) Precipitation of cadmium by Clostridium thermoaceticum. Appl Environ Microbiol 59(1):7–14
Dameron CT, Reese RN, Mehra RK, Kortan AR, Carroll PJ, Steigerwald ML, Brus LE, Winge DR (1989) Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature 338:596–597
Darnall DW, Greene B, Henzel MJ, Hosea M, McPherson RA, Sneddon J, Alexander MD (1986) Selective recovery of gold and other metal ions from an algal biomass. Environ SciTechnol 20:206–208
Das S, Das A, Guha A (2008) Adsorption behavior of mercury on functionalized Aspergillus versicolor mycelia: atomic force microscopic study. Langmuir 25:360–366
Das SK, Das AR, Guha AK (2009) Gold nanoparticles: microbial synthesis and application in water hygiene management. Langmuir 25:8192–8199
Das SK, Liang J, Schmidt M, Laffir F, Marsili E (2012) Biomineralization mechanism of gold by zygomycete fungi Rhizopous oryzae. ACS Nano 6:6165–6173
Das VL, Thomas R, Varghese RT, Soniya EV, Mathew J, Radhakrishnan EK (2014) Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 4(2):121–126
Debieux CM, Dridge EJ, Mueller CM, Splatt P, Paszkiewicz K, Knight I, Florance H, Love J, Titball RW, Lewis RJ, Richardson DJ (2011) A bacterial process for selenium nanosphere assembly. Proc Natl Acad Sci 108(33):13480–13485
Deljou A, Goudarzi S (2016) Green extracellular synthesis of the silver nanoparticles using Thermophilic Bacillus Sp. AZ1 and its antimicrobial activity against several human pathogenetic bacteria. Iran J Biotechnol 14(2):25–32
Dhanjal S, Cameotra SS (2010) Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb Cell Factories 9:52
Divya K, Kurian LC, Vijayan S, Jisha MS (2016) Green synthesis of silver nanoparticles by Escherichia coli: analysis of antibacterial activity. J Water Environ Nanotechnol 1(1):63–74. https://doi.org/10.7508/jwent.2016.01.008
Du L, Jiang H, Liu X, Wang E (2007) Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem Commun 9(5):1165–1170
Durán N, Marcato PD, Alves OL, de Souza, GIH, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol: https://doi.org/10.1186/1477-3155-3-8
Elblbesy MAA, Madbouly AK, Hamdan TAA (2014) Bio-synthesis of magnetite nanoparticles by bacteria. Am J Nano Res Appl 2(5):98–103
El-Naggar NEA, Mohamedin A, Hamza SS, Sherief AD (2016) Extracellular biofabrication, characterization, and antimicrobial efficacy of silver nanoparticles loaded on cotton fabrics using newly isolated Streptomyces sp. SSHH-1E. J Nanomater 2016:17. https://doi.org/10.1155/2016/3257359
El-Shanshoury AERR, Elsilk SE, Ateya PS, Ebeid EM (2012) Synthesis of lead nanoparticles by Enterobacter sp. and avirulent Bacillus anthracis PS2010. Ann Microbiol 62(4):1803–1810
Ersoy H, Rybicki FJ (2007) Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging 26(5):1190–1197
Fakhrullin RF, Zamaleeva AI, Morozov MV, Tazetdinova DI, Alimova FK, Hilmutdinov AK, Zhdanov RI, Kahraman M, Culha M (2009) Living fungi cells encapsulated in polyelectrolyte shells doped with metal nanoparticles. Langmuir 25(8):4628–4634
Fakhrullin RF, García-Alonso J, Paunov VN (2010) A direct technique for preparation of magnetically functionalised living yeast cells. Soft Matter 6(2):391–397
Fayaz M, Tiwary CS, Kalaichelvan PT, Venkatesan R (2010) Blue orange light emission from biogenic synthesized silver nanoparticles using Trichoderma viride. Colloids Surf B Biointerfaces 75:175–178
Feng Y, Yu Y, Wang Y, Lin X (2007) Biosorption and bioreduction of trivalent aurum by photosynthetic bacteria Rhodobacter capsulatus. CurrMicrobiol 55:402–408
Fernández-Llamosas H, Castro L, Blázquez ML, Díaz E, Carmona M (2016) Biosynthesis of selenium nanoparticles by Azoarcus sp. CIB Microb Cell Fact 15(1):109
Fouad H, Hongjie L, Yanmei D, Baoting Y, El-Shakh A, Abbas G, Jianchu M (2016) Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilisto control filarial vector Culexpipiens pallens and its antimicrobial activity. Artif Cells Nanomedicine Biotechnol 1–10
Gallardo C, Monrás JP, Plaza DO, Collao B, Saona LA, Venegas FA, Soto C, Ulloa G, Vásquez CC, Bravo D (2014) Low-temperature biosynthesis of fluorescent semiconductor nanoparticles (CdS) by oxidative stress resistant Antarctic bacteria. J Biotechnol 87:108–115
Gharieb M, Wilkinson S, Gadd G (1995) Reduction of selenium oxyanions by unicellular, polymorphic and filamentous fungi: cellular location of reduced selenium and implications for tolerance. J Ind Microbiol 14:300–311
Gong J, Zhang Z, Bai H, Yang G (2007) Microbiological synthesis of nanophase PbS by Desulfotomaculum sp. Sci China Ser E Technolo Sci 50(3):302–307
Gorobets SV, Gorobets OY, Demianenko IV (2011) Self-organization of magnetic nanoparticles when giving magnetic properties to yeast Saccharomyces cerevisiae. Res Bull NTUU KPI 3:27–33
Gorobets SV, Gorobets OY, Demianenko IV, Nikolaenko RN (2013) Self-organization of magnetite nanoparticles in providing Saccharomyces cerevisiae yeasts with magnetic properties. J Magn Magn Mater 337–338:53–57
Gregorio SD, Lampis S, Vallini G (2005) Selenite precipitation by a rhizospheric strain of Stenotrophomonassp. isolated from the root system of Astragalus bisulcatus: a biotechnological perspective. Environ Int 31:233–241
Guo L, Huang J, Zhang X, Li Y, Zheng L (2008) Bacterial magnetic nanoparticles as drug carriers. J Mater Chem 18(48):5993–5997
Gupta S, Bector S (2013) Biosynthesis of extracellular and intracellular gold nanoparticles by Aspergillus fumigatus and A. flavus. Anton Leeuw 103:1113–1123
Gupta A, Silver S (1998) Silver as a biocide: will resistance become a problem? Nat Biotechnol 16:888
Gurunathan S, Kalishwaralal K, Vaidyanathan R, Deepak V, Pandian SRK, Muniyandi J, Hariharan N, Eom SH (2009) Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloid Surf B 9:328–335
Hanzlik M, Winklhofer M, Petersen N (1996) Spatial arrangement of chains of magnetosomes in magnetotactic bacteria. Earth Planet Sci Lett 145(1–4):125–134
He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N (2007) Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett 61(18):3984–3987
Hennebel T, Van Nevel S, Verschuere S, De Corte S, De Gusseme B, Cuvelier C, Fitts JP, Van der Lelie D, Boon N, Verstraete W (2011) Palladium nanoparticles produced by fermentatively cultivated bacteria as catalyst for diatrizoate removal with biogenic hydrogen. Appl Microbiol Biotechnol 91(5):1435–1445
Holmes JD, Smith PR, Evans-Gowing R, Richardson DJ, Russell DA, Sodeau JR (1995) Energy-dispersive-X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch Microbiol 163:143–147
Hosseini-Abari A, Emtiazi G, Lee SH, Kim BG, Kim JH (2014) Biosynthesis of silver nanoparticles by Bacillus stratosphericus spores and the role of dipicolinic acid in this process. Appl Biochem Biotechnol 174(1):270–282
Huang CP, Juang CP, Morehart K, Allen L (1990) The removal of Cu (II) from dilute aqueous solutions by Saccharomyces cerevisiae. Water Res 24:433–439
Husain S, Sardar M, Fatma T (2015) Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J Microbiol Biotechnol 31(8):1279–1283
Husseiny MI, El-Aziz MA, Badr Y, Mahmoud MA (2007) Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A Mol Biomol Spectrosc 67(3–4):1003–1006
Ingle A, Gade A, Pierra S, Sönnichsen C, Rai M (2008) Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci 4:141–144
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9(6):385
Jayaseelan C, Rahuman AA, Roopan SM, Kirthi AV, Venkatesan J, Kim SK, Iyappan M, Siva C (2013) Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 107:82–89
Jha AK, Prasad K (2010) Ferroelectric BaTiO3 nanoparticles: biosynthesis and characterization. Colloid Surf B 75(1):330–334
Jha AK, Prasad K, Kulkarni AR (2009a) Synthesis of TiO2 nanoparticles using microorganisms. Colloid Surf B 71(2):226–229
Jha AK, Prasad K, Prasad K (2009b) A green low-cost biosynthesis of Sb2O3 nanoparticles. Biochem Eng J 43(3):303–306
Jiang S, Kim MG, Kim SJ, Jung HS, Lee SW, Sadowsky MJ, Hur HG (2011) Bacterial formation of extracellular U (VI) nanowires. Chem Commun 47(28):8076–8078
Juibari MM, Abbasalizadeh S, Jouzani GS, Noruzi M (2011) Intensified biosynthesis of silver nanoparticles using a native extremophilic Ureibacillus thermosphaericus strain. Mater Lett 65(6):1014–1017
Juzenas P, Chen W, Sun YP, Coelho MAN, Genralov R, Genralova N, Christensen L (2008) Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev 60:1600–1614
Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S (2008) Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloid Surf B 65(1):150–153
Kalishwaralal K, Deepak V, Ramakumarpandian S, Nellaiah H, Sangiliyandi G (2008) Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett 62:4411–4413
Kalishwaralal K, Deepak V, Pandian SRK, Gurunathan S (2009) Biological synthesis of gold nanocubes from Bacillus licheniformis. Bioresour Technol 100:5356–5358
Kang SH, Bozhilov KN, Myung NV, Mulchandani A, Chen W (2008) Microbial synthesis of CdS nanocrystals in genetically engineered E. coli. Angew Chem Int Ed 47(28):5186–5189
Kannan N, Selvaraj S, Murty RV (2010) Microbial production of silver nanoparticles. Dig J Nanomater Bios 5(1):135–140
Kapoor A, Viraraghavan T, Cullimore DR (1999) Removal of heavy metal using the fungus Aspergillus niger. Bioresour Technol 70:95–104
Kar PK, Murmu S, Saha S, Tandon V, Acharya K (2014) Anthelmintic efficacy of gold nanoparticles derived from a phytopathogenic fungus, Nigrospora oryzae. PLoS One 9(1):e84693
Kato H (2011) In vitro assays: tracking nanoparticles inside cells. Nat Nanotechnol 6(3):39–140
Khan MM, Kalathil S, Han TH, Lee J, Cho MH (2013) Positively charged gold nanoparticles synthesized by electrochemically active biofilm-a biogenic approach. Nanosci Nanotechnol 13:6079–6085
Khan I, Saeed K, Khan I (2017) Nanoparticles: properties, applications and toxicities. Arab J Chem. https://doi.org/10.1016/j.arabjc.2017.05.011
Khandel P, Shahi SK (2016) Microbes mediated synthesis of metal nanoparticles: current status and future prospects. Int J Nanomater Biostruct 6(1):1–24
Kitching M, Ramani M, Marsili E (2015) Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microbial Biotechnol 8(6):904–917
Klaus T, Joerger R, Olsson E, Granqvist CG (1999) Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci U S A 96:13611–13614
Klaus-Joerger T, Joerger R, Olsson E, Granqvist CG (2001) Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol 19(1):15–20
Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S (2004) Microbial synthesis of gold nanoparticles by metal reducing bacterium. Trans Mater Res Soc Jpn 29:2341–2343
Kora AJ, Rastogi L (2016) Biomimetic synthesis of selenium nanoparticles by Pseudomonas aeruginosa ATCC 27853: an approach for conversion of selenite. J Environ Manag 181:231–236
Korbekandi H, Mohseni S, MardaniJouneghani R, Pourhossein M, Iravani S (2016) Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif Cells Nanomed Biotechnol 44(1):235–239
Kovalenko MV, Manna L, Cabot A, Hens Z, Talapin DV, Kagan CR, Klimov XVI, Rogach AL, Reiss P, Milliron DJ, Guyot-Sionnnest P, Konstantatos G, Parak WJ, Hyeon T, Korgel BA, Murray CB, Heiss W (2015) Prospects of nanoscience with nanocrystals. ACS Nano 2:1012–1057
Kowshik M, Ashtaputre S, Kharrazi S, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2002a) Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14(1):95
Kowshik M, Deshmukh N, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2002b) Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol Bioeng 78(5):583–588
Kowshik M, Arhtaputre S, Kharrazi S, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2003) Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14:95–100
Kuber C, Souza SF (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigates. Colloid Surf B 47:160–164
Kumar SA, Ansary AA, Ahmad A, Khan MI (2007) Extracellular biosynthesis of CdSe quantum dots by the fungus, Fusarium oxysporum. J Biomed Nanotechnol 3:190–194
Kumar U, Shete A, Harle AS, Kasyutich O, Schwarzacher W, Pundle A, Poddar P (2008) Extracellular bacterial synthesis of protein-functionalized ferromagnetic Co3O4nanocrystals and imaging of self-organization of bacterial cells under stress after exposure to metal ions. Chem Mater 20(4):1484–1491
Labrenz M, Druschel GK, Thomsen-Ebert T, Gilbert B, Welch SA, Kemner KM (2000) Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 290:1744–1747
Lampis S, Zonaro E, Bertolini C, Bernardi P, Butler CS, Vallini G (2014) Delayed formation of zero-valent selenium nanoparticles by Bacillus mycoides SeITE01 as a consequence of selenite reduction under aerobic conditions. Microb Cell Factories 13(1):35
Lang C, Schuler D (2006) Biogenic nanoparticles: production, characterization, and application of bacterial magnetosomes. J Phys Condens Matter 18:S2815–S2828
Lengke MF, Fleet ME, Southam G (2006a) Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)− thiosulfate and gold (III)− chloride complexes. Langmuir 22(6):2780–2787
Lengke MF, Ravel B, Fleet ME, Wanger G, Gordon RA, Southam G (2006b) Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold (III)− chloride complex. Environ Sci Technol 40(20):6304–6309
Lengke MF, Fleet ME, Southam G (2006c) Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum (IV)− chloride complex. Langmuir 22(17):7318–7323
Li XZ, Nikaido H, Williams KE (1997) Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J Bacteriol 179:6127–6132
Li X, Chen S, Hu W, Shi S, Shen W, Zhang X, Wang H (2009) In situ synthesis of CdS nanoparticles on bacterial cellulose nanofibers. Carbohydr Polym 76(4):509–512
Li X, Xu H, Chen ZS, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater 2011:16. https://doi.org/10.1155/2011/270974
Li Y, Li Y, Li Q, Fan X, Gao J, Luo Y (2016a) Rapid biosynthesis of gold nanoparticles by the extracellular secretion of Bacillus niabensis 45: characterization and antibiofilm activity. J Chem 2016:7. https://doi.org/10.1155/2016/2781347
Li J, Li Q, Ma X, Tian B, Li T, Yu J, Hua Y (2016b) Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int J Nanomed 11:–5931
Liu T, Liu B, Zhang H, Wang Y (2005) The fluorescence bioassay platforms on quantum dots nanoparticles. J Fluoresc 15:729–733
Lloyd JR, Lovley DR (2000) Microbial detoxification of metals and radionuclides. Curr Opin Biotechnol 12:248–253
Magdi HM, Mourad MHE, Abd El Aziz MM (2014) Biosynthesis of silver nanoparticles using fungi and biological evaluation of mycosynthesized silver nanoparticles. Egypt J Exp Biol (Bot) 10(1):1–12
Mageswari A, Subramanian P, Ravindran V, Yesodharan S, Bagavan A, Rahuman AA, Karthikeyan S, Gothandam KM (2015) Synthesis and larvicidal activity of low-temperature stable silver nanoparticles from psychrotolerant Pseudomonas mandelii. Environ Sci Pollut Res 22(7):5383–5394
Malarkodi C, Rajeshkumar S, Vanaja M, Paulkumar K, Gnanajobitha G, Annadurai G (2013) Eco-friendly synthesis and characterization of gold nanoparticles using Klebsiellapneumoniae. J NanostructChem 3:30. https://doi.org/10.1186/2193-8865-3-30
Maliszewska I (2011) Microbial synthesis of metal nanoparticles. In: Mahendra R, Nelson D (eds) Metal nanoparticlesin microbiology. Springer, Berlin, pp 153–176
Mann S, Sparks NHC, Frankel RB, Bazylinski DA, Jannasch HW (1990) Biomineralization of ferrimagneticgreigite (Fe3O4) and iron pyrite (FeS2) in a magnetotactic bacterium. Nature 343:258–260
Marshall MJ, Beliaev AS, Dohnalkova AC, Kennedy DW, Shi L, Wang Z, Boyanov MI, Lai B, Kemner KM, McLean JS, Reed SB (2006) c-type cytochrome-dependent formation of U (IV) nanoparticles by Shewanellaoneidensis. PLoS Biol 4(8):e268
Merzlyak A, Lee SW (2006) Phage as template for hybrid materials and mediators for nanomaterials synthesis. Curr Opin Chem Biol 10:246–252
Mi C, Wang Y, Zhang J, Huang H, Xu L, Wang S, Fang X, Fang J, Mao C, Xu S (2011) Biosynthesis and characterization of CdS quantum dots in genetically engineered Escherichia coli. J Biotechnol 153(3):125–132
Mishra A, Tripathy S, Wahab R, Jeong SH, Hwang I, Yang YB et al (2011) Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C2C12 cells. Appl Microbiol Biotechnol 92:617–630
Mishra A, Kumari M, Pandey S, Chaudhry V, Gupta KC, Nautiyal CS (2014) Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour Technol 166:235–242
Moghaddam AB, Namvar F, Moniri M, Tahir P, Azizi S, Mohamad R (2015) Nanoparticles biosynthesized by fungi and yeast: a review of their preparation, properties, and medical applications. Molecules 20:16540–16565
Mohamedin A, El-Naggar NEA, Shawqi Hamza S, Sherief AA (2015) Green synthesis, characterization and antimicrobial activities of silver nanoparticles by Streptomyces viridodiastaticus SSHH-1 as a living nanofactory: statistical optimization of process variables. Curr Nanosci 11(5):640–654
Morcos SK (2007) Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol 80(950):73–76
Mouxing FU, Qingbiao LI, Daohua SUN, Yinghua LU, Ning HE, Xu D, Wang H, Huang J (2006) Rapid preparation process of silver nanoparticles by bioreduction and their characterizations. Chin J Chem Eng 14:114–117
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar PV, Alam M, Kumar R et al (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett 1:515–519
Musarrat J, Dwivedi S, Singh BR, Saquib Q, Al Khedhairy AA (2011a) Microbially synthesized nanoparticles: scope and applications. Agri Env App. https://doi.org/10.1007/978-1-4419-7931-5-5
Musarrat J, Dwivedi S, Singh BR, Saquib Q, Al-Khedhairy AA (2011b) Microbially synthesized nanoparticles: scope and applications. In: Microbes and microbial technology. Springer, New York, pp 101–126
Nadaf NY, Kanase SS (2016) Biosynthesis of gold nanoparticles by Bacillus marisflavi and its potential in catalytic dye degradation. Arab J Chem. https://doi.org/10.1016/j.arabjc.2016.09.02
Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO (2002) Biomimetic synthesisand patterning of silver nanoparticles. Nat Mater 1:169–172
Nair B, Pradeep T (2002) Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des 2:293–298. https://doi.org/10.1021/cg0255164
Nanda A, Saravanan M (2009) Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine 5(4):452–456
Nangia Y, Wangoo N, Goyal N, Shekhawat G, Suri CR (2009) A novel bacterial isolate Stenotrophomonasmaltophilia as living factory for synthesis of gold nanoparticles. Microb Cell Fact 8(1):39
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interf Sci 156(1):1–13
Parikh RY, Singh S, Prasad BLV, Patole MS, Sastry M, Shouche YS (2008) Extracellularsynthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: towards understanding biochemical synthesis mechanism. Chembiochem 9:1415–1422
Patel V, Berthold D, Puranik P, Gantar M (2015) Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep 5:112–119
Paulkumar K, Rajeshkumar S, Gnanajobitha G, Vanaja M, Malarkodi C, Annadurai G (2013) Biosynthesis of silver chloride nanoparticles using Bacillus subtilis MTCC 3053 and assessment of its antifungal activity. ISRN Nanomater 2013:8. https://doi.org/10.1155/2013/317963
Perez-Gonzalez T, Jimenez-Lopez C, Neal AL, Rull-Perez F, Rodriguez-Navarro A, Fernandez-Vivas A, Iañez-Pareja E (2010) Magnetite biomineralization induced by Shewanellaoneidensis. Geochimica Cosmochim Acta 74(3):967–979
Philip D (2009) Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim Acta Part A Mol Biomol Spectrosc 73:374–381
Plaza DO, Gallardo C, Straub YD, Bravo D, Pérez-Donoso JM (2016) Biological synthesis of fluorescent nanoparticles by cadmium and tellurite resistant Antarctic bacteria: exploring novel natural nanofactories. Microb Cell Factories 15:76
Posfai M, Moskowitz BM, Arato B et al (2006) Properties of intracellular magnetite crystals produced by Desulfovibriomagneticus strain RS – 1. Earth Planet Sci Lett 249(3–4):444–455
Prasad K, Jha AK (2009) ZnO nanoparticles: synthesis and adsorption study. Nat Sci 1:129–135
Prasad K, Jha AK (2010) Biosynthesis of CdS nanoparticles: an improved green and rapid procedure. J Colloid Interface Sci 342(1):68–72
Prasad K, Jha AK, Kulkarni AR (2007) Lactobacillus assisted synthesis of titanium nanoparticles. Nanoscale Res Lett 2:248–250
Priyadarshini S, Gopinath V, Priyadharsshini NM, Mubarak Ali D, Velusamy P (2013) Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloid Surf B 102:232–237
Rajakumar G, Rahuman A, Roopan SM, Khanna VG, Elango G, Kamaraj C, Zahir AA, Velayutham K (2012) Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta A Mol Biomol Spectrosc 91:23–29
Raza MA, Kanwal Z, Rauf A, Sabri AN, Riaz S, Naseem S (2016) Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 6(4):74
Riddin TL, Gericke M, Whiteley CG (2006) Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 17:3482–3489
Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A (2016) Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules 21(7):836
Sadhasivam S, Shanmugam P, Yun K (2010) Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloid Surf B 81:358–362
Sadowski Z, Maliszewskaih B, Grochowalska I, Polowczyk T, Kozlecki T (2008) Synthesis of silver nanoparticles using microorganisms. Mat Sci-Poland 26(2):419–424
Safarik I, Maderova Z, Pospiskova K, Baldikova E, Horska K, Safarikova M (2015) Magnetically responsive yeast cells: methods of preparation and applications. Yeast 32(1):227–237
Saifuddin N, Wong CW, Yasumira AA (2009) Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. J Chem 6(1):61–70
Salvadori MR, Ando RA, do Nascimento CA, Corrêa B (2014) Intracellular biosynthesis and removal of copper nanoparticles by dead biomass of yeast isolated from the wastewater of a mine in the Brazilian Amazonia. PLoS One 9(1):e87968
Sanghi R, Verma P (2009) A facile green extracellular biosynthesis of CdS nanoparticles by immobilized fungus. Chem Eng J 155(3):886–891
Sanjenbam P, Gopal JV, Kannabiran K (2014) Anticandidal activity of silver nanoparticles synthesized using Streptomyces sp. VITPK1. J Mycol Med 24(3):211–219
Sarangadharan S, Nallusamy S (2015) Biosynthesis and characterization of silver nanoparticles produced by Bacillus licheniformis. Int J Pharma Med Biol Sci 4(4):236
Saravanan M, Vemu AK, Barik SK (2011) Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Colloid Surf B 88(1):325–331
Sarkar J, Dey P, Saha S, Acharya K (2011) Mycosynthesis of selenium nanoparticles. Micro Nano Lett 6:599–602
Sarkar J, Ray S, Chattopadhyay D, Laskar A, Acharya K (2012) Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata. Bioprocess Biosyst Eng 35:637–643
Sastry M, Ahmad A, Islam Khan M, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci 85:162–170
Sawle BD, Salimath B, Deshpande R, Bedre MD, Prabhakar BK, Venkataraman A (2008) Biosynthesis and stabilization of Au and Au-Ag alloy nanoparticles by fungus, Fusarium semitectum. Sci Technol Adv Mater. https://doi.org/10.1088/1468-6996/9/3/035012
Schuler D, Frankel RB (1999) Bacterial magnetosomes: microbiology, biomineralization and biotechnological applications. Appl Microbiol Biotechnol 52:464–473
Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ (2015) Green synthesis of metallic nanoparticles via biological entities. Materials 8(11):7278–7308
Shahverdi AR, Minaeian S, Shahverdi HR, Jamalifar H, Nohi AA (2007a) Rapidsynthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem 42:919–923
Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S (2007b) Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 3(2):168–171. https://doi.org/10.1016/j.nano.2007.02.001
Shankar SS, Ahmad A, Pasricha R, Sastry M (2003) Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem 13:1822–1826
Sharma PK, Balkwill DL, Frenkel A, Vairavamurthy MA (2000) A new Klebsiella planticola strain (Cd-1) grows anaerobically at high cadmium concentrations and precipitates cadmium sulfide. Appl Environ Microbiol 66(7):3083–3087
Sharma N, Pinnaka AK, Raje M, Ashish FN, Bhattacharyya MS, Choudhury AR (2012) Exploitation of marine bacteria for production of gold nanoparticles. Microb Cell Factories 11(1):86
Shivaji S, Madhu S, Singh S (2011) Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem 46(9):1800–1807
Singh PK, Kundu S (2014) Biosynthesis of gold nanoparticles using bacteria. Proc Natl Acad Sci India Sect B Biol Sci 84(2):331–336
Singh S, Bhatta UM, Satyam PV, Dhawan A, Sastry M, Prasad BLV (2008) Bacterial synthesis of silicon/silica nanocomposites. J Mater Chem 18(22):2601–2606
Singh AV, Patil R, Anand A, Milani P, Gade WN (2010) Biological synthesis of copper oxide nano particles using Escherichia coli. Curr Nanosci 6(4):365–369
Singh S, Vidyarthi AS, Nigam VK, Dev A (2014) Extracellular facile biosynthesis, characterization and stability of gold nanoparticles by Bacillus licheniformis. Artif Cells Nanomed Biotechnol 42(1):6–12
Składanowski M, Wypij M, Laskowski D, Golińska P, Dahm H, Rai M (2016) Silver and gold nanoparticles synthesized from Streptomyces sp. isolated from acid forest soil with special reference to its antibacterial activity against pathogens. J Clust Sci 28:59–79. https://doi.org/10.1007/s10876-016-1043-6
Smith PR, Holmes JD, Richardson DJ, Russell DA, Sodeau JR (1998) Photophysical and photochemical characterization of bacterial semiconductor cadmium sulfideparticles. J Chem Soc Faraday Trans 94:1235–1241
Smith AM, Duan H, Mohs AM, Nie S (2008) Bioconjugated quantum dots for in vivomolecular and cellular imaging. Adv Drug Deliv Rev 60:1226–1240
Sneha K, Sathishkumar M, Mao J, Kwak IS, Yun YS (2010) Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem Eng Journal 162:989–996
Sonker AS, Richa JP, Rajneesh VK (2017) Characterization and in vitro antitumor, antibacterial and antifungal activities of green synthesized silver nanoparticles using cell extract of Nostoc sp. strain HKAR-2. Can J Biotech 1(1):26–37
Srinath BS, Rai VR (2015) Biosynthesis of highly monodispersed, spherical gold nanoparticles of size 4–10 nm from spent cultures of Klebsiella pneumoniae. 3Biotech 5(5):671–676
Srivastava P, Braganca JM, Kowshik M (2014) In vivo synthesis of selenium nanoparticles by Halococcus salifodinae BK18 and their anti-proliferative properties against HeLa cell line. Biotechnol Prog 30:1480–1487
Suresh AK, Pelletier DA, Wang W, Broich ML, Moon JW, Gu B, Allison DP, Joy DC, Phelps TJ, Doktycz MJ (2011) Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater 7(5):2148–2152
Swamy MK, Sudipta KM, Jayanta K, Balasubramanya S (2015a) The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl Nanosci 5(1):73–81. https://doi.org/10.1007/s13204-014-0293-6
Swamy MK, Akhtar MS, Mohanty SK, Sinniah UR (2015b) Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spec Acta Part A Mol Biomol Spectr 151:939–944
Sweeney RY, Mao C, Gao X, Burt JL, Belcher AM, Georgiou G, Iverson BL (2004) Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem Biol 11(11):1553–1559
Sweety A, Wadhwani, Shedbalkar UU, Singh R, Chopade BA (2016) Biogenic selenium nanoparticles: current status and future prospects. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-016-7300-7
Tarafdar JC, Raliya R (2013) Rapid, low-cost, and ecofriendly approach her for iron nanoparticle synthesis using Aspergillus oryzae TFR9. J Nanopart. https://doi.org/10.1155/2013/141274
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanotechnol Biol Med Nanomed 6:257–262
Thenmozhi M, Kannabiran K, Kumar R, Gopiesh KV (2013) Antifungal activity of Streptomyces sp. VITSTK7 and its synthesized Ag2O/Ag nanoparticles against medically important Aspergillus pathogens. J Med Mycol 23(2):–97, 103
Tollamadugu NVKV, Prasad T, Kambala VSR, Naidu R (2011) A critical review on biogenic silver nanoparticles and their antimicrobial activity. Curr Nanosci 7:531–544
Torres SK, Campos VL, León CG, Rodríguez-Llamazares SG, Rojas SM, Gonzalez M, Smith C, Mondaca MA (2012) Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J Nanopart Res 14(11):1236
Usha R, Prabu E, Palaniswamy M, Venil CK, Rajendran KR (2010) Synthesis of metal oxide nano particles by Streptomyces sp. for development of antimicrobial textiles. Global J Biotechnol Biochem 5:153–160
Vali H, Weiss B, Li YL, Sears SK, Kim SS, Kirschvink JL, Zhang CL (2004) Formation of tabular single-domain magnetite induced by Geobacter metallireducens GS-15. Proc Natl Acad Sci U S A 101(46):16121–16126
Velmurugan P, Shim J, You Y, Choi S, Kamala-Kannan S, Lee KJ, Kim HJ, Oh BT (2010) Removal of zinc by live, dead, and dried biomass of Fusarium spp. isolated from the abandoned-metal mine in South Korea and its perspective of producing nanocrystals. J Hazard Mater 182:317–324
Vetchinkina E, Loshchinina E, Kursky V, Nikitina V (2013) Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium. J Microbiol 51:829–835
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Waghmare SS, Deshmukh AM, Sadowski Z (2014) Biosynthesis, optimization, purification and characterization of gold nanoparticles. Afr J Microbiol Res 8:138–146
Wall JD, Krumholz LR (2006) Uranium reduction. Ann Rev Microbiol 60:149–166
Wang Y, Ju Z, Cao B, Gao X, Zhu Y, Qiu P, Xu H, Pan P, Bao H, Wang L et al (2015) Ultrasensitive rapid detection of human serum antibody biomarkers by biomarker-capturing viral nanofibers. ACS Nano 9:4475–4483
Watson JHP, Ellwood DC, Soper AK, Charrock J (1999) Nanosized strongly-magnetic bacterially-produced iron sulfide materials. J Magn Mater 203:69–72
Windt WD, Aelterman P, Verstraete W (2005) Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol 7(3):314–325
Xiang L, Wei J, Jianbo S, Guili W, Feng G, Ying L (2007) Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett Appl Microbiol 45:75–81
Xue B, He D, Gao S, Wang D, Yokoyama K, Wang L (2016) Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int J Nanomedicine 11:1899–1906
Yadav V, Sharma N, Prakash R, Raina KK, Bharadwaj LM, Prakash NT (2008) Generation of selenium containing nano-structures by soil bacterium, Pseudomonas aeruginosa. Biotechnology 7:299–304
Yee N, Ma J, Dalia A, Boonfueng T, Kobayashi DY (2007) Se (VI) reduction and the precipitation of Se (0) by the facultative bacterium Enterobacter cloacae SLD1a-1 are regulated by FNR. Appl Environ Microb 73(6):1914–1920
Zare B, Babaie S, Setayesh N, Shahverdi A (2012) Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles. Nanomed J 1:14–20
Zeng J, Ma Y, Jeong U, Xia Y (2010) Au(I): an alternative and potentially better precursor than Au(III) for the synthesis of Au nanostructures. J Mater Chem 20:2290–2301
Zhang H, Li Q, Lu Y, Sun D, Lin X, Deng X, He N, Zheng S (2005) Biosorption and bioreduction of diamine silver complex by Corynebacterium. J Chem Technol Biotechnol 80:285–290
Zhang X, He X, Wang K, Yang X (2011) Different active biomolecules involved in biosynthesis of gold nanoparticles by three fungus species. J Biomed Nanotechnol 7:245–254
Zhang XF, Shen W, Gurunathan S (2016) Biologically synthesized gold nanoparticles ameliorate cold and heat stress-Induced oxidative stress in Escherichia coli. Molecules 21(6):731
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Swamy, M.K., Rudramurthy, G.R., Patra, J.K., Sinniah, U.R. (2018). Microbe-Based Metallic Nanoparticles Synthesis and Biomedical Applications: An Update. In: Patra, J., Das, G., Shin, HS. (eds) Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-10-7140-9_19
Download citation
DOI: https://doi.org/10.1007/978-981-10-7140-9_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7139-3
Online ISBN: 978-981-10-7140-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)