Abstract
A total of 88 lactic acid bacteria (LAB) strains were isolated from Chinese traditional sourdough and five of them were selected based on their bile resistance. All the five strains were identified as Lactobacillus plantarum by 16S rRNA gene sequencing. In vitro probiotic properties of the L. plantarum strains including tolerance to simulated gastrointestinal conditions, aggregation activity, and cholesterol removal ability were assessed. Two representatives, L. plantarum ZJUFT34 and L. plantarum ZJUFT17, were intragastrically administered to male C57BL/6J mice of 4-week age for 6 weeks to evaluate their in vivo health-promoting effects. The results indicated that L. plantarum ZJUFT34, L. plantarum ZJUFHN9, and L. plantarum ZJUFAH5 could survive the 3-h incubation in simulated gastric juice with a pH value of 2.0, while L. plantarum ZJUFT32 and L. plantarum ZJUFT17 exhibited better autoaggregation activities and coaggregation activities with pathogens. All the strains showed a cholesterol removal ability in vitro. However, L. plantarum ZJUFT34 or L. plantarum ZJUFT17 administration did not significantly change the serum total cholesterol in vivo. But the ratio of high-density lipoprotein cholesterol to low-density lipoprotein cholesterol was significantly increased by the L. plantarum administration. Besides, L. plantarum ZJUFT17 significantly lowered serum tumor necrosis factor (TNF)-α concentrations. Furthermore, the administration of the LAB strains showed significant influences on lipid metabolism-related gut microbiota. These findings suggested that the L. plantarum strains may benefit the prevention of metabolic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are defined as live microorganisms, which, when administered in adequate amounts, confer a health benefit on the host (FAO/WHO 2002). Administration of probiotics is considered an effective approach to modulate gut microbiota and to maintain or restore human health (Collado et al. 2008), though their functional roles in maintaining and promoting human health have been challenged in some cases (Suez et al. 2018; Zmora et al. 2018). Beneficial probiotic effects on some disorders such as inflammatory bowel disease and irritable bowel syndrome have been supported by research results from clinical trials (Sanchez et al. 2017). Among the numerous researches on probiotics, various studies have demonstrated the beneficial effects of specific probiotics on the amelioration of metabolic syndrome (Cani and Van Hul 2015), symptoms of which include obesity, insulin resistance, hyperlipidemia, hyperglycemia, and hypertension (Eckel et al. 2005). Different probiotic strains have been reported possessing distinct capacities to modulate metabolic phenotypes, mainly involving Bifidobacterium spp. (Bernini et al. 2016; Minami et al. 2015) and Lactobacillus spp. (Barreto et al. 2014; Kadooka et al. 2010), as well as the so-called next-generation probiotics Akkermansia muciniphila (Everard et al. 2013). All the strains performed differently with respect to the impact on metabolic phenotypes such as body weight, fat mass, inflammatory markers, plasma lipid, and cholesterol levels.
To benefit human health, probiotics must survive from passage through the gastrointestinal tract (GIT) and exert functions in the gut environment (Ramos et al. 2013). Therefore, the abilities to withstand low pH, bile toxicity, digestive enzymes, to adhere to the intestinal epithelial cells for temporary colonization, and to repel pathogens may serve as criteria for selecting promising probiotics in vitro (Kilic et al. 2013). Undoubtedly, lactic acid bacteria (LAB) have been the most studied group for probiotics exploration in the past few decades (Angmo et al. 2016). LAB, generally regarded as safe, could be found in various traditional fermented foods (Liu et al. 2011). Sourdough has been used worldwide as a starter in making cereal-based fermented foods, e.g., Chinese steamed bread, a staple food in China for about 2000 years (Liu et al. 2016). Extensive research has revealed that LAB are the predominant bacteria in this ecological niche (De Vuyst et al. 2014; Gobbetti et al. 2016). Furthermore, it has been reported that sourdough microbiota and the lactic microbiota of human exhibit a remarkable overlap, indicating that intestinal microbiota may be a source of the sourdough LAB (De Vuyst et al. 2014; Gobbetti and Gänzle 2012). Though majority of the studies have focused on their technological properties, some efforts have recently been exerted to investigate the probiotic properties of sourdough LAB. Manini et al. (2016) investigated the probiotic properties of 13 LAB strains belonging to 7 species isolated from sourdough in terms of their pH and bile resistance, adhesion to Caco-2 cells, and anti-listeria activity. They found that some strains showed good probiotic potentials. Yang et al. (2016) investigated the effect of administration of 5 LAB strains isolated from traditional Chinese sourdough on the GIT microbiota of mice. Their results suggested that LAB from Chinese sourdough might be useful for strengthening the flora balance in GIT and improve nutrient metabolism. In another study, a probiotic evaluation was performed on the LAB strain Enterococcus faecium YF5, isolated from Chinese traditional sourdough (Tan et al. 2013). The ability of Enterococcus faecium YF5 to survive in low pH, bile salts, and in gastric and intestinal digestion in vitro, to adhere to HT-29 cells, and to inhibit the tested foodborne pathogens makes it a candidate for probiotics. All the studies have revealed that sourdough may serve as a reservoir of potential probiotics. The characterization of potential probiotics from sourdough generally involves acid and bile tolerance, resistance to simulated GIT, and adhesion to in vitro cells; however, the in vivo health-promoting effects, especially on metabolic syndrome, have been scarcely researched. In addition, although increasing attention has been paid to the isolation of probiotic strains from various food ecosystems, the exploration of candidate probiotic strains from sourdough is still rare.
In this study, 88 LAB isolates from Chinese traditional sourdough were screened for potential probiotics. The isolates were assessed based on a series of in vitro tests including bile resistance, simulated GIT tolerance, aggregation activity, and cholesterol removal activity. Then, representatives were further administered to animals to investigate their potential benefits for lipid-related metabolism in vivo by determining the serum lipid profile, inflammatory markers, and gut microbiota changes.
Materials and methods
Isolation of lactic acid bacteria from sourdough
Fifteen Chinese traditional sourdough samples were collected from different regions of China as previously reported (Liu et al. 2016). A 10-g sourdough sample was thoroughly suspended with 90 mL 0.85% sterile saline solution and was then decimally diluted. All dilutions were plated onto de Man, Rogosa and Sharp (MRS) agar and anaerobically incubated at 37 °C for 48 h. Then colonies were picked based on morphology and were purified by successive streaking on MRS agar for at least three times. All the purified isolates were subjected to gram staining and catalase test. The strains exhibiting gram-positive and catalase-negative properties were presumptively considered as LAB and stored in MRS broth at 4 °C for later use.
Bile tolerance screening
The LAB strains were subjected to a preliminary screening according to the method describe by Walker and Gilliland (1993) with some modifications. The purified strains were inoculated into MRS broth and incubated at 37 °C for 18 h, then subcultured into MRS-THIO broth (MRS broth supplemented with 0.2% sodium thioglycollate) containing porcine bile (0.3%, w/v) with an inoculum size of 1% (v/v) and incubated at 37 °C for 24 h. The difference of absorbance at 620 nm between 0 and 24 h (△OD620) was determined. LAB strains that showed relatively high biomass production (△OD620) during the 24-h incubation were selected.
Identification of the LAB strains
The total DNA of the selected strains was isolated using a DNA extraction kit (Axygen) by following the manufacturer’s instructions. The universal primer pairs, 27F and 1492R, were used for the amplication of the 16S rRNA gene (Weisburg et al. 1991). The PCR was performed as described in previous study (Liu et al. 2016), i.e., preliminary denaturation for 5 min at 94 °C, followed by 30 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 1 min, and terminated with an elongation step at 72 °C for 10 min. The amplicons were sequenced by a company (Shanghai Sangon Biotech, Shanghai, China) and the obtained sequences were compared with the GenBank database by a BLAST search.
In vitro resistance to simulated human GIT
The simulated gastrointestinal juices were prepared according to previous studies (Bao et al. 2010; Charteris et al. 1998). The simulated gastric juice was prepared by suspending pepsin (3.5 g/L) in 0.2% saline and adjusting the pH to 2.0, 2.5, and 3.0, respectively, with 1 mol/L HCl, and sterilized by filtering through a 0.45-μm Millipore filter. The simulated intestinal juice was prepared by suspending trypsin (1 g/L) and bile salts (18 g/L) in a sterile solution containing sodium bicarbonate (11 g/L) and sodium chloride (2 g/L). The solution pH was adjusted to 8.0 with 0.5 mol/L NaOH and sterilized by filtering through a 0.45-μm Millipore filter.
The LAB strains were incubated at 37 °C in MRS broth for 18 h and were harvested by centrifugation (10, 000g, 5 min). Then they were resuspended in sterile saline solution (0.85%, m/v) to gain a concentration of ca. 1–2 × 109 cfu/mL. An aliquot of 0.5 mL of the cell suspension was added into 4.5 mL of the simulated gastric juices (pH 2.0, 2.5, 3.0), followed by an incubation at 37 °C for 3 h. Total viable counts were determined at the time points of 0, 1, 2, and 3 h for the evaluation of tolerance in gastric juice. After the 3-h incubation in simulated gastric juice, an aliquot of 0.5 mL was taken and transferred to 4.5 mL of the simulated intestinal juice and then incubated at 37 °C for 24 h. The tolerance in simulated intestinal juice was assessed by the total viable counts recovered at 0, 8, and 24 h. The total viable counts were enumerated using pour plate method on MRS agar. The experiments were carried out in triplicate. The survival rate was calculated according to the equation below:
where N1 (cfu/mL) represents the total viable counts after treatment by simulated gastrointestinal juices and N0 (cfu/mL) represents the total viable counts before the treatment.
Aggregation activity
Aggregation activity including autoaggregation and coaggregation was measured according to the method described by Collado et al. (2008). Briefly, four pathogen strains Escherichia coli ATCC 25922, Salmonella typhimurium ATCC 14028, Staphylococcus aureus CMCC 26003, and Listeria monocytogenes ATCC 15313 and the LAB strains were separately incubated at 37 °C for 18 h and then were harvested by centrifugation (10,000g, 5 min). Finally, they were washed twice and resuspended in PBS buffer to gain a concentration of ca. 108 cfu/mL. For autoaggregation ability determination, 4 mL of LAB suspension was added into sterile tubes individually and thoroughly suspended. These tubes were placed at room temperature without agitation and an aliquot of 150 μL upper suspension was taken at different time points (0, 4, 8, 18, and 24 h) to determine their absorbance (600 nm) using a microplate reader (Infinite M200Pro, Tecan, Austria). Autoaggregation percentage was calculated using the equation:
where A0 is the absorbance at 0 h, and At is the absorbance of upper suspension at different time points.
As for the determination of coaggregation of the LAB strains with pathogens, equal volumes (2 mL) of LAB and the pathogen cultures were mixed in sterile tubes and vortexed completely. These tubes were placed at room temperature without agitation and an aliquot of 150 μL upper suspension was taken at 0 and 4 h to determine their absorbance (600 nm) using the microplate reader. Coaggregation percentage was calculated as follows:
where Apat and Aprobio are the absorbances of pathogens and LAB at 0 h, respectively, and Amix is the absorbance of the mixed suspensions at 4 h.
In vitro cholesterol removal capability
The cholesterol removal capability of the strains was determined according to the method described by Bordoni et al. (2013) and Feng et al. (1973), with some modifications. The MRS-CHOL broth was prepared by supplementing MRS broth with cholesterol at a concentration of 0.1 g/L, in which Tween 80 was added at 2 mL/L, followed by a sterilization (121 °C, 20 min). An aliquot of 0.1 mL of overnight cultures was added into 0.9 mL of MRS-CHOL broth and incubated anaerobically at 37 °C for 48 h. Meanwhile, 0.1 mL of MRS broth mixed with 0.9 mL of MRS-CHOL broth was used as a control. After the incubation, an aliquot of 0.2 mL of the LAB culture was added to 4.8 mL of ethanol followed by a centrifugation (3000g, 10 min, 4 °C). Then, 2 mL of the supernatant was transferred to a clean tube, into which 2 mL of ferric ammonium sulfate color reagent was slowly added. The absorbance was determined at 560 nm. Cholesterol removal rate (CRR) was calculated as follows:
where Acon and Ainoc are the absorbance of control and inoculated samples, respectively.
Animals and treatment
Male C57BL/6J mice (4 weeks of age) were obtained from SLAC Laboratory Animal (Shanghai, China) and housed in Laboratory Animal Research Center (SPF) of Zhejiang Chinese Medical University. Mice were kept in hard top cages with three mice per cage under 12-h light/dark conditions at 22 ± 2 °C and 50 ± 10% relative humidity with access to a mouse pellet diet and water ad libitum. The experiment was approved by the Animal Ethical Committee of Zhejiang Chinese Medical University (Number of resolution: ZSLL-2017-049).
After 1-week acclimation, 27 mice were equally divided into three groups. The T34 group and T17 group were intragastrically administered with 0.2 mL of saline containing 2 ± 1 × 108 cfu of L. plantarum ZJUFT34 or L. plantarum ZJUFT17, respectively. And the CON group was given 0.2 mL of sterilized saline as a negative control. All the animals were treated once a day in the morning for 6 weeks. After 6 weeks treatment, mice were fasted for 12 h, and blood samples were collected from retrobulbar, intraorbital, capillary plexus. Mice were then killed by cervical dislocation.
Analysis of serum cholesterol and cytokine levels
The levels of total serum cholesterol (T-CHO), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using a kit (Nanjin Jiangcheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. Serum concentrations of TNF-α, interleukin (IL)-1β, IL-10, and IL-6 were determined using commercial ELISA kits (eBioscience) following the manufacturers’ instructions.
Gut microbiota analysis
Genomic DNA was extracted from fecal samples of mice treated for 6 weeks using a QIAamp DNA Stool Mini Kit (QIAGEN, Venlo, Netherlands) following the manufacturer’s protocol. The concentration and quality of the extracted DNA were determined using a Thermo Scientific NanoDrop 2000c.The PCR amplification of 16S rDNA V3-V4 region and the subsequent high-throughput sequencing as well as the data pretreatment were performed in a company (Realbio Technology Inc. Shanghai, China) using a HiSeq2500 platform. The differences of microbiota between the groups were analyzed by calculating α-diversity and β-diversity (Kemp and Aller 2004).
Statistical analysis
Statistical analysis of more than two groups was conducted by one-way analysis of variance (ANOVA) followed by Tukey’s tests, while data comparison between two groups were analyzed by the unpaired t tests using the software GraphPad Prism (version 6). The data of in vitro experiments were expressed as mean ± standard deviation (SD), while in vivo experiment results were expressed as mean ± standard error of mean (SEM). p < 0.05 was considered statistically significant.
Accession numbers of the sequence data
The five strains in this study have been deposited at China Center for Type Culture Collection (CCTCC Nos.: M2017399, M2017342, M2017744, M2017341, and M2017743) and their 16S rRNA gene sequences have been submitted to GenBank with accession numbers from MG739430 to MG739434. The raw data of high-throughput sequencing of the mouse gut microbiota have been deposited at the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) with the accession numbers from SRX4408722 to SRX4408748.
Results
Bile tolerance screening and identification of the strains
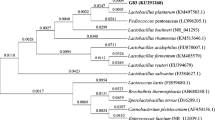
A total of 88 LAB strains were picked and purified from Chinese traditional sourdough samples, which were then subjected to a bile resistance screening (Table S1 and S2). Five strains were finally selected based on their growth capacities in bile solution after two times screening (Table 1). The five strains were all identified as Lactobacillus plantarum by 16S rRNA gene sequencing, namely L. plantarum ZJUFT34 (T34), L. plantarum ZJUFT17 (T17), L. plantarum ZJUFT32 (T32), L. plantarum ZJUFHN9 (HN9), and L. plantarum ZJUFAH5 (AH5).
Transit tolerance
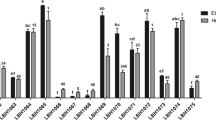
The effects of simulated gastric juice and intestinal transit on the survival rates of the five strains are shown in Fig. 1. After being incubated for 3 h in simulated gastric juice of pH 2.0, the survival rates of the strains were less than 50%, especially T32 and T17, which completely lost their vitalities, and after an incubation in simulated intestinal juice for 24 h, only the strain AH5 maintained some vitality. But when the pH value of the simulated gastric juice was raised to 2.5 or 3.0, the survival rates of the strains markedly improved. Especially, when the pH value of the simulated gastric juice increased to pH 3.0, almost no vitality loss was found among the five strains during a 3-h incubation, and the final survival rates were 77–89% after being exposed to the simulated intestinal juice for 24 h.
Aggregation activity
Autoaggregation of the five strains was measured at 4, 8, 18, and 24 h (Fig. 2a) and the aggregation rate increased over time. At 4 h, no significant difference was observed, but at 8 h, T32 showed a higher aggregation rate than other strains. After an 18-h aggregation, the aggregation rates of strains T17, T32, and AH5 were all higher than 98%, while strains T34 and HN9 were 84% and 62%, respectively. Finally, at 24 h, their aggregation rates showed the least divergence ranging from 80 to 100%. Among the LAB strains, T32 exhibited the highest coaggregation percentages with Salmonella typhimurium and Listeria monocytogenes, while T17 showed the highest coaggregation percentage with Escherichia coli (Fig. 2b).
In vitro reduction of cholesterol
The cholesterol removal rates of the L. plantarum strains are shown in Fig. 3. All of them possessed the capacity of lowering cholesterol in vitro, and interestingly, they showed a similar cholesterol removal rate, mostly ranging from 20 to 30%.
Serum levels of cholesterols and cytokines
The effects of L. plantarum supplementation on mice cholesterol profiles are shown in Fig. 4. The total cholesterol concentration in serum was not significantly affected. But the HDL-C and LDL-C concentrations of T17 group were significantly lower than those of CON group, and the HDL-C/LDL-C ratio was significantly higher in the L. plantarum-treated groups compared to the CON group (p < 0.05).
Effects of L. plantarum ZJUFT34 and L. plantarum ZJUFT17 on serum lipids including total cholesterol (T-CHO), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) as well as the HDL-C/LDL-C ratio. Data are expressed as mean ± SEM. Values with asterisk are significantly different compared to CON group (*p < 0.05, **p < 0.01, ***p < 0.001)
The levels of some cytokines in serum, including IL-1β, IL-6, IL-10, and TNF-α, are shown in Fig. 5. Generally, the two L. plantarum strains exerted nonsignificant effects on the four cytokines, except that the concentration of TNF-α in T17 group was significantly reduced compared to the CON group (p < 0.05).
Changes in gut microbiota
As shown in Fig. 6, the gut microbiota of mice administered with L. plantarum was profoundly changed, and the two L. plantarum strains exerted different influence on the bacterial consortium in mice intestine. The Venn diagram demonstrated that most of the operational taxonomic units (OTUs) were shared by the three groups; however, each group also had its unique ones (Fig. 6a). Bacteroidetes was identified as the dominant phylum in all the three groups followed by Firmicutes (Fig. 6b). Interestingly, the L. plantarum-treated groups had a higher relative abundance of Bacteroidetes, especially the T17 group. The sequencing data were further analyzed at the genus level. As shown in Fig. 6c, the α-diversity analysis indicated that the L. plantarum treatment did not significantly change the diversity of gut microbiota in mice. As for the β-diversity, principal coordinate analysis (PCoA) of the unweighted UniFrac distances demonstrated that the samples of the T17 group were clustered together on the plot, and the same was true for the CON group. In contrast, the samples in the T34 group were scattered (Fig. 6d). The rank sum test was used to identify genera that have significant differences in richness between groups, and based on that, a heat map was drawn to illustrate the differences of the groups (Fig. 6e). Genera that showed significant differences in relative abundance between the groups included Vampirovibrio, Parabacteroides, Odoribacter, Alloprevotella, Bacteroides, Butyricicoccus, Lachnospiracea_incertae_sedis, Alistipes, Akkermansia, Bifidobacterium, and Mucispirillum (Table 2). Particularly, no Alloprevotella was found in the CON group, while it had a high relative abundance in the L. plantarum-treated groups. On the contrary, the genus Vampirovibrio was observed in all the samples of CON group, while it was only detected in two out of nine samples in each of the L. plantarum-treated groups (data not shown). In addition, the relative abundance of Akkermansia, Bifidobacterium, Mucispirillum, and Odoribacter also decreased significantly in at least one of the L. plantarum-treated groups (p < 0.05). On the other hand, the relative abundance of Alistipes and Parabacteroides were significantly elevated in the T17 group (p < 0.05). Meanwhile, the relative abundance of Bacteroides, Butyricicoccus, and Lachnospiracea_incertae_sedis were significantly enhanced in the T34 group (p < 0.05).
The influences of L. plantarum administration on gut microbiota in mice. The Venn diagram of OTUs (a), bar plot of microbial community at phylum level (b), Chao 1’s diversity parameter of α-diversity (c), PCoA plot of unweighted UniFrac distances (d), and heat map based on the significantly different genera between the groups (e)
Discussion
Traditional sourdough, which accommodates a high density of LAB, may serve as a reservoir for probiotics. In this study, all the five L. plantarum strains could grow quite well not only at 30 °C, but also at 37 °C (Fig. S1), indicating that they are able to adapt to the higher in vivo temperature (37 °C). However, the strains must overcome the barriers such as low pH and bile in GIT, before they reach the desired body niches to exert their functions. In this study, all the five strains could well tolerate to pH as low as 2.5 in line with the previous reported probiotics (Guo et al. 2009). The pH value in stomach varies ranging from 1–2 (during fasting) up to 4–5 (after a meal) (Bao et al. 2010; Papadimitriou et al. 2015). That means the strains have the opportunity to survive the gastric acidity. From the transit tolerance assay, it could be observed that the effects of the simulated intestinal juices on the strains were correlated with their remaining vitalities after being treated in the simulated gastric juice. If the strains maintained high survival rates after being exposed to low pH conditions, a less vitality loss could be observed when treated with the simulated intestinal juices. A similar phenomenon was found in the research performed by Guo et al. (2009). Assumedly, the ability to tolerate low pH in stomach should be one of the decisive factors for the strains to maintain vitality through GIT. In addition to the low pH in stomach, the bile pressure in the intestine is another challenge for probiotics. Tolerance to bile concentrations between 0.15 and 0.5% has been recommended for in vitro evaluation of probiotics and the concentration of 0.3% is usually adopted by researchers (Guo et al. 2009; Papadimitriou et al. 2015; Tan et al. 2013). As porcine bile is similar to human bile with respect to the ratios of bile salt/cholesterol, phospholipid/cholesterol, and glycine/taurine (Legrand-Defretin et al. 1991; Thornton 1996), as well as viscosity, bilirubin, and cholylglycine (Kobayashi et al. 1998), porcine bile was chosen in our study for preliminary screening for potential probiotics. The selected five strains grew well in the bile solution, suggesting that they could tolerate the in vivo bile pressure. Apart from the acid and bile tolerance, being able to adhere to epithelial cells and mucosal surfaces is also a desirable probiotic trait. In previous researches, the human colon cancer cell line HT-29 or Caco-2 cells have been used to investigate the adhesion ability of potential probiotics from sourdough and they showed good adhesion to the cells (Manini et al. 2016; Tan et al. 2013). Alternatively, autoaggregation capacity of LAB has been reported to be correlated with their adhesion to host cells; therefore, it is applied as a desirable characteristic for probiotic screening in vitro (Malik et al. 2013). The high autoaggregation activities of the five L. plantarum strains may indicate their abilities to adhere to epithelial cells and mucosal surfaces (Malik et al. 2013; Trivedi et al. 2013). Meanwhile, it has been reported that the coaggregation of probiotics and pathogens helps to remove the pathogens from the GIT (Collado et al. 2008). As T17 and T32 possessed better coaggregation abilities, they may be capable of excluding pathogens in GIT.
The L. plantarum strains, especially T17, could improve serum cholesterol profile and may help ameliorate metabolic syndrome. After the 6-week administration, nonsignificant change was observed regarding the content of total cholesterol in serum between the tests and control group, agreeing to the previous research performed by Yang et al. (2016), in which case five LAB strains isolated from Chinese sourdough were fed to mice for 4 weeks. However, in this study, the LAB strains improved the composition of cholesterols in mice, which has not been reported before concerning the sourdough LAB. The levels of HDL-C decreased in L. plantarum-treated groups, while the LDL-C concentrations were more remarkably reduced. Consequently, the ratio of HDL-C to LDL-C of L. plantarum-treated groups, especially in the T17 group, was significantly higher than that of the CON group. It has been revealed that high LDL-C concentration had a positive relationship with the risk for coronary artery disease (CAD) (Costabile et al. 2017), and that high HDL-C/LDL-C ratio had a significantly lower event rate of major cardiovascular adverse events (Sekiguchi et al. 2018). Thus, the reduction of the LDL-C and the increase of HDL-C/LDL-C ratio indicated that the administration of the L. plantarum strains may help to lower the risk of CAD and related metabolic syndrome. Besides, the T17 administration significantly lowered the serum TNF-α concentration in comparison with the control group. TNF-α is an important mediator of insulin resistance in obesity and it induces insulin resistance via the attenuation of insulin receptor signaling (Hotamisligil et al. 1996). In addition, an elevated level of TNF-α may diminish lipid oxidation and increase lipid accumulation, which exacerbates obesity (Tse et al. 2017). Some Lactobacillus species have been reported to be able to produce soluble molecules which could suppress the production of TNF-α in activated macrophages (Pena and Versalovic 2003). In this study, T17 may be capable of generating certain metabolites that inhibited TNF-α production in vivo. Therefore, the result suggested that the strain L. plantarum ZJUFT17 may have a therapeutic potential in the prevention of TNF-α-mediated insulin resistance and obesity, as well as in the modulation of immune responses (LaDuca and Gaspari 2001).
The gut microbiota of mice was significantly altered after the administration with the L. plantarum strains and the strain T17 seemed to exert a greater influence than T34. Among the significantly changed gut bacteria, the genus Alloprevotella was reported to be associated with decreased lifetime cardiovascular disease risk profile (Kelly et al. 2016), and the increase of abundance of Alloprevotella could ameliorate intestinal dysbiosis in high-fat diet (HFD) mice (Shang et al. 2017). Meanwhile, the genus Vampirovibrio has been found to exacerbate the HFD-induced change (Lu et al. 2018). Therefore, the L. plantarum strains may be beneficial in preventing metabolic syndrome by increasing the relative abundance of Alloprevotella and decreasing relative abundance of Vampirovibrio. On the other hand, however, T34 significantly decreased the relative abundance of Bifidobacterium, which is considered as typical probiotics in the intestine. In addition, the relative abundance of genus Akkermansia was reduced in the L. plantarum-treated groups. A typical representative of this genus, Akkermansia muciniphila, a mucin-degrading bacterium residing in the mucus layer, could reverse HFD-induced metabolic disorders (Derrien et al. 2017). The results may suggest that the L. plantarum strains did not always modulate the gut bacteria in a desirable way. Considering the complexity of the gut microbiota and their unclear specific roles in host health, the probiotic properties of the L. plantarum strains still need further investigations.
The results in this study indicated that the two strains may be capable of attenuating metabolic syndrome, since they could improve serum cholesterol profile, reduce TNF-α content, and modulate the lipid metabolism-related gut microbiota. But the validation and mechanisms of action still need to be explored by using animal models. The research showed that Chinese traditional sourdough could be a useful resource for isolating probiotics.
References
Angmo K, Kumari A, Savitri BTC (2016) Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. Lwt-Food Sci Technol 66:428–435. https://doi.org/10.1016/j.lwt.2015.10.057
Bao Y, Zhang Y, Zhang Y, Liu Y, Wang S, Dong X, Wang Y, Zhang H (2010) Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 21:695–701. https://doi.org/10.1016/j.foodcont.2009.10.010
Barreto FM, Simão ANC, Morimoto HK, Lozovoy MAB, Dichi I, da Silva Miglioranza LH (2014) Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 30(7–8):939–942
Bernini LJ, Simão ANC, Alfieri DF, Lozovoy MAB, Mari NL, de Souza CHB, Dichi I, Costa GN (2016) Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: a randomized trial. Effects of probiotics on metabolic syndrome. Nutrition 32(6):716–719
Bordoni A, Amaretti A, Leonardi A, Boschetti E, Danesi F, Matteuzzi D, Roncaglia L, Raimondi S, Rossi M (2013) Cholesterol-lowering probiotics: in vitro selection and in vivo testing of bifidobacteria. Appl Microbiol Biotechnol 97(18):8273–8281
Cani PD, Van Hul M (2015) Novel opportunities for next-generation probiotics targeting metabolic syndrome. Curr Opin Biotechnol 32:21–27
Charteris W, Kelly P, Morelli L, Collins J (1998) Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 84(5):759–768
Collado MC, Meriluoto J, Salminen S (2008) Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol 226:1065–1073. https://doi.org/10.1007/s00217-007-0632-x
Costabile A, Buttarazzi I, Kolida S, Quercia S, Baldini J, Swann JR, Brigidi P, Gibson GR (2017) An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS One 12:e0187964. https://doi.org/10.1371/journal.pone.0187964
De Vuyst L, Van Kerrebroeck S, Harth H, Huys G, Daniel HM, Weckx S (2014) Microbial ecology of sourdough fermentations: diverse or uniform? Food Microbiol 37:11–29. https://doi.org/10.1016/j.fm.2013.06.002
Derrien M, Belzer C, de Vos WM (2017) Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106:171–181. https://doi.org/10.1016/j.micpath.2016.02.005
Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet 365(9468):1415–1428
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110(22):9066–9071. https://doi.org/10.1073/pnas.1219451110
FAO/WHO (2002) Working group report on drafting guidelines for the evaluation of probiotics in food. Ontario, London
Feng HW, Pi CM, Wang R, Chen LC (1973) Use of ferric ammonium sulfate in serum-cholesterol determination. Clin Chem 19:121–122
Gobbetti M, Gänzle M (2012) Handbook on sourdough biotechnology. Springer Science & Business Media
Gobbetti M, Minervini F, Pontonio E, Di Cagno R, De Angelis M (2016) Drivers for the establishment and composition of the sourdough lactic acid bacteria biota. Int J Food Microbiol 239:3–18. https://doi.org/10.1016/j.ifoodmicro.2015.05.022
Guo Z, Wang JC, Yan LY, Chen W, Liu XM, Zhang HP (2009) In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. Lwt-Food Sci Technol 42:1640–1646. https://doi.org/10.1016/j.lwt.2009.05.025
Hotamisligil GkS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271(5249):665–670
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T (2010) Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64(6):636–643
Kelly TN, Bazzano LA, Ajami NJ, He H, Zhao J, Petrosino JF, Correa A, He J (2016) Gut microbiome associates with lifetime cardiovascular disease risk profile among Bogalusa Heart Study participants. Circ Res 119:956–964. https://doi.org/10.1161/CIRCRESAHA.116.309219
Kemp PF, Aller JY (2004) Bacterial diversity in aquatic and other environments: what 16S rDNA libraries can tell us. FEMS Microbiol Ecol 47(2):161–177. https://doi.org/10.1016/S0168-6496(03)00257-5
Kilic GB, Kuleasan H, Somer VF, Akpinar D (2013) Determining potential probiotic properties of human originated Lactobacillus plantarum strains. Biotechnol Bioproc E 18:479–485. https://doi.org/10.1007/s12257-012-0785-8
Kobayashi T, Taniguchi S, Ye Y, Niekrasz M, Nour B, Cooper DKC (1998) Comparison of bile chemistry between humans, baboons, and pigs: implications for clinical and experimental liver xenotransplantation. Lab Anim Sci 48(2):197–200
LaDuca JR, Gaspari AA (2001) Targeting tumour necrosis factor alpha: new drugs used to modulate inflammatory diseases. Dermatol Clin 19:617–635. https://doi.org/10.1016/S0733-8635(05)70304-1
Legrand-Defretin V, Juste C, Henry R, Corring T (1991) Ion-pair high-performance liquid chromatography of bile salt conjugates: application to pig bile. Lipids 26(8):578–583
Liu S-N, Han Y, Z-j Z (2011) Lactic acid bacteria in traditional fermented Chinese foods. Food Res Int 44:643–651. https://doi.org/10.1016/j.foodres.2010.12.034
Liu T, Li Y, Chen J, Sadiq FA, Zhang G, Li Y, He G (2016) Prevalence and diversity of lactic acid bacteria in Chinese traditional sourdough revealed by culture dependent and pyrosequencing approaches. Lwt-Food Sci Technol 68:91–97. https://doi.org/10.1016/j.lwt.2015.12.025
Lu C, Sun T, Li Y, Zhang D, Zhou J, Su X (2018) Microbial diversity and composition in different gut locations of hyperlipidemic mice receiving krill oil. Appl Microbiol Biotechnol 102:355–366. https://doi.org/10.1007/s00253-017-8601-1
Malik S, Petrova MI, Claes IJJ, Verhoeven TLA, Busschaert P, Vaneechoutte M, Lievens B, Lambrichts I, Siezen RJ, Balzarini J, Vanderleyden J, Lebeer S (2013) The highly autoaggregative and adhesive phenotype of the vaginal Lactobacillus plantarum strain CMPG5300 is sortase dependent. Appl Environ Microbiol 79(15):4576–4585
Manini F, Casiraghi M, Poutanen K, Brasca M, Erba D, Plumed-Ferrer C (2016) Characterization of lactic acid bacteria isolated from wheat bran sourdough. Lwt-Food Sci Technol 66:275–283. https://doi.org/10.1016/j.lwt.2015.10.045
Minami J-I, Kondo S, Yanagisawa N, Odamaki T, Xiao J-z, Abe F, Nakajima S, Hamamoto Y, Saitoh S, Shimoda T (2015) Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci 4
Papadimitriou K, Zoumpopoulou G, Foligne B, Alexandraki V, Kazou M, Pot B, Tsakalidou E (2015) Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front Microbiol 6:58. https://doi.org/10.3389/fmicb.2015.00058
Pena JA, Versalovic J (2003) Lactobacillus rhamnosus GG decreases TNF-α production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell Microbiol 5:277–285. https://doi.org/10.1046/j.1462-5822.2003.t01-1-00275.x
Ramos CL, Thorsen L, Schwan RF, Jespersen L (2013) Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol 36:22–29. https://doi.org/10.1016/j.fm.2013.03.010
Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A (2017) Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res 61(1). https://doi.org/10.1002/mnfr.201600240
Sekiguchi H, Kawada-Watanabe E, Arashi H, Yamaguchi J, Ogawa H, Hagiwara N (2018) Association between the low-density lipoprotein-cholesterol/high-density lipoprotein-cholesterol ratio and clinical outcomes in patients with acute coronary syndrome and dyslipidemia: a subanalysis of the HIJ-PROPER study. J Am Coll Cardiol 71:A1757. https://doi.org/10.1016/S0735-1097(18)32298-8
Shang Q, Song G, Zhang M, Shi J, Xu C, Hao J, Li G, Yu G (2017) Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J Funct Foods 28:138–146. https://doi.org/10.1016/j.jff.2016.11.002
Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, Horn M, Cohen Y, Moor AE, Zeevi D, Korem T, Kotler E, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Pevsner-Fischer M, Shapiro H, Sharon I, Halpern Z, Segal E, Elinav E (2018) Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 174(6):1406–1423 e16. https://doi.org/10.1016/j.cell.2018.08.047
Tan Q, Xu H, Aguilar ZP, Peng S, Dong S, Wang B, Li P, Chen T, Xu F, Wei H (2013) Safety assessment and probiotic evaluation of Enterococcus faecium YF5 isolated from sourdough. J Food Sci 78:M587–M593. https://doi.org/10.1111/1750-3841.12079
Thornton GM (1996) Probiotic bacteria: selection of Lactobacillus and Bifidobacterium strains from the healthy human gastrointestinal tract; characterisation of a novel Lactobacillus-derived antibacterial protein. NUI
Trivedi D, Jena PK, Patel JK, Seshadri S (2013) Partial purification and characterization of a bacteriocin DT24 produced by probiotic vaginal Lactobacillus brevis DT24 and determination of its anti-uropathogenic Escherichia coli potential. Probiotics Antimicrob 5:142–151. https://doi.org/10.1007/s12602-013-9132-4
Tse MCL, Herlea-Pana O, Brobst D, Yang X, Wood J, Hu X, Liu Z, Lee CW, Zaw AM, Chow BKC, Ye K, Chan CB (2017) Tumor necrosis factor-alpha promotes phosphoinositide 3-kinase enhancer a and AMP-activated protein kinase interaction to suppress lipid oxidation in skeletal muscle. Diabetes 66:1858–1870. https://doi.org/10.2337/db16-0270
Walker DK, Gilliland SE (1993) Relationships among bile tolerance, bile salt deconjugation, and assimilation of cholesterol by Lactobacillus acidophilus. J Dairy Sci 76(4):956–961
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Yang D, Yu X, Wu Y, Chen X, Wei H, Shah NP, Xu F (2016) Enhancing flora balance in the gastrointestinal tract of mice by lactic acid bacteria from Chinese sourdough and enzyme activities indicative of metabolism of protein, fat, and carbohydrate by the flora. J Dairy Sci 99(10):7809–7820
Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB, Federici S, Cohen Y, Linevsky R, Rothschild D, Moor AE, Ben-Moshe S, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Shapiro H, Pevsner-Fischer M, Sharon I, Halpern Z, Segal E, Elinav E (2018) Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174(6):1388–1405 e21. https://doi.org/10.1016/j.cell.2018.08.041
Funding
This research was supported by the Hangzhou industry-university cooperation project [grant number 20161631E01] and Agricultural Technology Promotion Special Fund of Zhejiang University New Rural Development Institute [grant number 2017006].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 920 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Liu, T., Zhao, M. et al. In vitro and in vivo investigations of probiotic properties of lactic acid bacteria isolated from Chinese traditional sourdough. Appl Microbiol Biotechnol 103, 1893–1903 (2019). https://doi.org/10.1007/s00253-018-9554-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9554-8