Abstract
Acute liver failure is a drastic, unpredictable clinical syndrome with high mortality. Various preventive and adjuvant therapies based on modulating the gut flora have been proposed for hepatic injury. We aimed to explore the preventive and therapeutic effects of Bifidobacterium adolescentis CGMCC15058 on rat liver failure, as well as the potential microecological and immunological mechanisms of those effects. B. adolescentis CGMCC15058 (3 × 109 CFU), isolated from healthy human stool, was gavaged to Sprague–Dawley rats for 14 days. Acute liver injury was induced on the 15th day by intraperitoneal injection of d-galactosamine. After 24 h, liver and terminal ileum histology, liver function, plasma cytokines, bacterial translocation and gut microbiota composition were assessed. We found that pretreatment with B. adolescentis significantly relieved elevated serum levels of alanine aminotransferase (ALT), total bile acid and lipopolysaccharide-binding protein and enhanced the expression of mucin 4 and the tight junction protein zonula occludens-1. B. adolescentis exhibited anti-inflammatory properties as indicated by decreased levels of mTOR and the inflammatory cytokines TNF-α and IL-6, as well as elevated levels of the anti-inflammatory cytokine interleukins-10 in the liver. Similar anti-inflammatory signs were also found in plasma. B. adolescentis significantly altered the microbial community, depleting the common pathogenic taxon Proteus and markedly enriching the taxa Coriobacteriaceae, Bacteroidales and Allobaculum, which are involved in regulating the metabolism of lipids and aromatic amino acids. Our findings not only suggest B. adolescentis acts as a prospective probiotic against liver failure but also provide new insights into the prevention and treatment of liver disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute liver failure is a drastic, unpredictable clinical syndrome; it is manifested clinically as severe deterioration of liver function followed by hepatic encephalopathy, coagulopathy and, ultimately, multiorgan failure (Wlodzimirow et al. 2012). Acute liver failure can be caused by drug-induced liver injury, idiosyncratic reactions to medication, viral hepatitis, excessive alcohol intake, or autoimmune hepatitis, although the causes of most cases are indeterminate (Bernal et al. 2015). Acute liver failure carries high mortality, ranging between 60 and 80% (Wlodzimirow et al. 2012).
Accumulating evidence demonstrates strong correlations between the gut microbiome and acute liver failure (Tranah et al. 2013). Disruption of liver physiology contributes to the weakening of the intestinal barrier, followed by movement of microbes and their products from the intestinal lumen to the liver, which is likely to aggravate certain liver diseases by enhancing the spread of inflammation, tissue damage and sepsis; in some cases, spontaneous bacterial peritonitis occurs (Wiest and Garcia-Tsao 2005).
The genus Bifidobacterium, the predominant component of the intestinal microbiota, has various probiotic functions in healthy humans, including carbohydrate fermentation, vitamin synthesis, carcinogen detoxification, restoring the equilibrium of the enteric flora and augmenting the host’s immune responses (Arboleya et al. 2016). Several species of Bifidobacterium, including B. adolescentis, B. bifidum, B. longum subsp. infantis, B. longum subsp. longum, B. breve and B. catenulatum, have shown anti-inflammatory activity both in vitro (Heuvelin et al. 2009; Khokhlova et al. 2012) and in vivo (Frick et al. 2007). In addition, the culture supernatant from bifidobacteria has shown anti-inflammatory activity (Khokhlova et al. 2012).
Various interventions that modulate the gut flora and regulate metabolic energy have been studied as preventive or adjuvant therapies for hepatic injury. B. longum can potentially attenuate cirrhosis (Moratalla et al. 2016a), and B. pseudocatenulatum CECT7765 improves gut homeostasis and prevents gut-derived complications in experimental chronic liver disease (Moratalla et al. 2016b). B. pseudocatenulatum LI09 and B. catenulatum LI10 attenuate liver injury by modifying the gut microbiota (Fang et al. 2017). A combination of B. animalis VKL, B. animalis VKB and Lactobacillus casei IMV B-7280 are effective at decreasing the weight of obese mice, decreasing cholesterol levels, restoring normal liver morphology and beneficially modulating the gut microbiome in high-calorie-induced obesity (Bubnov et al. 2017). Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 improves acute liver injury in rats (Lv et al. 2014).
Furthermore, B. adolescentis ameliorated visceral fat accumulation and insulin sensitivity in a high-fat-diet-fed rat model of metabolic syndrome (Chen et al. 2012), and increased the production of short-chain fatty acids in cultured human microbiota (Belenguer et al. 2006), and regulated the homeostasis of gut microbiota compositions in vivo (Belenguer et al. 2006; Marquet et al. 2009). Enteral B. adolescentis supplementation improved the intestinal barrier and reduced bacterial translocation, thus attenuating parenteral nutrition (PN)-induced gut and liver injuries, in infant rabbits (Wu et al. 2010).
In this work, B. adolescentis CGMCC 15058 was isolated from human stools, and we aimed to explore the preventive and therapeutic effects of that bacterial strain on rat liver failure and its microecological and immunological mechanisms.
Methods
Strains and culture conditions
B. adolescentis CGMCC 15058, deposited at the China General Microbiological Culture Collection Center (CGMCC), was originally isolated from healthy volunteers and cultured in Trypticase–Phytone–Yeast Broth Medium (RiShui, Co., Ltd., Qingdao, China) anaerobically for 36 h at 37 °C. A standard approach was followed to revive the frozen bacterial strains as proved by Fang et al. (2017). Cells were collected by centrifugation at 8000×g for 10 min at 4 °C. These cells were washed twice and resuspended at 4 °C in phosphate buffer with a final concentration of 3 × 109 colony-forming units (CFU)/ml for further use (Fang et al. 2017).
Experimental design and model of liver injury
Male germ-free Sprague–Dawley (SD) rats, weighing 250 to 320 g, were assigned to three groups with randomised blocks of seven replications. A normal control group was gavaged with normal saline, labelled the NC (negative control) group; a d-galactosamine (d-GalN)-induced liver failure group was treated with normal saline, labelled the PC (positive control) group; and a d-GalN-induced liver failure was treated daily with B. adolescentis CGMCC 15058 by gavage, labelled the B. adolescentis group. One millilitre of freshly prepared bacterial suspension (at 3 × 109 CFU/ml) for the B. adolescentis group and same quantity of saline solution for the NC and PC groups was orally administered once daily for 14 days.
1.1 g/kg body weight of d-GalN (G0500, Sigma, Saint Louis, MO, USA) was intraperitoneally injected to induce acute liver failure on the 15th day in all groups except the NC group. All animals were fed regular chow and had ad libitum access to food and water. SD rats were housed at room temperature (20–22 °C) with a controlled 12-h:12-h light:dark cycle. The animals were sacrificed 24 hours after d-GalN challenge under aseptic conditions.
After 24 h of d-GalN injection, the animals were aseptically anaesthetised and sacrificed.
Biochemical indicators of hepatic function
Serum was extracted by centrifugalising the venous blood samples at 3000g for 10 min and prepared for analysis. Glycylproline dipeptidyl aminopeptidase (GPDA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), total bilirubin (TBil), total bile acid (TBA), albumin and globulin in serum were evaluated by the Hitachi 7600–210 automatic analyser (Hitachi 7600–210; Hitachi, Tokyo, Japan) following the standard methods (Cho et al. 2014).
Plasma cytokine analysis
The plasma (20 μl) was extracted by centrifugalising the blood at room temperature (3000g for 10 min), then stored at − 80 °C. A Bio-Plex Pro Rat Cytokine 24-Plex Panel magnetic bead suspension array (Bio-Rad, CA, USA) was used to analyse plasma cytokines following its instructions. The cytokines targeted by the kit included erythropoietin (EPO), vascular endothelial growth factor (VEGF), interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (C-C motif) ligand 5 (CCL5), macrophage colony-stimulating factor (M-CSF), macrophage inflammatory proteins (MIPs) including MIP-1α and MIP-3α, monocyte chemoattractant protein 1 (MCP-1) and interleukins (ILs) including IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 (p70), IL-13, IL-17A and IL-18. The samples were probed with Bio-Plex 200 analyser, (Bio-Plex 200, BioPlex, Luminex 200, CA, USA); then, data were analysed by software Bio-Plex Manager 6.0 (Rotstein 2014).
Bacterial translocation assay
Samples from left liver lobe, the kidneys and the mesenteric lymph nodes (MLNs) were collected, weighed and homogenated aseptically in glass homogenisers autoclaved at 121 °C for at least 15 min. Thoroughly homogenised samples were diluted with physiological saline and separately incubated in duplicate on BHI agar (Oxoid, Thermo Fisher Biochemicals Ltd., Beijing, China) at 37 °C for 48 h under aerobic and anaerobic conditions. The bacterial translocation (BT) percentages from each plate was counted and expressed as fractions in the form n/N.
Histological examination
For histological evaluation, the left lobe of liver and terminal ileum was taken from each anaesthetised rat, and then immediately fixed by 10% formaldehyde solution for 24 h and embedded in paraffin. The formalin fixed and paraffin embedded tissue (FFPET) was cut into sections at the length of 2 μm, then successively stained with haematoxylin-eosin staining (HE) and analysed by a pathologist who was unaware of the group situation. Each specimen was studied from at least three slides.
Liver tissue damage was assessed semiquantitatively by a histological score from the Histological Activity Index (Knodell et al. 1981), which consists of two categories. One is the intralobular degeneration and focal necrosis, which scored, (0–1 or 3–4) and the other one is portal inflammation standing for the acute liver, scored (0–1 or 3–4) damage. Intestinal mucosal lesion was classified as described by Chiu et al. (1970). A score 0 means normal mucosa, while a score of 1 represents the development of subepithelial Gruenhagen’s space at the tip of the villus. In tissue with a score of 2, the space is more extended. While with a score of 3, a substantial epithelial would lift down the side of the villus. In tissue that scored 4, the epithelium is denuded from the villus. A score of 5 is defined as the losing of the villus itself.
Electron microscopy
Terminal ileum specimens were immediately postfixed in glutaraldehyde (2.5%) at 4 °C, dehydrated in rising concentrations of alcohol solutions and finally embedded with epoxy resin. Next, ultrathin sections were obtained and stained, in turn, with 3% uranyl acetate then 0.2% lead citrate (Dias et al. 2017). A Philips Tecnai 10 electron microscope (Philips, Eindhoven, the Netherlands) was used to observed ileal mucosal ultrastructure. Degree of damage was assessed in terms of the diameter, length and linear density of microvilli (Brown Jr. 1962).
16S rRNA sequencing and PICRUSt analysis
According to the manufacturer’s instructions, a QIAamp® Fast DNA Stool Mini Kit (Qiagen, Valencia, USA) was used to extract total genomic DNA from tissue and faeces. The quality and quantity of DNA were verified with agarose gel electrophoresis and a NanoDrop spectrophotometer. PCR amplification of bacterial 16S rRNA genes was conducted with 1 ng/μl of DNA as a template, HiFi Hot Start Ready Mix (kk2601, Kapabiosystems, Beijing, China) and barcoded primers 343F (5′-TACGGRAGGCAGCAG-3′) and 798R (5′-AGGGTATCTAATCCT-3′). Universal primers 343F and 798R were amplified to the V3-V4 regions of the 16S rRNA gene in bacterial diversity analysis.
The amplicon amplified for another round of PCR was tested for quality by gel electrophoresis and purified with AMPure XP beads (Agencourt, Beckman Coulter, USA). After purification process again, a Qubit dsDNA assay kit (Invitrogen™, Eugene, OR, USA) was used to quantify the final amplicon. Subsequent sequencing was conducted with purified amplicon.
According to the manufacturer’s instructions, an Illumina MiSeq platform (Illumina, San Diego, CA) was used to sequence and raw data were recorded in FASTQ format. To detect and remove ambiguous bases (N), Trimmomatic software was used to preprocess paired-end reads (Bolger et al. 2014). Sequences were removed with an average quality score below 20 detected by a sliding-window trimming approach. The software FLASH (Fast Length Adjustment of Short Reads) were used to assemble paired-end reads (Reyon et al. 2012) after the trimming process. The assembly parameters were as follows: 10-bp minimal overlap, 200-bp maximum overlap and 20% maximum mismatch rate. The sequences were denoised as follows: reads with ambiguous, homologous sequences or below 200 bp were abandoned. Reads with 75% of bases above Q20 were retained. Then, chimaeras were detected and removed. Denoising and chimaera detection were both carried out using QIIME software (version 1.8.0) (Caporaso et al. 2010).
UPARSE software removed primer sequence from clean reads and clustered into operational taxonomic units (OTUs), with a 97% similarity cutoff (Edgar 2013). QIIME was used to select representative read of each OUT and representative reads were BLASTed and annotated against the SILVA database Version 123 (http://www.arb-silva.de/download/archive/qiime/) or Greengenes (16S rDNA) using the Ribosomal Database Project (RDP) classifier (confidence threshold, 70%) (Wang et al. 2007). Microbial diversity and the community richness were calculated by the Chao1 index and rarefaction curve was provided for quality control (Supplementary Fig. S1). In three large conical mat laminae, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt v1.0.06) analysis was conducted to predict metabolic functions of the microbial communities (Langille et al. 2013) by referencing the Kyoto Encyclopedia of Genes and Genome (KEGG) Orthology (KO) Database (Kanehisa et al. 2014) using the “predict_metagenomes.py” command in PICRUSt (v1.0.0) (Langille et al. 2013). Asymptotic confidence intervals with continuity correction were calculated, and the Benjamini–Hochberg procedure was used for multiple comparisons, with a P threshold of 0.05, to control the false discovery rate (FDR) (Li et al. 2014). Sequence data can be accessed with the number SRP135921 in the GenBank Sequence Read Archive.
Lipopolysaccharide binding protein measurement
Blood from the inferior vena cava were collected in pyrogen-free heparinised syringes. Serum was separated out by centrifuging at 1000×g for 10 min. A rat lipopolysaccharide binding protein, LBP ELISA Kit (GD-S1538-K, Guduo Biotechnology Co. Ltd., Shanghai, China) was used to detect the level of LBP.
Immunofluorescence staining
Sections of the terminal ileum (2 μm), cut on a cryostat, were dewaxed, rehydrated using standard methods and treated with 3% H2O2. The specimens were stained with zonula occludens-1 (ZO-1) (1:100, Invitrogen, Carlsbad, CA, USA) antibodies overnight at 4 °C, then incubated with secondary antibody FITC-conjugated goat anti-rabbit (Beyotime, Shanghai, China) with a concentration of 1:100 for 1 h at 20–22 °C. PBS and mounting medium (Beyotime, Shanghai, China) were separately used to wash and mount with the sections, which finally were shooted with a fluorescence microscope (Eclipse 80i; Nikon, Tokyo, Japan).
RNA extraction
An RNeasy Mini Kit (Qiagen, Valencia, USA) was used to extract total RNA from liver samples and intestinal segments according to the manufacturer’s protocol. Ethidium bromide staining was used to verify the integrity of RNA; then, extracts were stored at − 80 °C until further processing.
Reverse transcription polymerase chain reaction
The relative expression of total mRNA was measured in triplicate by comparison of cycle thresholds with an Applied Biosystems VIIA7 real-time PCR system using a One Step SYBR PrimeScript Plus RT-PCR Kit (Takara Biomedicals, Kusatsu, Japan). β-actin was used as the loading control. The primer pairs are listed in Supplementary Table S1.
Statistical analysis
Kolmogorov-Smirnov test was employed to examine the normality of biochemical assay results and histology scores. The Mann–Whitney U test (for non-normal distributions) or Student’s t test (for normal distributions) was performed to test for differences between two groups. Clustering of microbial communities was assessed using analysis of similarities (ANOSIM) on weighted and unweighted UniFrac distance matrices. The chi-squared test was used to compare the frequency of bacterial translocation. Spearman’s rank correlation coefficient was employed when linear correlation analysis was conducted. If normally distributed, the values were shown as the mean ± SEM; otherwise, they were presented as median with interquartile range. A two-tailed P of < 0.05 was considered significant. The data were analysed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism (Fang et al. 2017).
Results
B. adolescentis CGMCC 15058 alleviated d-GalN-induced liver injury
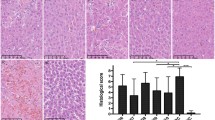
Twenty-four hours after the induction of liver damage, the morality of the PC group was 14.3%. None of the rats had died in the NC group or the B. adolescentis group. Hepatic histological abnormalities were observed in all groups except the NC group (Fig. 1a). Administration of B. adolescentis significantly ameliorated d-GalN-induced hepatic degeneration, necrosis and inflammatory infiltration compared to that of the PC group.
Bifidobacterium adolescentis CGMCC 15058 alleviated d-GalN-induced liver injury. a Representative images of hepatic haematoxylin and eosin (HE) staining. b Histological scores of livers based on these images; liver function; hepatic expression of mTOR and the inflammatory cytokines TNF-α, IL-6, IL-10, IL-17 and TGF-β. Gene expression was determined by quantitative PCR analysis of the total mRNA extracted from liver fragments. The results are expressed as fold change relative to the NC group. All data are given as the mean ± SEM. *P < 0.05 and **P < 0.01 vs. compared with the positive control (PC) group
Upon liver function analysis, serum alanine transaminase (ALT) (P = 0.01) and total bile acid (TBA) (P < 0.05) were observed to be significantly relieved in B. adolescentis-treated rats compared to PC rats. Interestingly, we found that the concentration of globulin in peripheral blood was significantly higher in the probiotic-treated group than in the PC group (P < 0.05).
B. adolescentis CGMCC 15058 reduced destruction of the intestinal barrier during d-GalN-induced liver injury
Plasma endotoxins contribute to the progression of liver damage, which serves as an indicator whether the integrity of the intestinal barrier is compromised (Nier et al. 2017) (Cani et al. 2008; Lebrun et al. 2017). Lipopolysaccharide (LPS)-binding protein (LBP), which binds to bacterial LPS to elicit an immune response (Muta and Takeshige 2001), has been established recently as a biological marker for diagnosis and prognosis of bacterial infections (Fang et al. 2012). The effects of the probiotic strain on endotoxaemia are shown in Table 1. Rats from the PC group (172.1 ± 15.4 EU/ml) showed significantly higher serum LBP levels than rats from the NC group (69.7 ± 15.9, P < 0.01). The B. adolescentis group was observed to have lower LBP levels than the PC group (P < 0.05).
Bacteria can penetrate the inner mucosal layer and contact the epithelium to elicit a beneficial or harmful effect, and the loosening of tight junctions is a major factor that contributes to pathological bacterial translocation (BT).
The translocation percentages of anaerobes were 50.0% vs. 0% (3/6 vs. 0/7, PC vs. B. adolescentis) in arterial blood (P < 0.05), 83.3% vs. 28.6% (5/6 vs. 2/7, PC vs. B. adolescentis) in the kidney (P < 0.05) and 100% vs. 42.9% (6/6 vs. 3/7, PC vs. B. adolescentis) in MLNs (P < 0.05). Similar trends were found for translocation of aerobic bacteria (100% vs. 85.7% in MLNs, 83.3% vs. 57.1% in arteries, 100% vs. 71.4% in the kidneys, PC vs. B. adolescentis). However, this difference was not significant owing to the insufficient sample size (Table 2).
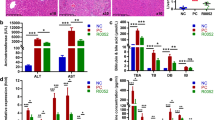
HE staining was conducted to explore whether the intestinal mucosa integrity was damaged by intraperitoneal injection of d-GalN and improved by probiotic bacterial strains. The results (Fig. 2b) showed that d-GalN-induced histological abnormalities of the terminal ileum were alleviated by pretreatment with B. adolescentis, with lower incidence of subepithelial Gruenhagen’s space, more complete villus architecture and significantly lower histological scores in the probiotic-treated group than in the PC group (Fig. 2d). Intestinal mucosal integrity was assessed by transmission electron microscopy; the microvilli of the intestinal epithelial cells in the PC groups were ruptured, sparse and stunted, while pretreatment with B. adolescentis reduced disruption of the brush border (Fig. 2a).
Treatment with Bifidobacterium adolescentis CGMCC 15058 improved intestinal barrier function. Representative images of a the ileal mucosal ultrastructure and histological alternations in the ileum assessed by b HE staining and c ileal ZO-1 immunofluorescence (× 20). d Ileal inflammation was monitored on the basis of histology scores, following the criteria presented in the “Methods” section. Ileal ZO-1, TLR-3 and MUC4 gene expression were determined by quantitative PCR. All data are given as the mean ± SEM. *P < 0.05 and **P < 0.01 vs. compared with the positive control (PC) group
To validate the impact of this strain on the intestinal barrier, we measured ileal tight junction protein and mucoprotein expression by quantitative PCR and immunofluorescence. Compared to the PC group, the B. adolescentis group showed elevated levels of MUC4 (P < 0.05) and ZO-1 (P < 0.05) mRNA expression (Fig. 2d). Breakdown of ZO-1 and relocation of the protein away from tight junctions were found in the PC group, but that group showed no significant difference in ZO-1 mRNA expression compared to the NC group (Fig. 2d). A clear, uniform distribution of ZO-1 protein was detected at the apical region of the intestinal epithelium with B. adolescentis (Fig. 2c).
B. adolescentis CGMCC 15058 exhibited overall anti-inflammatory characteristics during d-GalN-induced liver injury
Administration of B. adolescentis reduced d-GalN-induced inflammatory cytokines. In the liver, the expression levels of inflammatory and anti-inflammatory cytokines were measured by quantitative PCR. The d-GalN-induced increases in mTOR (P < 0.05) and the inflammatory cytokines TNF-α (P < 0.01) and IL-6 (P < 0.05) were significantly relieved after administration of this strain. IL-17 and TGF-β show similar decreasing trends. By contrast, the increase in the anti-inflammatory cytokine IL-10 (P < 0.05) in the liver under d-GalN was improved in the groups treated with this strain.
Furthermore, a total of 24 different plasma cytokines were examined to confirm their preventive effect on inflammation.
Twenty-four hours after d-GalN injection, plasma proinflammatory cytokines increased compared with the levels in the NC group, which may aggravate liver injury (Table 1). Administration of B. adolescentis significantly decreased the cytokines interleukin IL-1b (P < 0.05) and IL-7 (P < 0.05) and stimulated the anti-inflammatory cytokines IL-10 (P < 0.01) and IL-13 (P < 0.05) (Table 1). Among the chemokines, the increases in VEGF (P < 0.01), M-CSF (P < 0.01), MCP (P < 0.01) and GRO/KC (P < 0.01) were attenuated when the rats were treated with B. adolescentis (P < 0.01, P < 0.01, P < 0.05 and P < 0.05). Compared with the PC group, the group treated with this strain (P < 0.01) showed decreased levels of the chemokine RANTES. These results support the point that chemokines may play an important role in improving liver injury.
B. adolescentis ameliorated microbiome dysbiosis and restored the gut microbiota
Next, we sought to gain further insights into the changes in the gut microbiota upon administration of these strains during d-GalN-induced liver injury. Next-generation sequencing of 16S rRNA V3–V4 regions was conducted to investigate the impact of the probiotic on the microbiota composition of caecal content.
A total of 976,241 valid tags from 34 samples of rat caecum contents were filtered for downstream analysis. On the basis of ≥ 97% sequence identity, 1776 qualified OTUs were clustered.
The overall microbial diversity and the community richness, as calculated by the Chao1 index, were not significantly different across experimental groups (P < 0.05) (Fig. 3a). The overall structural changes in the microbial communities were analysed by Bray–Curtis principal coordinate analysis (PCoA) (Fig. 3b), which explained 27.75% of the total variation on PC1 and 13.89% on PC2. ANOSIM showed that the PC samples were roughly separated from the NC samples (P < 0.05). When the rats were treated with B. adolescentis before d-GalN administration, the gut microbiome was distinctly separated from that of the PC samples (P < 0.05), showing a distinct role of B. adolescentis in modulating the overall structures of the commensal bacterial communities in the three groups.
Effects of pretreatment with Bifidobacterium adolescentis CGMCC 15058 on the overall structural changes in gut microbial communities during d-GalN-induced acute liver injury. a The α-diversity of the gut microbiome, determined by the Chao1 diversity index of the probiotic group compared with that of the PC group. b The principal coordinate analysis (PCoA) plot shows the β-diversity of the gut microbiome with Bray–Curtis dissimilarity derived from 16S sequencing data. c Comparison of taxa at the bacterial family and d genus levels between the PC and B. adolescentis groups. Data are given as the median with first and third quartiles. *P < 0.05 and **P < 0.01 compared with the positive control (PC) group
To further characterise phenotypic changes in the taxonomic composition, relative taxon abundance was compared at the family and genus levels and showed that intestinal microbial structure was affected by B. adolescentis in rats with acute hepatic injury (Fig. 3c, d). Oral administration of B. adolescentis ameliorated the d-GalN-induced depletion of Erysipelotrichaceae. Compared with the PC group, the B. adolescentis group showed significant enrichment of the families Desulfovibrionaceae, Erysipelotrichaceae, Peptostreptococcaceae and Bacteroides (Fig. 3c).
At the genus level, acute liver injury had a wide-ranging impact on the gut microbiome (Fig. 3d). The common pathogens Proteus (Luo et al. 2016) and Runella, belonging to the family Cytophagaceae, showed increased abundance in the PC group. Administration of B. adolescentis considerably promoted the abundance of Coriobacteriaceae, Bacteroidales and Allobaculum, which are associated with the metabolism of lipids and aromatic amino acids (Raza et al. 2017). However, the abundance of the genera Romboutsia, Marivita, Dorea, Desulfovibrio and Erysipelatoclostridium in the B. adolescentis group was not supported related to liver disease.
Linear discriminant analysis effect size (LEfSe) was performed to compare the estimated phylotypes of the probiotic-group and PC-group microbiomes and identify differentially abundant biomarkers with biological consistency between the two groups (Segata et al. 2011). LEfSe demonstrated that the phylum Nitrospirae, class Betaproteobacteria and order Nitrospirales were enriched in the PC group compared to the NC group. At the family level, the NC group show significant enrichment of Prevotellaceae and Acidaminococcaceae (Fig. 4a). At the genus level, the NS group, characterised by a preponderance of Fastidiosipila and Romboutsia, was similar to the B. adolescentis group (Fig. 4a, b). Christensenellaceae was depleted in the PC group, and administration of B. adolescentis attenuated this Christensenellaceae depletion and ameliorated the d-GalN-induced enrichment of Porphyromonadaceae and Enterobacteriaceae. In addition, Acidaminococcaceae, Coprococcus_1 and Prevotellaceae were enriched in the NC group, and Corynebacteriaceae, Erysipelotrichales, Coriobacteriaceae_UCG_002, Dorea, Marivita, Parasutterella, Allobaculum and Ruminiclostridium_6 enriched in the B. adolescentis group (Fig. 4b).
Effects of pretreatment with Bifidobacterium adolescentis on the alterations of gut bacterial taxonomic abundance during d-GalN-induced acute liver injury. Bacterial taxa identified as differentially abundant between groups according to linear discriminant analysis effect size (LEfSe). The bacterial taxa of the probiotic group were compared with those of the PC group at different levels. Green indicates bacterial taxa whose abundance was higher in the PC group, and red indicates bacterial taxa whose abundance was higher in the other group
Several significant functional annotations were found in the microbiome of the three groups. d-GalN inhibits peptidoglycan biosynthesis. Notably, the microbiome of B. adolescentis group functions in aminoacyl-tRNA, peptidoglycan biosynthesis, betalain biosynthesis, 1,1,1-trichloro-2,2-bis (4-chlorophenyl) ethane (DDT) degradation (P = 0.047) and basic molecular biological processes (Fig. 5a, b).
Correlation-network analysis between gut bacterial genera and indexes, between gut bacterial genera and gut bacterial genera and between indexes and indexes in rats with d-GalN-induced liver failure with/without B. adolescentis
Correlations of gut bacterial genera with endotoxin, gut barrier marker and inflammatory cytokines indicate important roles in the regulation of liver failure. To verify that the alterations in the gut microbial composition play an important role in alleviating the increase in inflammatory cytokines, correlation-network analysis was used to identify key linkages between the involved inflammatory cytokines and gut bacterial genera in acute liver injury (Fig. 6a). We found that Erysipelatoclostridium, which was enriched in the B. adolescentis group, was positively correlated with IL-10 (r = 0.557), IL-13 (r = 0.627) and MUC4 (r = 0.646), while negatively correlated with Toll-like receptor (TLR) 4 (r = −0.574, TNF-α (r = −0.692) and IL-6 (r = −0.574). Furthermore, we found positive correlations between Gemmatimonadaceae, which was enriched in the PC group, and TLR4 in the liver (r = 0.644), as well as mTOR (r = 0.57) and TNF-α (r = 0.685).
Correlation-network analysis between gut bacterial genera and indexes, between gut bacterial genera and gut bacterial genera in rats with d-GalN-induced liver failure rats with/without B. adolescentis. A Spearman correlation analysis was performed, and only correlations with P < 0.05 and r > 0.5 are displayed. a Correlation-network analysis between gut bacterial genera and indexes. b Correlation-network analysis between gut bacterial genera and gut bacterial genera. Blue nodes represent bacterial genera, yellow nodes represent tissue cytokines and pink nodes represent blood indexes. “Ra” represents plasma cytokines detected by a Bio-Plex rat cytokine assay. A red line connecting nodes represents a positive correlation, and a green line represents a negative correlation. The value of the corresponding correlation coefficient is indicated by the thickness of the line: the thicker the line, the greater the coefficient
Allobaculum was positively correlated with IL-10 in plasma (r = 0.621) and liver (r = 0.626), globulin (r = 0.812), MUC4 (r = 0.714) and MCP-1 (r = 0.692) while being negatively correlated with mTOR (r = − 0.61), TNF-α (r = − 0.703), IL-6 (r = − 0.703), IL-7 (r = − 0.643) and VEGF (r = − 0.604). Interestingly, we identified positive correlations between Coriobacteriaceae_UCG_002 and the anti-inflammatory cytokine IL-10 (r = 0.624), as well as between LBP and Anaerotruncus (r = 0.59) and between TBA and Aeromonas (r = 0.669).
Coriobacteriaceae_UCG_002 (P = 0.003, r = 0.544) and Ruminiclostridium were related to Roseburia (P = 0.001, r = 0.583), and Enterococcus were related to Prevotella_2 (P = 0.004, r = 0.530) (Fig. 6b).
Among the various relationships, a network was found in which inflammatory cytokines (Supplementary Fig. S2) were closely correlated with other inflammation markers; for example, TLR signalling has been shown to act as a bridge between bacteria and the epithelial barrier, playing a central role in the relationship between the gut and its microbiota. TLR3 was positively correlated with globulin (r = 0.554), IL-10 (r = 0.742), MUC4 (r = 0.769), MCP-1 (r = 0.56) and Ra IL-10 (r = 0.72), while it was negatively correlated with mTOR (r = − 0.588), Ra IL-7 (r = − 0.599) and TNF-α (r = − 0.67). The anti-inflammatory-related molecule TLR3 was negatively correlated with the inflammatory-related molecule TLR4 (r = 0.593). TLR4 was highly positively correlated with IL-6 (r = 0.72) and negatively correlated with IL-10 (r = − 0.604) and IL-13 (r = − 0.615). The liver inflammation indexes TNF-α and ALT were positively correlated with each other (r = 0.702) and were strongly associated with the plasma cytokines Ra IL-10 (r = − 0.681) and Ra IL-7 (r = 0.604).
mRNA expression of the tight junction protein ZO-1 was positively correlated with that of MUC4 (r = 0.648), globulin (r = 0.57) and IL-10 (r = 0.632) and negatively correlated with the expression of mTOR (r = − 0.599). mTOR was strongly associated with ALT (r = 0.702), Ra GRO/KC (r = 0.626), Ra IL-10 (r = − 0.863), Ra IL-13 (r = − 0.632) and Ra VEGF (r = 0.665).
Discussion
Liver disease development can be influenced by several factors including gender, body mass and poor lifestyle choices, such as diet (Lang and Beier 2018). Ethanol causes hepatotoxicity, liver injury and alcoholic liver disease (ALD) in humans (Shah and John 2018). High-calorie diet and/or obesity, especially the diet rich in saturated fatty acids as well as cholesterol, induces non-alcoholic fatty liver disease (NAFLD). A study by Allman et al. described that mice concomitantly exposed to a Western diet and carbon tetrachloride had worse hepatic steatosis and enhanced injury (Allman et al. 2010). Obesity is the most common cause of fatty liver disease in the USA (Lang and Beier 2018). Overweight patients are more susceptible for acute liver failure (Canbay et al. 2005). Significant differences between sexes were found that Kupffer cells (hepatic tissue macrophages) of female animals were shown more active in sequestering circulating virions, which protects hepatocytes more efficiently than those of males (Ying et al. 2018). Nevertheless, due to the sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, female mice are more susceptible to non-alcoholic fatty liver disease (Spruss et al. 2012). Acute liver failure is an organ-damaging and life-threatening disease caused by various factors (Davies and Banares 2015). d-GalN-induced male rats acute liver injury is a very well-reproducible experimental model for the study of liver disease (Lu et al. 2014). d-GalN induces a loss of uridine triphosphate via the galactose pathway and inhibits RNA and protein synthesis (Wang et al. 2014), thus resulting in hepatic necrosis and apoptosis due to metabolic changes (Yang et al. 2014).
Exposure to translocated gut microbiota and their metabolites may affect the liver in both health and disease (Woodhouse et al. 2018). Promoting the growth of “healthy” bacteria with probiotics may ameliorate dysbiosis and alter prognosis. The impact of probiotics on hepatic encephalopathy was assessed through a meta-analysis, which showed that probiotics improved both minimal and overt hepatic encephalopathy (Zhao et al. 2015). A phase I study on the effect of probiotics on cirrhosis, published in 2014 (Bajaj et al. 2014), shows that Lactobacillus GG (LGG) is safe and well tolerated and is associated with a reduction in endotoxaemia. The efficacy of a 7-day treatment with L. subtilis/Streptococcus faecium in alcoholic liver disease hints that endotoxaemia may be lowered as well (Hill et al. 2016; Wiest et al. 2017).
In experimental rats, oral administration of B. pseudocatenulatum or B. catenulatum had extensive beneficial effects on d-GalN-induced liver damage by reducing hepatic inflammation and necrosis and attenuating systemic inflammatory responses (Fang et al. 2017). Lv et al. (2014) reported that L. salivarius LI01 was beneficial in the prevention of acute liver failure.
Our study shows that in experimental rats with d-GalN-induced liver damage, oral administration of B. adolescentis decreases ALT and TBA levels, as well as hepatic inflammation and necrosis, and downregulates the expression of TNF-α, mTOR and IL-6; meanwhile, the expression of the anti-inflammatory mediator IL-10 was increased. Probiotic supplements not only attenuated systemic inflammatory responses but also stimulated expression of tight junction proteins and improved the intestinal flora. To the best of our knowledge, this study is the first to explore the effect and mechanism of B. adolescentis in a d-GalN-induced experimental rat model of liver damage.
d-GalN inhibited mRNA and protein synthesis (Keppler et al. 1974), biochemically evidenced by increases in ALT and the levels of MCP-1, VEGF and GRO/KC. MCP-1 recruit and activate monocytes/macrophages to the injured tissue area and regulate proinflammatory cytokines and adhesion molecules (Stanley et al. 1983). LPS induces macrophages secrete VEGF, which acts as a proinflammatory cytokine, and contributes to the development of SIRS in human subjects (Aspinall et al. 2011). The GRO/KC is thought to play a role in the rat similar to that played by IL-8 in humans (Dong et al. 2012). For ALF patients, infection is the major challenges and inflammatory state could be a target for future therapies (Mehta et al. 2014).
LPS of Gram-negative bacteria in the systemic circulation is captured and cleared by macrophages and hepatic Kupffer’s cells (Maitra et al. 1981), then results in the activation of the innate immune system and the secretion of high levels of proinflammatory cytokines (Galanos et al. 1979). LPS induces the secretion of acute-phase reactants and inflammatory cytokines, which is promoted by TGF-β in liver cells, in agreement with our network analysis (Quigley et al. 2013). LBP binds to bacterial LPS to elicit an immune response (Muta and Takeshige 2001). In our study, not only the inflammatory indexes ALT, IL-6 and TNF-α but also the anti-inflammatory indexes IL-7 and IL-10 were associated with LBP.
Tight junction proteins form a continuous seal around the apical aspect of adjoining epithelial cells in the apical-most strata to prevent the free passage of molecules between adjacent epithelial cells (paracellular pathway) (Sugrue and Zieske 1997). d-GalN impairs liver metabolism and increases the susceptibility of laboratory animals to LPS-induced shock (Lehmann et al. 1987). Circulating LBP, a marker of leaky-gut-associated endotoxaemia (Cani et al. 2008), significantly decreased after B. adolescentis intervention (P < 0.05). A high level of serum endotoxins is associated with intestinal barrier dysfunction (Yang et al. 2016). Probiotic interventions have been shown to effectively support intestinal barrier integrity in animals by tempering chronic inflammation and metabolic disorders (Cani and Delzenne 2009). Our finding of increased mRNA expression of MUC4 and the tight junction protein ZO-1 is in agreement with previous studies showing that Bifidobacterium plays a vital role in maintaining the intestinal mucosal barrier and diminishing BT in models of liver damage (Adawi et al. 2001; Madsen et al. 2001).
The reorganisation of ZO-1 in tight junctions is activated by Toll-like receptors (TLRs) (Cario et al. 2004). Intestinal epithelial cells from patients with inflammatory bowel diseases have similar or lower expression of TLR3 than those from control individuals (Abreu 2010). Here, our data showed that B. adolescentis stimulates the expression of TLR3, which was positively correlated with the barrier-function-related proteins ZO-1 and MUC4. Combined with the relevant literature, these data suggest that TLRs are important for increasing barrier function during injury (Abreu 2010).
In the clinic, systemic inflammatory response syndrome (SIRS) in ALF patients plays a particular role in their clinical course and outcome, resulting from massive hepatic cell necrosis and release of proinflammatory cytokines (Antoniades et al. 2008).
A network in our study was found in inflammatory cytokines (Supplementary Fig. S2) and relationships between factors were confirmed in other studies. Breakdown of intestinal barrier function allows the translocation of bacteria and bacterial products, including endotoxins (LPS)and bacterial DNA, to the liver (Hassoun et al. 2001) and MLNs, which were involved in intestinal failure-associated liver disease pathogenesis and activate hepatic macrophages via TLRs (Lee and Sokol 2015). TLR3 in our study was positively correlated with IL-10 both in plasma and liver. The pathway of gut microbiota-TLRs-IL-10 was demonstrated by Hackstein et al. (2017). IL-10, as a factor contributing to reduced antibacterial immunity during liver inflammation, reduced in blood and liver. Meanwhile, inflammatory cytokines IL-1β, IL-6 and IL-13 response strongly in our animal model during the acute liver injury progression.
LPS induces cytokines such as the proinflammatory cytokine TNF-α, triggering the hepatic migration of neutrophils and monocytes (Singh et al. 2011). TNF-α plays an irreplaceable role in the overwhelming systemic inflammatory intestinal barrier function and endotoxin response (Akashi-Takamura et al. 2006). TNF-α activated gut mucosal dysfunction in endotoxaemia (Woodhouse et al. 2018), such as functional disruption of epithelial tight junctions (Fink 2003). mRNA expression of mTOR was negatively correlated with that of TNF-α, which demonstrated TNF-α-mediated apoptotic signalling mechanisms (Jaeschke et al. 1998).
Our results are consistent with other studies in which probiotics showed anti-inflammatory effects on experimental d-GalN-induced liver failure (Fang et al. 2017; Lv et al. 2014; Yu et al. 2017). The oral treatment of mice with probiotics resulted in significantly enhanced basal levels of IL-10 RNA in comparison with the levels seen in wild-type control mice. Mice also demonstrated decreased IL-10 RNA in response to d-GalN injection. Liver function is indicated by ALT and TBA levels, which were reduced in the groups treated with B. adolescentis (P < 0.05 for both parameters), implying the alleviation of both liver inflammation and hepatocyte damage. In conclusion, the downregulation of inflammatory cytokine and circulating chemokine expression may be related to a decrease in endotoxin from the gut. Our findings indicate that B. adolescentis is a promising probiotic and may be useful as a preventive or therapeutic approach for combating acute liver failure.
Multiple pathogenic factors that accelerate liver injuries are associated with gastrointestinal microbiota dysbiosis. Pathological BT and increased endotoxin uptake result from gut dysbiosis, which disrupts the usual mechanisms of protection. Gut dysbiosis with over-representation of pathogenic bacteria can cause advanced liver disease (Woodhouse et al. 2018).
The alterations in the gut microbiota of the PC group included an increased abundance of pathogenic bacteria, e.g. Alcaligenaceae, Anaerostipes and Corynebacteriales, whereas beneficial bacteria, such as Prevotellaceae and Coprococcus_1, were decreased. It has been reported that the presence of Alcaligenaceae in the stool is associated with poor cognition in the overt (OHE) stages of hepatic encephalopathy (Bajaj 2014). Anaerostipes has been shown in patients with paratuberculosis infection (Arrazuria et al. 2016), and an increased relative abundance of Anaerotruncus species has been associated with ageing and age-associated inflammation with elevated proinflammatory chemokines in a mouse model (Zinkernagel et al. 2017). Consistent with our results, Anaerostipes has been found to be positively related to inflammatory IL-6, TNF-α, endotoxin receptors, LBP and MCP-1. MCP-1, an important member of the CC-chemokine family, recruits and activates monocytes/macrophages to the injured tissue area and regulates proinflammatory cytokines and adhesion molecules, which have been reported to play an important role in the early inflammatory response (Fang et al. 2017).
The family Prevotellaceae also tended to be more abundant in healthy subjects than in non-alcoholic fatty liver disease (NAFLD) patients (Shen et al. 2017). Prevotella is suggested to exert protective effects against the development of NAFLD and to be more abundant in healthy subjects than that in NAFLD patients (Kovatcheva-Datchary et al. 2015). In our study, Prevotellaceae was positively related to the anti-inflammatory cytokine IL-13. Although there was no difference in Bifidobacterium among groups, Bifidobacterium treatment interacted with Prevotella 9, Prevotellaceae_UCG_003, Prevotellaceae and Ruminococcaceae and Ruminiclostridium-6, all of which were enriched in the B. adolescentis group; additionally, the family Ruminococcaceae, a short-chain fatty acid (SCFA) producer, is negatively associated with injury-related factors (IL-12p40, IFN-γ, DR5) (Wu et al. 2017) and more abundant in healthy subjects than in non-alcoholic fatty liver disease (NAFLD) patients (Reddivari et al. 2017). SCFAs seem to calibrate the immune system and exert anti-inflammatory effects by activating regulatory T cells (Tregs) (Velasquez-Manoff 2015).
Specifically, B. adolescentis promoted intestinal health by alleviating microbial dysbiosis such as the depletion of Corynebacterium_1, Clostridium_sensu_stricto_1 and Aerococcaceae. Corynebacterium striatum may be associated with native valve endocarditis (Rufael and Cohn 1994). Dorea and Coriobacteriaceae_UCG_002 were enriched in the B. adolescentis group. Additionally, increased levels of Dorea (Del Chierico et al. 2017) were associated with good cognition and decreased inflammation in hepatic encephalopathy mice (Bajaj et al. 2012). Coriobacteriaceae was revealed to have strong associations with both hepatic triglyceride, glucose, and glycogen levels; a lean phenotype; and the metabolism of xenobiotics (Raza et al. 2017). In short, administration of probiotic B. adolescentis modifies the gut microbiota, enriching beneficial microbial taxa and inhibiting opportunistic pathogens, and this effect may help ameliorate liver failure.
Changes in microbial function, rather than abundance, could also account for the progression of liver damage. Predicted metabolic functions on Kyoto Encyclopedia of Genes and Genomes (KEGG) level 3 showed that the NC group had a high number of sequences assigned to cysteine and methionine metabolism, which is in agreement with Tomoko Okada’s study showing that hepatic cysteine concentration is significantly higher with d-GalN treatment than without; meanwhile, methionine concentration showed the opposite trend (Okada et al. 2011). Metabolic functions in the B. adolescentis group may focus on aminoacyl-tRNA, peptidoglycan biosynthesis and even DDT degradation. DDT, as an organochlorine pesticide, has been shown to have antiandrogenic activity (Yamada et al. 2004) and to display developmental toxicity in male rats (You et al. 1998).
For human nutrition, probiotics are defined as “live microbial food supplements or components of bacteria which benefit human health” (Sarao and Arora 2017). Genera such as Enterococcus, Streptococcus, Escherichia, Saccharomyces, Lactobacillus and Bifidobacterium have been marketed as probiotics. Lactic acid bacteria (LAB) of probiotics have several clinically proved health effects, consequently Lactobacillus and Bifidobacterium; these two main genera were used extensively as probiotics (Holzapfel et al. 2001). Following probiotic criteria that probiotics should be isolated from the same species as its intended host (Sarao and Arora 2017). B. adolescentis CGMCC 15058 was originally isolated from healthy volunteers and has potential for translation to humans. B. adolescentis alleviates liver injury and may be appropriate probiotic to the treatment on the human body.
Certain limitations existed in our study that there are still large gaps regarding the animal model and clinical disease; further studies are needed to explore the protective effect of B. adolescentis in human. Due to the sequence length, taxa at the species level were not investigated. Although significant biomarkers were identified, potential mechanisms may still exist and need to be fully explored.
In conclusion, our findings demonstrated that Bifidobacterium adolescentis CGMCC15058 alleviates d-GalN-induced liver injury in rats, which might be correlated with reduced cytotoxic factors and inflammatory cytokines along with enhanced apical tight junction. These protective effects may be driven by modified composition of the gut microbiota. Our findings provide new insights into the prevention and treatment of hepatic disease, suggesting Bifidobacterium adolescentis CGMCC15058 as a prospective probiotic against liver failure.
References
Abreu MT (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10:131–144. https://doi.org/10.1038/nri2707
Adawi D, Ahrne S, Molin G (2001) Effects of different probiotic strains of Lactobacillus and Bifidobacterium on bacterial translocation and liver injury in an acute liver injury model. Int J Food Microbiol 70:213–220
Akashi-Takamura S, Furuta T, Takahashi K, Tanimura N, Kusumoto Y, Kobayashi T, Saitoh S, Adachi Y, Doi T, Miyake K (2006) Agonistic antibody to TLR4/MD-2 protects mice from acute lethal hepatitis induced by TNF-alpha. J Immunol 176:4244–4251
Allman M, Gaskin L, Rivera CA (2010) CCl4-induced hepatic injury in mice fed a Western diet is associated with blunted healing. J Gastroenterol Hepatol 25:635–643. https://doi.org/10.1111/j.1440-1746.2009.06112.x
Antoniades CG, Berry PA, Wendon JA, Vergani D (2008) The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol 49:845–861. https://doi.org/10.1016/j.jhep.2008.08.009
Arboleya S, Watkins C, Stanton C, Ross RP (2016) Gut Bifidobacteria populations in human health and aging. Front Microbiol 7:1204. https://doi.org/10.3389/fmicb.2016.01204
Arrazuria R, Elguezabal N, Juste RA, Derakhshani H, Khafipour E (2016) Mycobacterium avium subspecies paratuberculosis infection modifies gut microbiota under different dietary conditions in a rabbit model. Front Microbiol 7:446. https://doi.org/10.3389/fmicb.2016.00446
Aspinall RJ, Weis SM, Barnes L, Lutu-Fuga K, Bylund DJ, Pockros PJ, Cheresh DA (2011) A Src family kinase inhibitor improves survival in experimental acute liver failure associated with elevated cerebral and circulating vascular endothelial growth factor levels. Liver Int 31:1222–1230. https://doi.org/10.1111/j.1478-3231.2011.02554.x
Bajaj JS (2014) The role of microbiota in hepatic encephalopathy. Gut Microbes 5:397–403. https://doi.org/10.4161/gmic.28684
Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM (2012) Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol 303:G675–G685. https://doi.org/10.1152/ajpgi.00152.2012
Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs M, Thacker LR, Wade JB, Daita K, Sistrun S, White MB, Noble NA, Thorpe C, Kakiyama G, Pandak WM, Sikaroodi M, Gillevet PM (2014) Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther 39:1113–1125. https://doi.org/10.1111/apt.12695
Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ (2006) Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol 72:3593–3599. https://doi.org/10.1128/AEM.72.5.3593-3599.2006
Bernal W, Lee WM, Wendon J, Larsen FS, Williams R (2015) Acute liver failure: a curable disease by 2024? J Hepatol 62:S112–S120. https://doi.org/10.1016/j.jhep.2014.12.016
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Brown AL Jr (1962) Microvilli of the human jejunal epithelial cell. J Cell Biol 12:623–627
Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Demchenko OA, Nechypurenko OV, Spivak MY (2017) Comparative study of probiotic effects of Lactobacillus and Bifidobacteria strains on cholesterol levels, liver morphology and the gut microbiota in obese mice. EPMA J 8:357–376. https://doi.org/10.1007/s13167-017-0117-3
Canbay A, Chen SY, Gieseler RK, Malago M, Karliova M, Gerken G, Broelsch CE, Treichel U (2005) Overweight patients are more susceptible for acute liver failure. Hepatogastroenterology 52:1516–1520
Cani PD, Delzenne NM (2009) The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 15:1546–1558
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481. https://doi.org/10.2337/db07-1403
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Cario E, Gerken G, Podolsky DK (2004) Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127:224–238
Chen J, Wang R, Li XF, Wang RL (2012) Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br J Nutr 107:1429–1434. https://doi.org/10.1017/S0007114511004491
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN (1970) Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 101:478–483
Cho SM, Lee SG, Kim HS, Kim JH (2014) Establishing pediatric reference intervals for 13 biochemical analytes derived from normal subjects in a pediatric endocrinology clinic in Korea. Clin Biochem 47:268–271. https://doi.org/10.1016/j.clinbiochem.2014.09.010
Davies NA, Banares R (2015) A new horizon for liver support in acute liver failure. J Hepatol 63:303–305. https://doi.org/10.1016/j.jhep.2015.05.020
Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis C, Gnani D, Furlanello C, Zandona A, Paci P, Capuani G, Dallapiccola B, Miccheli A, Alisi A, Putignani L (2017) Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 65:451–464. https://doi.org/10.1002/hep.28572
Dias G, Dallai R, Carapelli A, Almeida JP, Campos LA, Faroni LR, Lino-Neto J (2017) First record of gregarines (Apicomplexa) in seminal vesicle of insect. Sci Rep 7:175. https://doi.org/10.1038/s41598-017-00289-3
Dong F, Du YR, Xie W, Strong JA, He XJ, Zhang JM (2012) Increased function of the TRPV1 channel in small sensory neurons after local inflammation or in vitro exposure to the pro-inflammatory cytokine GRO/KC. Neurosci Bull 28:155–164. https://doi.org/10.1007/s12264-012-1208-8
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Fang H, Liu A, Dirsch O, Sun J, Jin H, Lu M, Yang D, Dahmen U (2012) Serum LBP levels reflect the impaired synthetic capacity of the remnant liver after partial hepatectomy in rats. J Immunol Methods 382:68–75. https://doi.org/10.1016/j.jim.2012.05.006
Fang D, Shi D, Lv L, Gu S, Wu W, Chen Y, Guo J, Li A, Hu X, Guo F, Ye J, Li Y, Li L (2017) Bifidobacterium pseudocatenulatum LI09 and Bifidobacterium catenulatum LI10 attenuate D-galactosamine-induced liver injury by modifying the gut microbiota. Sci Rep 7:8770. https://doi.org/10.1038/s41598-017-09395-8
Fink MP (2003) Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care 9:143–151
Frick JS, Fink K, Kahl F, Niemiec MJ, Quitadamo M, Schenk K, Autenrieth IB (2007) Identification of commensal bacterial strains that modulate Yersinia enterocolitica and dextran sodium sulfate-induced inflammatory responses: implications for the development of probiotics. Infect Immun 75:3490–3497. https://doi.org/10.1128/IAI.00119-07
Galanos C, Freudenberg MA, Reutter W (1979) Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A 76:5939–5943
Hackstein CP, Assmus LM, Welz M, Klein S, Schwandt T, Schultze J, Förster I, Gondorf F, Beyer M, Kroy D, Kurts C, Trebicka J, Kastenmüller W, Knolle PA, Abdullah Z (2017) Gut microbial translocation corrupts myeloid cell function to control bacterial infection during liver cirrhosis. Gut 66:507–518. https://doi.org/10.1136/gutjnl-2015-311224
Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA (2001) Post-injury multiple organ failure: the role of the gut. Shock 15:1–10
Heuvelin E, Lebreton C, Grangette C, Pot B, Cerf-Bensussan N, Heyman M (2009) Mechanisms involved in alleviation of intestinal inflammation by Bifidobacterium breve soluble factors. PLoS One 4:e5184. https://doi.org/10.1371/journal.pone.0005184
Hill C, Scott K, Klaenhammer TR, Quigley E, Sanders ME (2016) Probiotic nomenclature matters. Gut Microbes 7:1–2. https://doi.org/10.1080/19490976.2015.1127484
Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U (2001) Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr 73:365S–373S. https://doi.org/10.1093/ajcn/73.2.365s
Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA (1998) Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol 160:3480–3486
Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M (2014) Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42:D199–D205. https://doi.org/10.1093/nar/gkt1076
Keppler DO, Pausch J, Decker K (1974) Selective uridine triphosphate deficiency induced by D-galactosamine in liver and reversed by pyrimidine nucleotide precursors. Effect on ribonucleic acid synthesis. J Biol Chem 249:211–216
Khokhlova EV, Smeianov VV, Efimov BA, Kafarskaia LI, Pavlova SI, Shkoporov AN (2012) Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol 56:27–39. https://doi.org/10.1111/j.1348-0421.2011.00398.x
Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J (1981) Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431–435
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F (2015) Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 22:971–982. https://doi.org/10.1016/j.cmet.2015.10.001
Lang AL, Beier JI (2018) Interaction of volatile organic compounds and underlying liver disease: a new paradigm for risk. Biol Chem. https://doi.org/10.1515/hsz-2017-0324
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. https://doi.org/10.1038/nbt.2676
Lebrun LJ, Lenaerts K, Kiers D, Pais de Barros JP, Le Guern N, Plesnik J, Thomas C, Bourgeois T, Dejong CHC, Kox M, Hundscheid IHR, Khan NA, Mandard S, Deckert V, Pickkers P, Drucker DJ, Lagrost L, Grober J (2017) Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep 21:1160–1168. https://doi.org/10.1016/j.celrep.2017.10.008
Lee WS, Sokol RJ (2015) Intestinal microbiota, lipids, and the pathogenesis of intestinal failure-associated liver disease. J Pediatr 167:519–526. https://doi.org/10.1016/j.jpeds.2015.05.048
Lehmann V, Freudenberg MA, Galanos C (1987) Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med 165:657–663
Li LG, Cai L, Zhang XX, Zhang T (2014) Potentially novel copper resistance genes in copper-enriched activated sludge revealed by metagenomic analysis. Appl Microbiol Biotechnol 98:10255–10266. https://doi.org/10.1007/s00253-014-5939-5
Lu Y, Wang WJ, Song YZ, Liang ZQ (2014) The protective mechanism of schisandrin A in D-galactosamine-induced acute liver injury through activation of autophagy. Pharm Biol 52:1302–1307. https://doi.org/10.3109/13880209.2014.890232
Luo M, Yang XX, Tan B, Zhou XP, Xia HM, Xue J, Xu X, Qing Y, Li CR, Qiu JF, Li YL (2016) Distribution of common pathogens in patients with pyogenic liver abscess in China: a meta-analysis. Eur J Clin Microbiol Infect Dis 35:1557–1565. https://doi.org/10.1007/s10096-016-2712-y
Lv LX, Hu XJ, Qian GR, Zhang H, Lu HF, Zheng BW, Jiang L, Li LJ (2014) Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 improves acute liver injury induced by D-galactosamine in rats. Appl Microbiol Biotechnol 98:5619–5632. https://doi.org/10.1007/s00253-014-5638-2
Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C (2001) Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580–591
Maitra SK, Rachmilewitz D, Eberle D, Kaplowitz N (1981) The hepatocellular uptake and biliary excretion of endotoxin in the rat. Hepatology 1:401–407
Marquet P, Duncan SH, Chassard C, Bernalier-Donadille A, Flint HJ (2009) Lactate has the potential to promote hydrogen sulphide formation in the human colon. FEMS Microbiol Lett 299:128–134. https://doi.org/10.1111/j.1574-6968.2009.01750.x
Mehta G, Gustot T, Mookerjee RP, Garcia-Pagan JC, Fallon MB, Shah VH, Moreau R, Jalan R (2014) Inflammation and portal hypertension - the undiscovered country. J Hepatol 61:155–163. https://doi.org/10.1016/j.jhep.2014.03.014
Moratalla A, Caparros E, Juanola O, Portune K, Puig-Kroger A, Estrada-Capetillo L, Bellot P, Gomez-Hurtado I, Pinero P, Zapater P, Gonzalez-Navajas JM, Such J, Sanz Y, Frances R (2016a) Bifidobacterium pseudocatenulatum CECT7765 induces an M2 anti-inflammatory transition in macrophages from patients with cirrhosis. J Hepatol 64:135–145. https://doi.org/10.1016/j.jhep.2015.08.020
Moratalla A, Gomez-Hurtado I, Moya-Perez A, Zapater P, Peiro G, Gonzalez-Navajas JM, Gomez Del Pulgar EM, Such J, Sanz Y, Frances R (2016b) Bifidobacterium pseudocatenulatum CECT7765 promotes a TLR2-dependent anti-inflammatory response in intestinal lymphocytes from mice with cirrhosis. Eur J Nutr 55:197–206. https://doi.org/10.1007/s00394-015-0837-x
Muta T, Takeshige K (2001) Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem 268:4580–4589
Nier A, Engstler AJ, Maier IB, Bergheim I (2017) Markers of intestinal permeability are already altered in early stages of non-alcoholic fatty liver disease: studies in children. PLoS One 12:e0183282. https://doi.org/10.1371/journal.pone.0183282
Okada T, Kawakami S, Nakamura Y, Han KH, Ohba K, Aritsuka T, Uchino H, Shimada K, Sekikawa M, Ishii H, Fukushima M (2011) Amelioration of D-galactosamine-induced acute liver injury in rats by dietary supplementation with betaine derived from sugar beet molasses. Biosci Biotechnol Biochem 75:1335–1341. https://doi.org/10.1271/bbb.110105
Quigley EM, Stanton C, Murphy EF (2013) The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol 58:1020–1027. https://doi.org/10.1016/j.jhep.2012.11.023
Raza GS, Putaala H, Hibberd AA, Alhoniemi E, Tiihonen K, Makela KA, Herzig KH (2017) Polydextrose changes the gut microbiome and attenuates fasting triglyceride and cholesterol levels in Western diet fed mice. Sci Rep 7:5294. https://doi.org/10.1038/s41598-017-05259-3
Reddivari L, Veeramachaneni DNR, Walters WA, Lozupone C, Palmer J, Hewage MKK, Bhatnagar R, Amir A, Kennett MJ, Knight R, Vanamala JKP (2017) Perinatal bisphenol a exposure induces chronic inflammation in rabbit offspring via modulation of gut bacteria and their metabolites. mSystems 2. https://doi.org/10.1128/mSystems.00093-17
Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK (2012) FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol 30:460–465. https://doi.org/10.1038/nbt.2170
Rotstein OD (2014) Circulating cytokines in predicting development of severe acute pancreatitis. Crit Care 18:575. https://doi.org/10.1186/s13054-014-0575-0
Rufael DW, Cohn SE (1994) Native valve endocarditis due to Corynebacterium striatum: case report and review. Clin Infect Dis 19:1054–1061
Sarao LK, Arora M (2017) Probiotics, prebiotics, and microencapsulation: a review. Crit Rev Food Sci Nutr 57:344–371. https://doi.org/10.1080/10408398.2014.887055
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. https://doi.org/10.1186/gb-2011-12-6-r60
Shah NJ, John S (2018) Liver failure, acute on chronic. StatPearls Publishing, Treasure Island
Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG (2017) Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 16:375–381. https://doi.org/10.1016/S1499-3872(17)60019-5
Singh R, Bullard J, Kalra M, Assefa S, Kaul AK, Vonfeldt K, Strom SC, Conrad RS, Sharp HL, Kaul R (2011) Status of bacterial colonization, Toll-like receptor expression and nuclear factor-kappa B activation in normal and diseased human livers. Clin Immunol 138:41–49. https://doi.org/10.1016/j.clim.2010.09.006
Spruss A, Henkel J, Kanuri G, Blank D, Puschel GP, Bischoff SC, Bergheim I (2012) Female mice are more susceptible to nonalcoholic fatty liver disease: sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol Med 18:1346–1355. https://doi.org/10.2119/molmed.2012.00223
Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmez SH (1983) CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem 21:151–159. https://doi.org/10.1002/jcb.240210206
Sugrue SP, Zieske JD (1997) ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Exp Eye Res 64:11–20. https://doi.org/10.1006/exer.1996.0175
Tranah TH, Vijay GK, Ryan JM, Shawcross DL (2013) Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis 28:1–5. https://doi.org/10.1007/s11011-012-9370-2
Velasquez-Manoff M (2015) Gut microbiome: the peacekeepers. Nature 518:S3–S11. https://doi.org/10.1038/518S3a
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Wang Y, Gao LN, Cui YL, Jiang HL (2014) Protective effect of danhong injection on acute hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. Evid Based Complement Alternat Med 2014:153902–153908. https://doi.org/10.1155/2014/153902
Wiest R, Garcia-Tsao G (2005) Bacterial translocation (BT) in cirrhosis. Hepatology 41:422–433. https://doi.org/10.1002/hep.20632
Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R (2017) Targeting the gut-liver axis in liver disease. J Hepatol 67:1084–1103. https://doi.org/10.1016/j.jhep.2017.05.007
Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA (2012) Systematic review: acute liver failure - one disease, more than 40 definitions. Aliment Pharmacol Ther 35:1245–1256. https://doi.org/10.1111/j.1365-2036.2012.05097.x
Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL (2018) Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther 47:192–202. https://doi.org/10.1111/apt.14397
Wu J, Wang X, Cai W, Hong L, Tang Q (2010) Bifidobacterium adolescentis supplementation ameliorates parenteral nutrition-induced liver injury in infant rabbits. Dig Dis Sci 55:2814–2820. https://doi.org/10.1007/s10620-009-1101-0
Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L (2017) Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front Microbiol 8:1804. https://doi.org/10.3389/fmicb.2017.01804
Yamada T, Kunimatsu T, Miyata K, Yabushita S, Sukata T, Kawamura S, Seki T, Okuno Y, Mikami N (2004) Enhanced rat Hershberger assay appears reliable for detection of not only (anti-)androgenic chemicals but also thyroid hormone modulators. Toxicol Sci 79:64–74. https://doi.org/10.1093/toxsci/kfh093
Yang XF, He Y, Li HY, Liu X, Chen H, Liu JB, Ji WJ, Wang B, Chen LN (2014) Hepatoprotective effects of erythropoietin on D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Mol Med Rep 10:555–559. https://doi.org/10.3892/mmr.2014.2164
Yang PJ, Yang WS, Nien HC, Chen CN, Lee PH, Yu LC, Lin MT (2016) Duodenojejunal bypass leads to altered gut microbiota and strengthened epithelial barriers in rats. Obes Surg 26:1576–1583. https://doi.org/10.1007/s11695-015-1968-0
Ying B, Spencer JF, Tollefson AE, Wold WSM, Toth K (2018) Male Syrian hamsters are more susceptible to intravenous infection with species C human adenoviruses than are females. Virology 514:66–78. https://doi.org/10.1016/j.virol.2017.10.015
You L, Casanova M, Archibeque-Engle S, Sar M, Fan LQ, Heck HA (1998) Impaired male sexual development in perinatal Sprague-Dawley and Long-Evans hooded rats exposed in utero and lactationally to p,p'-DDE. Toxicol Sci 45:162–173
Yu L, Zhao XK, Cheng ML, Yang GZ, Wang B, Liu HJ, Hu YX, Zhu LL, Zhang S, Xiao ZW, Liu YM, Zhang BF, Mu M (2017) Saccharomyces boulardii administration changes gut microbiota and attenuates D-Galactosamine-induced liver injury. Sci Rep 7:1359. https://doi.org/10.1038/s41598-017-01271-9
Zhao LN, Yu T, Lan SY, Hou JT, Zhang ZZ, Wang SS, Liu FB (2015) Probiotics can improve the clinical outcomes of hepatic encephalopathy: an update meta-analysis. Clin Res Hepatol Gastroenterol 39:674–682. https://doi.org/10.1016/j.clinre.2015.03.008
Zinkernagel MS, Zysset-Burri DC, Keller I, Berger LE, Leichtle AB, Largiader CR, Fiedler GM, Wolf S (2017) Association of the intestinal microbiome with the development of neovascular age-related macular degeneration. Sci Rep 7:40826. https://doi.org/10.1038/srep40826
Funding
This study was supported by the National Science Foundation of China (NSFC) (81330011, 81790631 and 81570512) and the National Basic Research Program of China (973 program) (2013CB531401).
Author information
Authors and Affiliations
Contributions
Y.L. and L. Lv designed and conceived the experiments. Y.L., J. Ye., D.F. and D.S. performed the experiments and collected samples. L.Y. and X.B. performed DNA extractions. X.J. oversaw the RT-PCR and immunohistochemical staining. Y.L., L. Lv and J. Ye performed library construction and sequencing and designed the analysis. Q.W., P. CG and J. Wu analysed the data. D.S. drafted the manuscript. All authors contributed to and approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures were performed according to the 2011 National Institutes of Health Guide for the care and use of laboratory animals and were approved by the Animal Care and Use Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University.
Informed consent
All participants in this study provided a written informed consent before sample collection. The research is in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of the First Affiliated Hospital of Zhejiang University.
Electronic supplementary material
ESM 1
(PDF 400 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Lv, L., Ye, J. et al. Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in d-galactosamine-treated rats. Appl Microbiol Biotechnol 103, 375–393 (2019). https://doi.org/10.1007/s00253-018-9454-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9454-y