Abstract
Microorganisms can produce a number of different bioproducts from the sugars in plant biomass. One challenge is devising processes that utilize all of the sugars in lignocellulosic hydrolysates. D-xylose is the second most abundant sugar in these hydrolysates. The microbial conversion of D-xylose to ethanol has been studied extensively; only recently, however, has conversion to bioproducts other than ethanol been explored. Moreover, in the case of yeast, D-xylose may provide a better feedstock for the production of bioproducts other than ethanol, because the relevant pathways are not subject to glucose-dependent repression. In this review, we discuss how different microorganisms are being used to produce novel bioproducts from D-xylose. We also discuss how D-xylose could be potentially used instead of glucose for the production of value-added bioproducts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant biomass potentially provides a renewable source for the production of different fuels and chemicals. One promising route involves the use of native or engineered microorganisms, which can convert the sugars present in plant biomass into a wide range of value-added products (Isikgor and Becer 2015). Much of this work has focused on the conversion of glucose, the most abundant sugar in plant biomass. However, many other sugars are also present in plant biomass (Isikgor and Becer 2015; Jagtap et al. 2012, 2013; Rubin 2008; Tai et al. 2016). Key among them is D-xylose, a pentose (five-carbon) sugar, hereafter referred to simply as xylose. It represents 30–40% of the sugars recoverable from plant biomass (Dhiman et al. 2012, 2013; Hamacher et al. 2002; Himmel et al. 2007; Jagtap et al. 2014a, b; Jeffries 1983; Jeffries and Shi 1999; Rubin 2008). Not surprising, many different microorganisms have been explored for the conversion of xylose to different products (Jeffries 1983, 2006; Jeffries et al. 2007; Johnsen et al. 2009; Moysés et al. 2016; Nunn et al. 2010; Stephens et al. 2007). Much of this work has focused on the conversion of xylose to ethanol (Gong et al. 1981; Jeffries 2006; Millati et al. 2005; Mohd Azhar et al. 2017; Toivola et al. 1984; Zhang et al. 1995; Zhou et al. 2016). However, more recently, a number of studies have shown that microbes can convert xylose to other valuable fuels and chemicals—in some cases with better results than those obtained from glucose (Brat and Boles 2013; Feng et al. 2015; Guo et al. 2016b; Jordan et al. 2016; Kim et al. 2014; Kwak et al. 2017; Lee et al. 2015; Lian and Zhao 2015; Mohd Azhar et al. 2017; Moysés et al. 2016; Tippmann et al. 2013; Turner et al. 2015). Moreover, some of these chemicals, such as sugar alcohols, are natural by-products of xylose metabolism, indicating that xylose may provide the ideal source for their production (Jagtap and Rao 2018; Jeffries 2006; Jeppsson et al. 2006; Kwon et al. 2006; Lin et al. 2005; Moysés et al. 2016; Rafiqul and Sakinah 2013).
While xylose represents an abundant and renewable feedstock for many microbial conversion processes, its metabolism is not native to many production strains such as Saccharomyces cerevisiae and Zymomonas mobilis (Jeffries 2006; Toivari et al. 2004; van Zyl et al. 1989; Zhang et al. 1995). As a consequence, these microorganisms need to be genetically engineered to utilize xylose, a process that involves many genetic modifications in addition to expressing the heterologous enzymes. Even in microorganisms where xylose metabolism is present, it is often inefficient as compared to glucose metabolism and thus often requires further optimization.
In addition, developing efficient and economical processes for the conversion of plant biomass into different fuels and chemicals will require microorganisms capable of utilizing both glucose and xylose, the two principle sugars in plant biomass. However, xylose metabolism is often subject to glucose catabolite repression, where the cells will first consume glucose before they consume the xylose (Farwick et al. 2014; Gancedo 1998; Görke and Stülke 2008; Meijer et al. 1998). This phenomenon reduces the productivity of fermentation processes involving mixtures of glucose and xylose, because it lengthens the fermentation times. Ideally, microorganisms could be identified or engineered to simultaneously consume these two sugars (Kim et al. 2012).
Not surprisingly, the development of microorganisms capable of efficiently converting xylose to different fuels and chemicals has attracted significant attention over the years and still is a vibrant area of research (Jagtap and Rao 2018; Ledesma-Amaro et al. 2016; Liu et al. 2015; Park et al. 2017; Zhang et al. 2016a, b). Much of this effort, as noted above, has focused on engineering microorganisms capable of converting xylose into ethanol. A number of excellent reviews have already been written on this topic (Hahn-Hagerdal et al. 2007a; Jordan et al. 2012; Lin and Tanaka 2006; Matsushika et al. 2009; Mohd Azhar et al. 2017; Zaldivar et al. 2001). As a consequence, we limit our discussion to the conversion of xylose to products other than ethanol. Our review mostly focuses on yeast and fungi for brevity’s sake, aside from discussing the different pathways involved in xylose metabolism. Of course, bacteria also provide promising hosts for xylose conversion, particularly in case of the production host and native xylose-utilizer Escherichia coli. In addition, multiple strains of the bacterium Zymomonas mobilis—which are capable of producing ethanol at high rates, yields, and titers—have been engineered to efficiently utilize xylose (Agrawal et al. 2011; Dunn and Rao 2014, 2015; Jeon et al. 2005).
Xylose metabolism
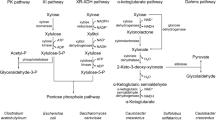
Microorganisms primarily catabolize xylose through three different pathways (Fig. 1). Bacteria most commonly employ the isomerase pathway for conversion of xylose to D-xylulose using xylose isomerase (XI). D-xylulose is then phosphorylated by xylulose kinase (XK), yielding D-xylulose-5-phosphate, which then enters central metabolism through the pentose phosphate pathway. The XI pathway is found in diverse prokaryotes (Jeffries 1983; Lajoie et al. 2016; Schellenberg et al. 1984; Umemoto et al. 2012). In addition, it is also found in a few anaerobic fungi such as Piromyces and Orpinomyces (Brat et al. 2009; Gárdonyi and Hahn-Hägerdal 2003; Harhangi et al. 2003; Madhavan et al. 2008; Nierman et al. 2001; Sarthy et al. 1987; Walfridsson et al. 1996).
Xylose metabolic pathways in microorganisms. XR, D-xylose reductase; XDH, xylitol dehydrogenase; XI, D-xylose isomerase; XK, xylulokinase; PK, phosphoketolase; PTA, phosphotransacetylase; ACK, acetate kinase; ACS, acetyl-CoA synthetase; XDH, D-xylose dehydrogenase; XL, xylonolactonase; XAD, xylonate dehydratase; KDXA, 2-oxo-3-deoxy xylonate aldolase; KDXD, 2-oxo-3-deoxy xylonate dehydratase; and αKGSADH, α-oxoglutaric semialdehyde dehydrogenase
Archaea, along with some bacteria such as Caulobacter crescentus, possess oxidative xylose metabolic pathways called the Weimberg and Dahms pathways (Johnsen et al. 2009; Nunn et al. 2010; Stephen Dahms 1974; Weimberg 1961). In the Weimberg pathway, xylose is oxidized to D-xylonolactone by D-xylose dehydrogenase, followed by a lactonase to hydrolyze the lactone to D-xylonate. Xylonate dehydratase then acts on D-xylonate to produce 2-keto-3-deoxy-D-xylonate and a second dehydratase forms the α-keto-semialdehyde. The α-keto-semialdehyde is subsequently oxidized to alpha-ketoglutarate, an intermediate of the tricarboxylic acid cycle (TCA) (Fig. 1) (Weimberg 1961). The Dahms pathway is similar to the Weimberg pathway except that the 2-keto 3-deoxy-D-xylonate is cleaved by an aldolase to form pyruvate and glycoaldehyde (Stephen Dahms 1974).
Xylose-utilizing yeasts and some filamentous fungi employ an oxido-reductive pathway consisting of two enzymatic reactions for xylose utilization (Fig. 1) (Jeffries 2006; Moysés et al. 2016). First, xylose is reduced to D-xylitol by xylose reductase (XR) using NADH or NADPH as a cofactor, depending on the enzyme. Some XRs solely utilize NADPH, whereas others can utilize both NADPH and NADH, typically with higher specificity for the former (Lee 1998; Schneider et al. 1989). The ability of some yeasts to utilize xylose anaerobically has been attributed to this dual cofactor specificity (Bruinenberg et al. 1984). Next, D-xylitol is oxidized to D-xylulose by the strictly NAD+-dependent xylitol dehydrogenase (XDH) (Kotter et al. 1990). D-xylulose is then phosphorylated to D-xylulose-5-phosphate by xylulokinase (XK) (Jin et al. 2002). Xylulose-5-phosphate is a metabolic intermediate of the nonoxidative pentose phosphate pathway (Stincone et al. 2015). Yeasts metabolize D-xylulose-5-phosphate to various phosphorylated sugars such as fructose-6-phosphate and glyceraldehyde-3-phosphate, which enters into glycolysis pathway (Jeffries 2006; Moysés et al. 2016). The difference in cofactor specificity in the first two steps of xylose metabolism can result in cofactor imbalance when oxygen or respiration is limiting (Dijken and Scheffers 1986). The redox imbalances during conversion of xylose into xylulose form a bottleneck in pentose fermentation for many yeasts.
Some oleaginous yeasts such as Rhodotorula graminis and Rhodotorula glutinis use the phosphoketolase (PK) pathway to convert xylulose-5-phosphate to glyceraldehyde-3-phosphate and acetyl phosphate (Whitworth and Ratledge 1977). Glyceraldehyde-3-phosphate further enters in glycolysis pathway. Acetate kinase (ACK) and acetyl-CoA synthase (ACS) convert acetyl phosphate into acetyl-CoA (Ingram-Smith et al. 2006).
Xylitol production
Xylitol is a five-carbon sugar alcohol used as a natural sweetener and sugar substitute. In addition, it has been shown to prevent tooth decay and, as a consequence, is often used as a sugar substitute for chewing gum (Saha et al. 2007). It is also one of the Department of Energy’s top 12 bio-based building block chemicals (Werpy et al. 2004). Xylitol can be produced chemically from xylose (Rafiqul and Sakinah 2012). It can also be produced using biological methods (Albuquerque et al. 2014; Jordan et al. 2012; Kwak and Jin 2017; Lane et al. 2018; Rafiqul and Sakinah 2013; Saha and Bothast 1997). A number of microorganisms naturally produce xylitol during growth on xylose (Table 1). In particular, many yeasts employ the XR-XDH pathway for xylose metabolism. Xylitol is a metabolic intermediate in this pathway, the product of the NADPH-dependent xylose reductase (XR) that catalyzes the conversion of xylose to xylitol. Xylitol is then oxidized to xylulose by the NAD+-dependent xylitol dehydrogenase (Jeffries 2006; Moysés et al. 2016). Xylitol production in these yeasts is known to result from a redox imbalance associated with the different cofactor specificities for these two enzymes. These imbalances tend to be magnified during growth under oxygen-limited conditions due to the inability of the cells to regenerate NAD. Many studies have focused on five main engineering strategies for improving xylitol production from xylose: overexpression of heterologous or native genes involved in xylitol production pathway, altering the cofactor specificity by deleting or overexpressing relevant genes, disruption of XDH gene, expressing heterologous xylose transporters, and optimizing culture conditions (Pal et al. 2016).
Fungi
Multiple filamentous fungi produce xylitol during aerobic growth on xylose (Table 1). In one study, the authors screened 11 different fungi for their ability to produce xylitol during growth on 11.5 g/L xylose (Sampaio et al. 2003). All were found to produce xylitol with titers ranging from 0.14 to 0.52 g/L. While these titers are relatively low, other fungi have been found to produce xylitol at far higher titers. For example, Petromyces albertensis produces 36.8 g/L xylitol from 100 g/L xylose (Dahiya 1991). The addition of methanol was found to further increase xylitol production to 39.8 g/L xylitol. The oxidation of methanol yields NADH, which is utilized for the reduction of xylose to xylitol by the NADH-dependent xylose reductase (Dahiya 1991).
In addition to native production, filamentous fungi have also been engineered to produce xylitol from xylose. For example, Ashbya gossypii was engineered to produce 22.6 g/L xylitol from 80 g/L xylose by overexpressing the native genes encoding for xylose reductase (GRE3), xylitol dehydrogenase (XYL2), and xylulose kinase (XKS1) (Díaz-Fernández et al. 2017). Trichomderma reesei has also been engineered for xylitol production by reducing the activity of XDH. In one study, RNA interference was used to reduce the expression of XDH in T. reesei, which increased xylitol production 5-fold and yielded titers of 2.37 g/L (Wang et al. 2005). In a second study, T. reesei was engineered to produce xylitol by deleting XDH and overexpressing XR, which increased xylitol production from 0 to 3.72 g/L (Hong et al. 2014).
Yeast
Multiple yeasts from diverse genera such as Candida, Clavispora, Hansenula, Issatchenkia, Kluyveromyces, and Pichia have been have shown to produce xylitol during aerobic growth on xylose in defined media (Barbosa et al. 1988; Gong et al. 1981; Kim et al. 2004; Oh and Kim 1998; Rao et al. 2007; Sampaio et al. 2008; Suryadi et al. 2000; Vandeska et al. 1995; Wang et al. 2005). Candida tropicalis and Candida guilliermondi exhibit some of the best yields, with reported values of 0.80 and 0.74 g/g, respectively (Table 1) (Barbosa et al. 1988; Gong et al. 1981). The highest xylitol productivity (12 g/L/h) from xylose using a submerged membrane bioreactor was reported in Candida tropicalis with glucose as co-substrate (Kwon et al. 2006).
In addition to native production, yeasts have also been engineered to produce xylitol. For example, the expression of heterologous XR, disruption of XDH, and overexpression of heterologous transporters in Candida tropicalis and Kluyveromyces marxianus produced higher xylitol as compared to wild-type strains (Jeon et al. 2012, 2013; Lee et al. 2003b; Zha et al. 2013; Zhang et al. 2013, 2014, 2015). A codon-optimized XR from Neurospora crassa was expressed in C. tropicalis LNG2 where the gene encoding xylitol dehydrogenase (XYL2) was disrupted (Jeon et al. 2012). The resulting strain produced 48 g/L of xylitol during fed-batch growth (Jeon et al. 2012). In another study, the NADH preferring XR from C. parapsilosis was expressed in C. tropicalis BN1. The resulting strain produced 280 g/L xylitol with productivity of 5.09 g/L/h during fed-batch growth (Lee et al. 2003b). In another study, the xylose transporter gene CoXT2 from Arabidopsis thaliana was integrated into the genome of a C. tropicalis strain where XYL2 was disrupted and the xylose reductase from N. crassa expressed. This strain produced xylitol with productivity of 1.14 g/L/h (Jeon et al. 2013). The three transporter genes KmFPS1 encoding aquaglyceroporin from Kluyveromyces marxianus, CiGXF1 encoding Candida intermedia glucose/xylose facilitator, and CiGXS1 encoding glucose/xylose symporter gene were expressed in K. marxianus YZJ074 (Zhang et al. 2014). This strain produced 71.5 g/L xylitol from 100 g/L xylose with productivity of 1.49 g/L/h at 42 °C (Zhang et al. 2014).
Native strains of S. cerevisiae cannot grow on xylose (Jeffries 1983). S. cerevisiae has been engineered for xylose utilization and transport, yielding strains capable of producing high levels of xylitol (Hahn-Hagerdal et al. 2007b; Jeppsson et al. 2006; Saloheimo et al. 2007). The δ-integration vector for chromosome integration and YRp-based episomal plasmid vector were used to introduce XR in S. cerevisae (Chung et al. 2002). Chromosomal integration showed better mitotic stability of the XR gene along with a high expression level in S. cerevisae and yielded 1.7-fold improvement in xylitol productivity in fed-batch culture (Chung et al. 2002). S. cerevisiae was also engineered to produce xylitol by overexpressing XR and XDH from Pichia stipitis and overexpressing the native transaldolase (TAL) and transketolase (TKL). This strain produced 2 g/L xylitol with a yield of 0.82 g/g xylose (Walfridsson et al. 1997). Expression of xylose reductase from P. stipitis in a S. cerevisiae strain produced 19.2 g/L xylitol (Zha et al. 2013). S. cerevisiae has also been engineered to produce xylitol by expressing CDT1, GH1-1, a β-glucosidase from N. crassa, and two copies of XYL1 from P. stipitis. This strain is able to consume both xylose and cellobiose to produce xylitol with a yield of 1 g/g xylose (Oh et al. 2013; Zha et al. 2013).
Bacteria
A few bacteria possess the XR-XDH pathway for producing of xylitol from xylose (Rangaswamy and Agblevor 2002; Yoshitake et al. 1973). For example, Enterobacter liquefaciens produced 28 g/L xylitol during growth on 100 g/L xylose as the sole carbon source (Yoshitake et al. 1973). Corynebacterium sp. B424 has been shown to produce 10 g/L xylitol, the highest among 17 screened cultures (Rangaswamy and Agblevor 2002).
Escherichia coli has also been engineered to produce xylitol from xylose (Häcker et al. 1999; Suzuki et al. 1999). Replacement of the native cyclic AMP receptor protein (CRP) with a cyclic AMP-independent mutant (CRP*) facilitated xylose utilization in mixtures of xylose and glucose, where glucose serves as source of cell growth and reducing equivalents (Cirino et al. 2006). In addition, E. coli expressing XR genes from Candida tenuis and C. tropicalis produced 13.3 g/L xylitol from 50 g/L xylose (Häcker et al. 1999; Suzuki et al. 1999).
A number of other bacteria have been engineered to produce xylitol from xylose (Kim et al. 2010; Nyyssölä et al. 2005; Sasaki et al. 2010; Suzuki et al. 1999; Yoshitake et al. 1973). Corynebacterium glutamicum was engineered to produce xylitol by integrating the pentose transporter gene (araE), disrupting the native lactate dehydrogenase gene (ldhA) and expressing a mutant XR (K274R) (Sasaki et al. 2010). The resulting strain produced 26.5 g/L xylitol. The genes encoding xylulokinase (XylB) and the phosphoenolpyruvate-dependent fructose phosphotransferase (PTSfru) were also expressed in this strain to eliminate intracellular xylitol phosphate formation. The resulting strain produced 166 g/L xylitol with productivity of 7.9 g/L/h from a mixture of xylose and glucose during fed-batch growth (Sasaki et al. 2010). Lactococcus was also engineered to produce 160 g/L xylitol with productivity of 1.75 g/L/h during fed-batch growth from a mixture of xylose and glucose by overexpressing the native xylose transporter and XYL1 from P. stipitis (Nyyssölä et al. 2005).
Arabitol production
D-arabitol is a five-carbon sugar alcohol, derived from xylose, that can be also used as a natural sweetener and sugar substitute (Werpy et al. 2004). It is also listed, along with xylitol, as one of the Department of Energy’s top 12 bio-based building block chemicals. Multiple yeasts are known to produce arabitol during growth on glucose (Bernard et al. 1981; Bisping et al. 1996; Blakley and Spencer 1962; Escalante et al. 1990; Hajny 1964; Moran and Witter 1979; Van Eck et al. 1993; Wilson and Mortlock 1973). Far fewer are reported to produce arabitol from xylose (Lin et al. 2005; Saha et al. 2007). However, arabitol is linked to xylose metabolism through arabitol dehydrogenase, which reduces xylulose to arabitol using NADH as the cofactor. In these regards, arabitol can potentially be produced as an overflow metabolite associated with redox imbalances during growth on xylose, where the production of arabitol by arabitol dehydrogenase regenerates NAD. In support of this mechanism, a recent study demonstrated that the oleaginous yeast Rhodosporidium toruloides produces 49 g/L arabitol during growth on 150 g/L xylose in rich media; however, during growth on low-nitrogen media, which is commonly used to induce lipid production in oleaginous yeast, no arabitol was produced (Table 2) (Jagtap and Rao 2018). These differences were associated with the rates of xylose utilization. R. toruloides consumes xylose at far greater rate in rich medium than in low-nitrogen medium. Likely, these high rates of xylose utilization induce a redox imbalance that results in the production of arabitol. This would also suggest that additional yeasts produce arabitol, assuming they have arabitol dehydrogenase.
Erythritol production
Erythritol is a four-carbon polyol produced by microorganisms as an osmoprotectant (Blomberg and Adler 1992; Carly and Fickers 2018; Spencer and Sallans 1956). Erythritol is a noncaloric sweetener, about 60–70% as sweet as sucrose with similar taste and texture (Carly and Fickers 2018). The human body cannot metabolize erythritol, despite being quickly absorbed by the small intestine, and is rapidly excreted in urine. Unlike other polyols, erythritol is not a laxative (Hiele et al. 1993; Livesey 2001). Erythritol is also noncariogenic and shown to prevent dental plaque on a long-term basis (Falony et al. 2016; Mäkinen et al. 2005).
Erythritol can be produced chemically from xylose (Nabors and Gelardi 2001). It can also be produced by bacteria, yeasts, and fungi during growth on xylose (Guo et al. 2016a; Jovanović et al. 2014; Veiga-da-Cunha et al. 1993). In bacteria, xylose is converted to xylulose-5-phosphate using the XI-XK pathway. Xylulose-5-phosphate enters into pentose phosphate pathway to generate fructose-6-phosphate. The phosphoketolase enzyme splits fructose-6-phosphate into erythrose-4-phosphate and acetyl phosphate. An erythrose-4-phosphatase converts erythrose-4-phosphate to erythrose, which is further converted into erythritol by erythrose reductase (ER) (Moon et al. 2010; Veiga-da-Cunha et al. 1993). Acetyl phosphate is either converted into acetate by ACK or into acetyl-CoA by phosphate acetyltransferase (PTA).
In yeasts, xylose is converted to xylulose-5-phosphate by an action of XR, XDH, and XK. Xylulose-5-phosphate enters pentose phosphate pathway to synthesize erythrose-4-phosphate. Erythrose-4-phosphatase dephosphorylates erythrose-4-phosphate into erythrose. An erythrose reductase converts erythrose into erythritol with NADPH as the cofactor (Mirończuk et al. 2017; Rzechonek et al. 2018). The genes encoding erythritol reductase have been identified in multiple yeast (Janek et al. 2017; Kobayashi et al. 2013; ; Lee et al. 2002, a).
Four different yeast strains were screened for erythritol production from xylose (Guo et al. 2016a). Aureobasidium pullulans CGMCC3.0837 showed the highest erythritol production (11.5 g/L) and the most efficient xylose consumption among the screened strains (Table 2) (Guo et al. 2016a). A. pullulans CGMCC3.0837 was subjected to UV mutagenesis, and erythritol production increased by 51% in the A. pullulans mutant ER35. Furthermore, response surface methods were used for medium composition optimization to increase erythritol synthesis by A. pullulans ER35. The erythritol production respectively increased 2.6-fold (29.6 g/L) and 2.7-fold (31.8 g/L) as compared to the parent strain during growth in a shake flask and a fermentor (Table 2).
Filamentous fungi produce very low concentrations of erythritol during aerobic growth on xylose (Jovanović et al. 2014). The err1 gene encoding erythrose reductase from Trichoderma reesei has been overexpressed in T. reesei QM6a and T. reesei Rut-C30 (Jovanović et al. 2014). The overexpression strains QPEC1 and RPEC1 produced 3.2-fold and 1.4-fold higher erythritol as compared to wild-type T. reesei QM6a and T. reesei Rut-C30, respectively. Erythritol production was less than 5 mg/L, which is very low as compared to bacteria and yeasts (Table 2).
2,3-Butanediol production
2,3-Butanediol (2,3-BDO) can be used to produce cosmetics, foods, fumigants, antifreeze agent, transport fuels, medicines, and polymers (Celińska and Grajek 2009). Wild-type S. cerevisiae has an endogenous 2,3-BDO biosynthetic pathway (Kim and Hahn 2015; Kim et al. 2017a). Pyruvate is first converted into α-acetolactate by α-acetolactate synthase (ALS) in the mitochondria. The α-acetolactate then spontaneously decaboxylates into diacetyl in the presence of oxygen. The diacetyl is reduced to 2,3-BDO via acetoin by 2,3-butanediol dehydrogenase (BDH) (Fig. 2) (Kim and Hahn 2015; Kim et al. 2013, 2017a, b). In comparison to the bacterial 2,3-BDO pathway, S. cerevisiae does not possess the α-acetolactate decarboxylase (ALDC), which is responsible for direct production of acetoin from α-acetolactate. Native acetoin production is very low in S. cerevisiae, and pyruvate is preferentially used for ethanol synthesis instead of 2,3-BDO. Therefore, S. cerevisiae produces a trace amount of 2,3-BDO.
Xylose pathway engineering for biofuel and chemical production. XR, D-xylose reductase; XDH, xylitol dehydrogenase; XI, D-xylose isomerase; ADH, D-arabitol dehydrogenase; XK, xylulokinase; RPE, ribulose-5-phosphate epimerase; PRK, phosphoribulokinase; RuBisCO, ribulose-1,5-bisphosphate carboxylase/oxygenase; PDC, pyruvate decarboxylase; ALD, alcohol dehydrogenase; ADH, aldehyde dehydrogenase; ACS, acetyl-CoA synthetase; LDH, lactate dehydrogenase; ALS, acetolactate synthase; ALDC, acetolactate decarboxylase; BDH, 2,3-butanediol dehydrogenase; KARI, ketoacid reductoisomerase; DHAD, dihydroxyacid dehydratase; KIVD, α-ketoisovalerate decarboxylase, Adh, alcohol dehydrogenase, ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; FAR, fatty acyl-CoA reductase FAR; ACAT, acetyl-CoA C-acetyltransferase; HMGCS, hydroxymethylglutaryl-CoA synthase; HMGR, hydroxymethylglutaryl-CoA reductase; MVK, mevalonate kinase; PMVK, phosphomevalonate kinase; MVD, diphosphomevalonate decarboxylase; IIPI, isopentenyl-diphosphate delta-isomerase; FDPS, farnesyl diphosphate synthase; SQS, squalene synthase; ADS, amorphadiene synthase
Pyruvate is also a precursor for ethanol synthesis; hence, it is important to redirect flux from ethanol to 2,3-BDO. The deletion of ADH and PDC genes eliminated ethanol production and increased 2,3-BDO production (Kim et al. 2017b). The ALS from B. subtilis has higher affinity for pyruvate than the endogenous ALS encoded by IIV2 in yeast. Acetolactate decarboxylase (ALSD) from B. subtilis catalyzes α-acetolactate to acetoin (Kim et al. 2013). The overexpression of endogenous BDH, ALS, and ALSD from B. subtilis has increased 2,3-BDO production from xylose in S. cerevisiae. The engineered S. cerevisiae BD4X strain produced 20.7 g/L 2,3-BDO with yield 0.27 g/g xylose. The BD4X strain produced 43.6 g/L 2,3-BDO during fed-batch fermentation (Kim et al. 2017b). In addition, overexpressing transaldolase (TAL), xylose reductase, and an NADH oxidase in a PDC-deficient yeast strain enhanced the yield of 2,3-BDO. The engineered BD5X-TXmNP strain produced 69.2 g/L 2,3-BD in batch fermentation and 96.8 g/L 2,3-BDO with productivity of 0.58 g/L/h in fed-batch fermentation (Table 3) (Kim et al. 2017b). Xylose appears to be a better substrate than glucose to produce 2,3-BDO, because the 2,3-BDO pathway is regulated by glucose repression. The deregulation of glucose-dependent repression redirects the metabolic flow from ethanol to 2,3-BD (Alff-Tuomala et al. 2016; Kwak and Jin 2017).
Isobutanol production
Isobutanol is a branched-chain alcohol that can be used to make transportation fuels, and its derivatives are used in tires, plastic bottles, carpets, and clothing (Felpeto-Santero et al. 2015). Isobutanol is naturally produced in low quantities by S. cerevisiae as a degradation product of valine (Chen et al. 2011).
Overexpression of the genes involved in valine metabolism has been used to increase the isobutanol production in S. cerevisiae (Chen et al. 2011). Mitochondrial valine biosynthesis enzymes such as ALS, keto-acid reductoisomerase (KARI), and dihydroxyacid dehydratase (DHAD) have been truncated and expressed in the cytosol to increase isobutanol production. The cytosolic isobutanol pathway was overexpressed in S. cerevisiae, which was previously engineered for xylose consumption. The resulting S. cerevisiae strain utilized 12 g/L xylose and produced 1.36 mg/L isobutanol with a yield of 0.16 mg/g xylose (Table 3) (Brat and Boles 2013). The consumption of nonfermentable sugars deregulates glucose-dependent repression on the development of mitochondria. The reverse localization of cytosolic pathways into mitochondria would be a better strategy to increase isobutanol production from xylose (DeRisi et al. 1997; Egner et al. 2002). Optimization of the xylose metabolic pathway and eliminating the competing pathways could enhance isobutanol production from xylose (Brat and Boles 2013).
Citric acid production
Citric acid is a widely used organic acid in the food industry (Berovic and Legisa 2007). For example, citric acid is used in carbonated beverages to provide taste and complement fruit flavors. The chelating and pH-adjusting properties of citric acid have been used to increase the stability of frozen food products by enhancing the activity of antioxidants. It is also used to inhibit color and flavor deterioration in frozen fruits. In addition, citric acid is used in oral pharmaceutical liquids and to maintain the activity of preservatives (Berovic and Legisa 2007).
The filamentous fungus Aspergillus niger has been used for decades to produce citric acid (Berovic and Legisa 2007). Traditionally, citric acid is produced from starch or sucrose. However, A. niger can also naturally produce citric acid from xylose. After 14 days of fermentation, A. niger produced 22 g/L citric acid from 71 g/L xylose with a yield of 0.31 g/g (Maddox et al. 1985). A. niger iMA871 produced 22 g/L citric acid from 160 g/L xylose within 8 days, and production was commenced upon a switch to phosphate-limited growth (Upton et al. 2017). The wild-type Aspergillus carbonarius produced citric acid at yield of 3 g/g dry cell mass and the engineered strain A. carbonarius SXR1, in which an NADH-dependent xylose reductase was expressed, produced citric acid at 13.8 g/g dry cell mass (Weyda et al. 2014).
The oleaginous yeast Yarrowia lipolytica naturally produces high concentrations of citric acid during growth on glucose. However, it does not grow well on xylose (Ryu et al. 2015). Y. lipolytica strain Po1g was engineered and adapted to grow on xylose by overexpressing XR and XDH from P. stipitis (Ledesma-Amaro et al. 2016; Ryu et al. 2015). This strain efficiently produced 80 g/L citric acid with a yield 0.53 g/g xylose (Ledesma-Amaro et al. 2016). Moreover, the production titer was similar to previously reported citric acid overproducing strains growing in glucose and glycerol (Lazar et al. 2011; Rywińska et al. 2010).
Lactic acid production
Lactate has a wide range of applications in the production of polylactic acid, biodegradable plastics, textile fibers, and additives in cosmetic and pharmaceutical industries (Yang et al. 2013). Lactic acid bacteria are generally used for lactate production (Papagianni 2012). The low pH during lactic acid fermentation, however, is inhibitory for bacteria, whereas S. cerevisiae is more tolerant to acidic pH.
L-lactate dehydrogenase (LDH) has been overexpressed in yeasts for the production of lactic acid from pyruvate (Dequin and Barre 1994; Porro et al. 1995). The LDH reaction is reversible, and LDH is allosterically inhibited by lactate (Papagianni 2012). Pyruvate is the branch metabolite between lactate and ethanol pathways. LDH has higher affinity for pyruvate and it competes with PDC for pyruvate. LDH could direct more metabolic flux to lactate from pyruvate than PDC because of weak glycolytic metabolism on xylose.
In ethanol-fermenting yeasts, pyruvate is converted into ethanol via acetaldehyde by PDC and alcohol dehydrogenase (ADH), respectively. Ethanol is still a major by-product produced with lactate in engineered S. cerevisiae. The deletion of aldehyde dehydrogenase (ALDH) and PDC reduced the metabolic flux toward ethanol and increased metabolic flux toward lactate (Baek et al. 2016).
The expression of LDH in xylose-fermenting S. cerevisiae produced lactic acid 60 g/L with a yield of 0.67 g/g xylose with low ethanol accumulation (0.01 g/g xylose) (Table 3) (Turner et al. 2015, 2017). The expression of lactate transporter gene JEN1 in S. cerevisiae has increased lactate transport outside the cell and led to increased lactate productivity (Branduardi et al. 2006).
LDH from Lactobacillus helveticus has been expressed in P. stipitis and the resulting strain produced 58 g/L lactate with a yield of 0.58 g/g xylose. This engineered strain cultured on xylose was better for lactate production than the best reported S. cerevisiae strains on glucose (Colombié et al. 2003; Ishida et al. 2005; Skory 2003).
Isoprenoid production
Isoprenoids are a class of natural products and main constituents of essential oils from plants and flowers (Chang and Keasling 2006). Extraction methods of isoprenoids from natural sources are inefficient and require a large quantity of natural resources (Nevoigt 2008). The isoprenoid squalene is linked to reduce serum cholesterol levels and is used in cosmetic, pharmaceutical, and food industries (Zhang et al. 2002). The isoprenoid amorphadiene is a plant derived isoprenoid and a precursor of antimalarial drug artemisinin (Chang and Keasling 2006). Metabolic engineering approaches have been used for the overproduction of the isoprenoids squalene and amorphadiene from xylose by S. cerevisiae. Xylose is preferred over glucose because of increased oxidative metabolism and reduced fermentative metabolism (Alff-Tuomala et al. 2016).
The pyruvate dehydrogenase bypass in S. cerevisiae has been engineered by overexpressing ALDH and ACS from Salmonella enterica to increase the supply of acetyl-CoA to the mevalonate pathway resulting in enhanced amorphadiene production (Shiba et al. 2007). The overexpression of truncated HMG-CoA reductase isozyme 1 (tHMG1) in an engineered S. cerevisiae capable of xylose utilization has also been an effective strategy for isoprenoid overproduction. TAL1, acetyl-CoA C-acetyltransferase (ACAT), and hydroxymethylglutaryl-CoA reductase (HMGR) were overexpressed using constitutive promoters in S. cerevisiae to increase xylose consumption and increase the metabolic fluxes from acetyl-CoA to mevalonate (MEV) pathway (Fig. 2). The co-expression of truncated ACAT and HMGR in S. cerevisiae has produced higher squalene from xylose than glucose, because of ethanol reassimilation with xylose utilization and metabolic flux transfer to cytosolic acetyl-CoA synthesis. Finally, 532 mg/L squalene was produced with a productivity of 11.3 mg/L/h from xylose in engineered S. cerevisiae (Table 3) (Kwak et al. 2017). Squalene synthase (SQS) downregulation and overexpression of ACAT, HMGR, and amorphadiene synthase (ADS) further increased amorphadiene titers. The HX4HEA-ES strain produced 254 mg/L amorphadiene under xylose culture conditions (Table 3) (Kwak et al. 2017).
Carotenoid production
Carotenoids are naturally occurring lipid soluble pigments displaying yellow, orange, and red color synthesized via the mevalonate pathway. Phytoene acts a precursor for the biosynthesis of specific carotenoids such as β-carotene, torulene, astaxanthin, and torularhodin in pigmented yeasts (Moliné et al. 2012). These pigments act as vitamin A precursors and have coloring and antioxidant properties. Rhodotorula spp. can synthesize carotenoids such as β-carotene, torulene, and torularhodin (Liu et al. 2018; Moliné et al. 2012).
The carotenogenic genes from Erwinia uredovora, Agrobacterium aurantiacum, and Xanthophyllomyces dendrorhus have been introduced into S. cerevisiae, Candida utilis, and Y. lipolytica for the production of carotenoids such as β-carotene, lycopene, or astaxanthin (Lange and Steinbuchel 2011; Matthaus et al. 2014; Misawa and Shimada 1997). The red yeast Phaffia rhodozyma NRRL Y-17268 has produced 5.2 mg/L astaxanthin from xylose-grown media (Parajo et al. 1997; Parajó et al. 1998).
Lipid production
Microbial lipid production has several advantages over plant oils, such as faster growth in defined media, lower land usage, better adaptability to market demands, and the ability to be produced from multiple substrates (Park et al. 2017). These lipids can be used to produce fuels, lubricants, and surfactants (Beopoulos et al. 2014; Blazeck et al. 2014; Matthaus et al. 2014; Ratledge 2004; Xue et al. 2013). Multiple microbes can accumulate large amounts of lipids, ranging from 20 to 70% of dry cell weight, during growth on many different sugars (Díaz et al. 2018; Huang et al. 2013; Kosa and Ragauskas 2011; Kurosawa et al. 2013; Shi and Zhao 2017; Yaguchi et al. 2018; Zhang et al. 2016a). Typically, these microbes accumulate lipid when cultured in excess sugar relative to other essential nutrients such nitrogen or phosphorous. As they cannot use the sugar for growth, they instead store it as lipid (Wang et al. 2018).
The theoretical yield of lipid production from xylose is 0.34 g/g, which is higher than glucose 0.32 g/g (Papanikolaou and Aggelis 2011). However, the practical yield of lipid is lower (Jin et al. 2015; Li et al. 2008; Weete 2012). Multiple strategies have been used to enhance lipid production such as overexpressing native or heterologous genes involved in sugar uptake and assimilation, overexpressing genes of fatty acid biosynthesis and TAG pathway, increasing availability of precursors for lipid synthesis, and deleting lipolysis pathway and β-oxidation pathway (Ferreira et al. 2018; Ledesma-Amaro et al. 2016; Li and Alper 2016; Niehus et al. 2018; Xu et al. 2016; Zhang et al. 2016a).
Bacteria
A few bacteria are able to produce high quantities of lipids during growth on xylose (Kurosawa et al. 2013; Xiong et al. 2012). For example, the actinomycetes genera of Rhodococcus, Streptomyces, and Gordonia accumulate lipids up to 20–30% of dry cell weight during growth on xylose (Castro et al. 2016; Waltermann and Steinbuchel 2005).
Bacteria such as Rhodococcus opacus PD630 and Rhodococcus jostii RHA1 can efficiently convert glucose into lipids but are unable to consume xylose naturally (Alvarez 2006; Hernández et al. 2008). XylA encoding xylose isomerase and xylB encoding xylulokinase from Streptomyces lividans TK23 were overexpressed into both R. opacus PD630 and R. jostii RHA1. Under nitrogen-limiting conditions, R. opacus PD630 XYLB and R. jostii RHA1 XYLB strains produced lipid up to 52.5 and 68.3% of dry cell weight, respectively. In another study, the xylose metabolic pathway genes from Streptomyces padanus MITKK-103 were expressed in R. opacus PD630 (Kurosawa et al. 2013). The resulting strain has produced 12 g/L lipid during growth on 120 g/L xylose.
Fungi
Oleaginous fungi can accumulate lipids up to 70% of dry cell weight (Chatzifragkou et al. 2010; Dey et al. 2011; Fakas et al. 2009; Mamatha et al. 2008; Ruan et al. 2012; Zheng et al. 2012). In one study, 11 filamentous fungi were investigated for lipid production during growth on xylose (Zheng et al. 2012) (Table 4). The lipid production ranged from 0.15 to 3.12 g/L, and lipid content ranged from 4 to 51% of dry cell weight (Gao et al. 2013; Zheng et al. 2012). The highest 50.9% lipid content was achieved by Mortierella isabellina during growth on xylose (Zheng et al. 2012). M. isabellina produced 18.5 g/L lipids with 64.3% lipid content during growth on 100 g/L xylose (Gao et al. 2013).
Yeast
Oleaginous yeasts such as R. toruloides, Lipomyces starkeyi, Cryptococcus curvatus, and Cutaneotrichosporon oleaginosus can accumulate intracellular lipids in the form of triacylglycerol (TAGs) as high as 40–60% of their cell dry weight from xylose under nitrogen-limited condition (Díaz et al. 2018; Shi and Zhao 2017; Yaguchi et al. 2018; Zhang et al. 2016b). R. toruloides IFO0880 produced 7.1 g/L lipid from xylose in low nitrogen medium (Zhang et al. 2016b). L. starkeyi AS 2.1560 and R. toruloides AS 2.138 produced 7.4 g/L and 5.3 g/L lipid, respectively, from pure xylose solution without any other nutrients in a two-stage fermentation process (Table 4) (Lin et al. 2014).
In R. toruloides strains, UV mutagenesis combined with a selection using cerulenin was used to increase lipid productivity (Yamada et al. 2017). A high-lipid-producing (1.86 g/L lipid) mutant of R. toruloides 87663-11C 2-53C was selected, and transcriptional levels of genes related to xylose utilization and lipid production were profiled (Yamada et al. 2017). The transcription levels of three (XYL1, XYL2, and XKS1) xylose metabolic genes in the mutant were the same as parental strain. Meanwhile, the transcription six genes (ACL1, FAS1, FAS2, GDH1, MAE1, and PYC1) related to lipid production in mutant strain were 4-fold upregulated (Yamada et al. 2017). In another study, overexpressing the native ACC1 and diacylglycerol acyltransferase (DGA1) enzymes involved in lipid production in R. toruloides IFO0880 increased lipid production from 7.1 to 9.5 g/L from xylose (Table 4) (Zhang et al. 2016a).
The overexpression of the native XDH, XR, and XK genes in Y. lipolytica allowed for growth on xylose as a sole carbon source for lipid production (Ledesma-Amaro et al. 2016; Li and Alper 2016; Niehus et al. 2018; Rodriguez et al. 2016). The resulting ylXYL+ strain produced 2.35 g/L lipid during growth on 60 g/L xylose (Table 4) (Niehus et al. 2018). The ylXYL+ strain was further engineered to obtain ylXYL-Obese-XA strain by overexpressing and deleting a number of genes involved in lipid production. The resulting ylXYL-Obese-XA strain produced 16.5 g/L lipid, 8.3 times higher than wild type in fed-batch fermentation. The maximum lipid content and productivity were 67% and 1.85 g/L/h, respectively.
Fatty alcohol production
Fatty alcohols are used in the production of cosmetics, detergents, lubricants, esters, and emulsifiers (Feng et al. 2015). Fatty alcohols can be extracted from natural plant oils or chemically synthesized from petrochemical sources. Both methods have limitations due to environmental concern or competition with food supply (Rupilius and Ahmad 2007). Microbial production of fatty alcohols from xylose may provide a better alternative than traditional methods.
As compared to glucose, xylose metabolism in engineered strains of S. cerevisiae appears to be more amendable for fatty alcohol production. The cytosolic acetyl-CoA is more efficiently produced under xylose compared to glucose (Jin et al. 2004; Salusjärvi et al. 2008). Many genes encoding the TCA cycle and respiratory enzymes in mitochondria are upregulated during growth on xylose and enhance ATP and citrate production. As a consequence, acetyl-CoA generation is enhanced by the action of ACS and ATP citrate lyase (CSL) (Jin et al. 2004; Salusjärvi et al. 2008). In addition, xylose culture conditions prevent the inhibition of fatty acid synthesis and deregulation of acyl-CoA because xylose does not induce SNF1, a protein that downregulates the expression of acetyl-CoA carboxylase (ACC1) and upregulates β-oxidation (Brink et al. 2016; Feng et al. 2015; Lee et al. 2015).
The overexpression of structural genes and deletion of negative regulators involved in downregulation of lipid synthesis in yeasts have been demonstrated to increase acetyl-CoA availability to improve 1-hexadecanol production. Two families of transcriptional factors, AMP-activated serine/threonine protein kinase (SNF1) and regulators of inositol-3-phosphate synthase (INO1), have been knocked-out to improve fatty acid synthesis in yeasts (Seip et al. 2013; Shi et al. 2014).
The overexpression of fatty acyl-CoA reductase (FAR), ACC1, ACL, and deletion of this histone deacetylase RPD3 improved 1-hexadecanol production from 45 mg/L to 1.1 g/L through fed-batch fermentation of glucose (Feng et al. 2015). Furthermore, when a xylose utilization pathway was introduced into the engineered S. cerevisiae strain, it produced 0.4 g/L 1-hexadecanol from xylose. Promoter engineering of xylose pathway increased 1-hexadecanol titer by 1.7-fold. The evolved strain produced over 1.2 g/L 1-hexadecanol using xylose during fed-batch fermentation (Table 3) (Guo et al. 2016b).
Conclusions
In this review, we discussed how different microorganisms are being used to produce diverse chemicals and fuels from xylose, an abundant sugar derived from plant biomass. Some of these products, such as sugar alcohols, are directly linked to xylose metabolism and, as such, represent ideal products derived from xylose. Others, such as lipid-based chemicals, are derived from core metabolic intermediates. The viability of making these products from xylose depends on the efficiency of xylose utilization, which is often less efficient than glucose utilization. In these regards, multiple opportunities exist for increasing the efficiency of xylose utilization such as the discovery and engineering of improved transporters and enzymes involved in xylose utilization. In addition, engineering microorganisms capable of simultaneously utilizing multiple sugars found in plant biomass would greatly increase the potential of plant biomass as a renewable feedstock for the production of diverse chemicals and fuels. While different strategies to address these challenges were not discussed in this review due to space limitations, they nonetheless represent a fertile area of active and future research.
We conclude by noting that xylose, despite limitations in utilization efficiency, may be a better feedstock than glucose, particularly in the case of yeast. Many pathways associated with nonfermentative metabolism are repressed during growth on glucose but not on xylose in engineered yeast. This means that it is often easier to redirect metabolic flux away from ethanol and toward other products during growth on xylose than it is on glucose (Alff-Tuomala et al. 2016; Kwak and Jin 2017). Thus, many nonethanol products are easier to produce from xylose than from glucose (Alff-Tuomala et al. 2016; Brat et al. 2009; Feng et al. 2015; Jin et al. 2004; Kim et al. 2017b; Kwak et al. 2017; Niehus et al. 2018; Salusjärvi et al. 2008; Shiba et al. 2007). In these regards, xylose may represent the ideal sugar for producing novel bioproducts from renewable biomass.
References
Agrawal M, Mao Z, Chen RR (2011) Adaptation yields a highly efficient xylose-fermenting Zymomonas mobilis strain. Biotechnol Bioeng 108(4):777–785. https://doi.org/10.1002/bit.23021
Albuquerque TL, da Silva IJ, de Macedo GR, Rocha MVP (2014) Biotechnological production of xylitol from lignocellulosic wastes: a review. Process Biochem 49(11):1779–1789. https://doi.org/10.1016/j.procbio.2014.07.010
Alff-Tuomala S, Salusjarvi L, Barth D, Oja M, Penttila M, Pitkanen JP, Ruohonen L, Jouhten P (2016) Xylose-induced dynamic effects on metabolism and gene expression in engineered Saccharomyes cerevisiae in anaerobic glucose-xylose cultures. Appl Microbiol Biotechnol 100(2):969–985. https://doi.org/10.1007/s00253-015-7038-7
Alvarez HM (2006) Bacterial triacylglycerols. In: Welson LT (ed) Triglycerides and cholesterol research, vol 6. Nova Science, New York, pp 159–176
Baek S-H, Kwon EY, Kim YH, Hahn J-S (2016) Metabolic engineering and adaptive evolution for efficient production of D-lactic acid in Saccharomyes cerevisiae. Appl Microbiol Biotechnol 100(6):2737–2748. https://doi.org/10.1007/s00253-015-7174-0
Barbosa MF, de Medeiros MB, de Mancilha IM, Schneider H, Lee H (1988) Screening of yeasts for production of xylitol fromd-xylose and some factors which affect xylitol yield in Candida guilliermondii. J Ind Microbiol 3(4):241–251
Beopoulos A, Verbeke J, Bordes F, Guicherd M, Bressy M, Marty A, Nicaud JM (2014) Metabolic engineering for ricinoleic acid production in the oleaginous yeast Yarrowia lipolytica. Appl Microbiol Biotechnol 98(1):251–262. https://doi.org/10.1007/s00253-013-5295-x
Bernard EM, Christiansen KJ, Tsang SF, Kiehn TE, Armstrong D (1981) Rate of arabinitol production by pathogenic yeast species. J Clin Microbiol 14(2):189–194
Berovic M, Legisa M (2007) Citric acid production. Biotechnol Annu Rev 13:303–343
Bisping B, Baumann U, Simmering R (1996) Effects of immobilization on polyol production by Pichia farinosa. Prog Biotechnol 11:395–401
Blakley ER, Spencer JF (1962) Studies on the formation of D-arabitol by osmophilic yeasts. Can J Biochem Physiol 40:1737–1748
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, Otoupal P, Alper HS (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5:3131. https://doi.org/10.1038/ncomms4131
Blomberg A, Adler L (1992) Physiology of osmotolerance in fungi. Adv Microb Physiol 33:145–212
Branduardi P, Sauer M, De Gioia L, Zampella G, Valli M, Mattanovich D, Porro D (2006) Lactate production yield from engineered yeasts is dependent from the host background, the lactate dehydrogenase source and the lactate export. Microb Cell Factories 5(1):4. https://doi.org/10.1186/1475-2859-5-4
Brat D, Boles E (2013) Isobutanol production from D-xylose by recombinant Saccharomyes cerevisiae. FEMS Yeast Res 13(2):241–244. https://doi.org/10.1111/1567-1364.12028
Brat D, Boles E, Wiedemann B (2009) Functional expression of a bacterial xylose isomerase in Saccharomyes cerevisiae. Appl Environ Microbiol 75(8):2304–2311. https://doi.org/10.1128/aem.02522-08
Brink DP, Borgström C, Tueros FG, Gorwa-Grauslund MF (2016) Real-time monitoring of the sugar sensing in Saccharomyes cerevisiae indicates endogenous mechanisms for xylose signaling. Microb Cell Factories 15(1):183. https://doi.org/10.1186/s12934-016-0580-x
Bruinenberg PM, de Bot PHM, van Dijken JP, Scheffers WA (1984) NADH-linked aldose reductase: the key to anaerobic alcoholic fermentation of xylose by yeasts. Appl Microbiol Biotechnol 19(4):256–260. https://doi.org/10.1007/bf00251847
Carly F, Fickers P (2018) Erythritol production by yeasts: a snapshot of current knowledge. Yeast 35(7):455–463
Castro AR, Rocha I, Alves MM, Pereira MA (2016) Rhodococcus opacus B4: a promising bacterium for production of biofuels and biobased chemicals. AMB Express 6(1):35. https://doi.org/10.1186/s13568-016-0207-y
Celińska E, Grajek W (2009) Biotechnological production of 2,3-butanediol—current state and prospects. Biotechnol Adv 27(6):715–725. https://doi.org/10.1016/j.biotechadv.2009.05.002
Chang MC, Keasling JD (2006) Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol 2(12):674–681. https://doi.org/10.1038/nchembio836
Chatzifragkou A, Fakas S, Galiotou-Panayotou M, Komaitis M, Aggelis G, Papanikolaou S (2010) Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. Eur J Lipid Sci Technol 112(9):1048–1057
Chen X, Nielsen KF, Borodina I, Kielland-Brandt MC, Karhumaa K (2011) Increased isobutanol production in Saccharomyes cerevisiae by overexpression of genes in valine metabolism. Biotechnol Biofuels 4:21. https://doi.org/10.1186/1754-6834-4-21
Chung Y-S, Kim M-D, Lee W-J, Ryu Y-W, Kim J-H, Seo J-H (2002) Stable expression of xylose reductase gene enhances xylitol production in recombinant Saccharomyes cerevisiae. Enzym Microb Technol 30(6):809–816
Cirino PC, Chin JW, Ingram LO (2006) Engineering Escherichia coli for xylitol production from glucose-xylose mixtures. Biotechnol Bioeng 95(6):1167–1176
Colombié S, Dequin S, Sablayrolles JM (2003) Control of lactate production by Saccharomyes cerevisiae expressing a bacterial LDH gene. Enzym Microb Technol 33(1):38–46. https://doi.org/10.1016/S0141-0229(03)00082-6
Dahiya JS (1991) Xylitol production by Petromyces albertensis grown on medium containing D-xylose. Can J Microbiol 37(1):14–18. https://doi.org/10.1139/m91-003
Dequin S, Barre P (1994) Mixed lactic acid–alcoholic fermentation by Saccharomyes cerevisiae expressing the lactobacillus casei L(+)–LDH. Nat Biotechnol 12:173–177. https://doi.org/10.1038/nbt0294-173
DeRisi JL, Iyer VR, Brown PO (1997) Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278(5338):680–686
Dey P, Banerjee J, Maiti MK (2011) Comparative lipid profiling of two endophytic fungal isolates—Colletotrichum sp. and Alternaria sp. having potential utilities as biodiesel feedstock. Bioresour Technol 102(10):5815–5823
Dhiman SS, Jagtap SS, Jeya M, Haw J-R, Kang YC, Lee J-K (2012) Immobilization of Pholiota adiposa xylanase onto SiO2 nanoparticles and its application for production of xylooligosaccharides. Biotechnol Lett 34(7):1307–1313. https://doi.org/10.1007/s10529-012-0902-y
Dhiman SS, Kalyani D, Jagtap SS, Haw J-R, Kang YC, Lee J-K (2013) Characterization of a novel xylanase from Armillaria gemina and its immobilization onto SiO2 nanoparticles. Appl Microbiol Biotechnol 97(3):1081–1091. https://doi.org/10.1007/s00253-012-4381-9
Díaz T, Fillet S, Campoy S, Vázquez R, Viña J, Murillo J, Adrio JL (2018) Combining evolutionary and metabolic engineering in Rhodosporidium toruloides for lipid production with non-detoxified wheat straw hydrolysates. Appl Microbiol Biotechnol 102(7):3287–3300. https://doi.org/10.1007/s00253-018-8810-2
Díaz-Fernández D, Lozano-Martínez P, Buey RM, Revuelta JL, Jiménez A (2017) Utilization of xylose by engineered strains of Ashbya gossypii for the production of microbial oils. Biotechnol Biofuels 10(1):3. https://doi.org/10.1186/s13068-016-0685-9
Dijken J, Scheffers A (1986) Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol Rev 1(3–4):199–224
Dunn KL, Rao CV (2014) Expression of a xylose-specific transporter improves ethanol production by metabolically engineered Zymomonas mobilis. Appl Microbiol Biotechnol 98(15):6897–6905. https://doi.org/10.1007/s00253-014-5812-6
Dunn KL, Rao CV (2015) High-throughput sequencing reveals adaptation-induced mutations in pentose-fermenting strains of Zymomonas mobilis. Biotechnol Bioeng 112(11):2228–2240. https://doi.org/10.1002/bit.25631
Egner A, Jakobs S, Hell SW (2002) Fast 100-nm resolution three-dimensional microscope reveals structural plasticity of mitochondria in live yeast. Proc Natl Acad Sci U S A 99(6):3370–3375. https://doi.org/10.1073/pnas.052545099
Escalante J, Caminal G, Figueredo M, de Mas C (1990) Production of arabitol from glucose by Hansenula polymorpha. J Ferment Bioeng 70(4):228–231. https://doi.org/10.1016/0922-338X(90)90053-Y
Fakas S, Papanikolaou S, Batsos A, Galiotou-Panayotou M, Mallouchos A, Aggelis G (2009) Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 33(4):573–580
Falony G, Honkala S, Runnel R, Olak J, Nõmmela R, Russak S, Saag M, Mäkinen P-L, Mäkinen K, Vahlberg T (2016) Long-term effect of erythritol on dental caries development during childhood: a posttreatment survival analysis. Caries Res 50(6):579–588
Farwick A, Bruder S, Schadeweg V, Oreb M, Boles E (2014) Engineering of yeast hexose transporters to transport d-xylose without inhibition by d-glucose. Proc Natl Acad Sci U S A 111(14):5159–5164. https://doi.org/10.1073/pnas.1323464111
Felpeto-Santero C, Rojas A, Tortajada M, Galán B, Ramón D, García JL (2015) Engineering alternative isobutanol production platforms. AMB Express 5:32. https://doi.org/10.1186/s13568-015-0119-2
Feng X, Lian J, Zhao H (2015) Metabolic engineering of Saccharomyes cerevisiae to improve 1-hexadecanol production. Metab Eng 27:10–19. https://doi.org/10.1016/j.ymben.2014.10.001
Ferreira R, Teixeira PG, Siewers V, Nielsen J (2018) Redirection of lipid flux toward phospholipids in yeast increases fatty acid turnover and secretion. Proc Natl Acad Sci U S A 115:1262–1267. https://doi.org/10.1073/pnas.1715282115
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62(2):334–361
Gao D, Zeng J, Zheng Y, Yu X, Chen S (2013) Microbial lipid production from xylose by Mortierella isabellina. Bioresour Technol 133:315–321. https://doi.org/10.1016/j.biortech.2013.01.132
Gárdonyi M, Hahn-Hägerdal B (2003) The Streptomyces rubiginosus xylose isomerase is misfolded when expressed in Saccharomyes cerevisiae. Enzym Microb Technol 32(2):252–259. https://doi.org/10.1016/S0141-0229(02)00285-5
Gong C-S, Chen LF, Tsao GT (1981) Quantitative production of xylitol from D-xylose by a high-xylitol producing yeast mutant Candida tropicalis HXP2. Biotechnol Lett 3(3):125–130. https://doi.org/10.1007/bf00127364
Görke B, Stülke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624. https://doi.org/10.1038/nrmicro1932
Guo J, Li J, Chen Y, Guo X, Xiao D (2016a) Improving erythritol production of Aureobasidium pullulans from xylose by mutagenesis and medium optimization. Appl Biochem Biotechnol 180(4):717–727. https://doi.org/10.1007/s12010-016-2127-3
Guo W, Sheng J, Zhao H, Feng X (2016b) Metabolic engineering of Saccharomyes cerevisiae to produce 1-hexadecanol from xylose. Microb Cell Factories 15:24. https://doi.org/10.1186/s12934-016-0423-9
Häcker B, Habenicht A, Kiess M, Mattes R (1999) Xylose utilisation: cloning and characterisation of the xylose reductase from Candida tenuis. Biol Chem 380(12):1395–1403
Hahn-Hagerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF (2007a) Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74(5):937–953. https://doi.org/10.1007/s00253-006-0827-2
Hahn-Hagerdal B, Karhumaa K, Jeppsson M, Gorwa-Grauslund MF (2007b) Metabolic engineering for pentose utilization in Saccharomyes cerevisiae. Adv Biochem Eng Biotechnol 108:147–177. https://doi.org/10.1007/10_2007_062
Hajny GJ (1964) D-arabitol production by Endomycopsis chodati. Appl Environ Microbiol 12(1):87–92
Hamacher T, Becker J, Gardonyi M, Hahn-Hägerdal B, Boles E (2002) Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiol 148(9):2783–2788
Harhangi HR, Akhmanova AS, Emmens R, van der Drift C, de Laat WTAM, van Dijken JP, Jetten MSM, Pronk JT, Op den Camp HJM (2003) Xylose metabolism in the anaerobic fungus Piromyces sp. strain E2 follows the bacterial pathway. Arch Microbiol 180(2):134–141. https://doi.org/10.1007/s00203-003-0565-0
Hernández MA, Mohn WW, Martínez E, Rost E, Alvarez AF, Alvarez HM (2008) Biosynthesis of storage compounds by Rhodococcus jostii RHA1 and global identification of genes involved in their metabolism. BMC Genomics 9(1):600. https://doi.org/10.1186/1471-2164-9-600
Hiele M, Ghoos Y, Rutgeerts P, Vantrappen G (1993) Metabolism of erythritol in humans: comparison with glucose and lactitol. Br J Nutr 69(1):169–176
Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315(5813):804–807. https://doi.org/10.1126/science.1137016
Hong Y, Dashtban M, Kepka G, Chen S, Qin W (2014) Overexpression of d-xylose reductase (xyl1) gene and antisense inhibition of d-xylulokinase (xyiH) gene increase xylitol production in Trichoderma reesei. Biomed Res Int 2014:8–8. https://doi.org/10.1155/2014/169705
Huang C, X-f C, Xiong L, Ma L-l, Chen Y (2013) Single cell oil production from low-cost substrates: the possibility and potential of its industrialization. Biotechnol Adv 31(2):129–139
Ingram-Smith C, Martin SR, Smith KS (2006) Acetate kinase: not just a bacterial enzyme. Trends Microbiol 14(6):249–253. https://doi.org/10.1016/j.tim.2006.04.001
Ishida N, Saitoh S, Tokuhiro K, Nagamori E, Matsuyama T, Kitamoto K, Takahashi H (2005) Efficient production of L-lactic acid by metabolically engineered Saccharomyes cerevisiae with a genome-integrated L-lactate dehydrogenase gene. Appl Environ Microbiol 71(4):1964–1970. https://doi.org/10.1128/aem.71.4.1964-1970.2005
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6(25):4497–4559. https://doi.org/10.1039/C5PY00263J
Jagtap SS, Rao CV (2018) Production of D-arabitol from D-xylose by the oleaginous yeast Rhodosporidium toruloides IFO0880. Appl Microbiol Biotechnol 102(1):143–151. https://doi.org/10.1007/s00253-017-8581-1
Jagtap SS, Dhiman SS, Jeya M, Kang YC, Choi J-H, Lee J-K (2012) Saccharification of poplar biomass by using lignocellulases from Pholiota adiposa. Bioresour Technol 120:264–272. https://doi.org/10.1016/j.biortech.2012.06.002
Jagtap SS, Dhiman SS, Kim T-S, Li J, Lee J-K, Kang YC (2013) Enzymatic hydrolysis of aspen biomass into fermentable sugars by using lignocellulases from Armillaria gemina. Bioresour Technol 133:307–314. https://doi.org/10.1016/j.biortech.2013.01.118
Jagtap SS, Dhiman SS, Kim T-S, Kim I-W, Lee J-K (2014a) Characterization of a novel endo-β-1,4-glucanase from Armillaria gemina and its application in biomass hydrolysis. Appl Microbiol Biotechnol 98(2):661–669. https://doi.org/10.1007/s00253-013-4894-x
Jagtap SS, Woo SM, Kim T-S, Dhiman SS, Kim D, Lee J-K (2014b) Phytoremediation of diesel-contaminated soil and saccharification of the resulting biomass. Fuel 116:292–298. https://doi.org/10.1016/j.fuel.2013.08.017
Janek T, Dobrowolski A, Biegalska A, Mirończuk AM (2017) Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis. Microb Cell Factories 16(1):118
Jeffries TW (1983) Utilization of xylose by bacteria, yeasts, and fungi. In: Chan YK, Fiechter A, Gong C-S, Jansen NB, Janshekar H, Jeffries TW, Kurtzman CP, Maleszka R, McCracken LD, Neirinck L, Schneider H, Szczesny T, Tsao GT, Veliky IA, Volesky B, Wang PY (eds) Pentoses and lignin. Springer, Berlin, pp 1–32
Jeffries TW (2006) Engineering yeasts for xylose metabolism. Curr Opin Biotechnol 17(3):320–326. https://doi.org/10.1016/j.copbio.2006.05.008
Jeffries TW, Shi N-Q (1999) Genetic engineering for improved xylose fermentation by yeasts. In: Tsao GT, Brainard AP, Bungay HR, Cao NJ, Cen P, Chen Z, Du J, Foody B, Gong CS, Hall P, Ho NWY, Irwin DC, Iyer P, Jeffries TW, Ladisch CM, Ladisch MR, Lee YY, Mosier NS, Mühlemann HM, Sedlak M, Shi NQ, Tsao GT, Tolan JS, Torget RW, Wilson DB, Xia L (eds) Recent progress in bioconversion of lignocellulosics. Springer, Berlin, pp 117–161
Jeffries TW, Grigoriev IV, Grimwood J, Laplaza JM, Aerts A, Salamov A, Schmutz J, Lindquist E, Dehal P, Shapiro H, Jin YS, Passoth V, Richardson PM (2007) Genome sequence of the lignocellulose-bioconverting and xylose-fermenting yeast Pichia stipitis. Nat Biotechnol 25(3):319–326. https://doi.org/10.1038/nbt1290
Jeon YJ, Svenson CJ, Rogers PL (2005) Over-expression of xylulokinase in a xylose-metabolising recombinant strain of Zymomonas mobilis. FEMS Microbiol Lett 244(1):85–92. https://doi.org/10.1016/j.femsle.2005.01.025
Jeon WY, Yoon BH, Ko BS, Shim WY, Kim JH (2012) Xylitol production is increased by expression of codon-optimized Neurospora crassa xylose reductase gene in Candida tropicalis. Bioprocess Biosyst Eng 35(1–2):191–198
Jeon WY, Shim WY, Lee SH, Choi JH, Kim JH (2013) Effect of heterologous xylose transporter expression in Candida tropicalis on xylitol production rate. Bioprocess Biosyst Eng 36(6):809–817
Jeppsson M, Bengtsson O, Franke K, Lee H, Hahn-Hägerdal B, Gorwa-Grauslund MF (2006) The expression of a Pichia stipitis xylose reductase mutant with higher KM for NADPH increases ethanol production from xylose in recombinant Saccharomyes cerevisiae. Biotechnol Bioeng 93(4):665–673. https://doi.org/10.1002/bit.20737
Jin YS, Jones S, Shi NQ, Jeffries TW (2002) Molecular cloning of XYL3 (D-xylulokinase) from Pichia stipitis and characterization of its physiological function. Appl Environ Microbiol 68(3):1232–1239
Jin YS, Laplaza JM, Jeffries TW (2004) Saccharomyes cerevisiae engineered for xylose metabolism exhibits a respiratory response. Appl Environ Microbiol 70(11):6816–6825. https://doi.org/10.1128/aem.70.11.6816-6825.2004
Jin M, Slininger PJ, Dien BS, Waghmode S, Moser BR, Orjuela A, Sousa Lda C, Balan V (2015) Microbial lipid-based lignocellulosic biorefinery: feasibility and challenges. Trends Biotechnol 33(1):43–54. https://doi.org/10.1016/j.tibtech.2014.11.005
Johnsen U, Dambeck M, Zaiss H, Fuhrer T, Soppa J, Sauer U, Schonheit P (2009) D-xylose degradation pathway in the halophilic archaeon Haloferax volcanii. J Biol Chem 284(40):27290–27303. https://doi.org/10.1074/jbc.M109.003814
Jordan DB, Bowman MJ, Braker JD, Dien BS, Hector RE, Lee CC, Mertens JA, Wagschal K (2012) Plant cell walls to ethanol. Biochem J 442(2):241–252. https://doi.org/10.1042/bj20111922
Jordan P, Choe JY, Boles E, Oreb M (2016) Hxt13, Hxt15, Hxt16 and Hxt17 from Saccharomyes cerevisiae represent a novel type of polyol transporters. Sci Rep 6:23502. https://doi.org/10.1038/srep23502
Jovanović B, Mach RL, Mach-Aigner AR (2014) Erythritol production on wheat straw using Trichoderma reesei. AMB Express 4:34–34. https://doi.org/10.1186/s13568-014-0034-y
Kim S, Hahn J-S (2015) Efficient production of 2,3-butanediol in Saccharomyes cerevisiae by eliminating ethanol and glycerol production and redox rebalancing. Metab Eng 31:94–101. https://doi.org/10.1016/j.ymben.2015.07.006
Kim T-B, Lee Y-J, Kim P, Kim CS, Oh D-K (2004) Increased xylitol production rate during long-term cell recycle fermentation of Candida tropicalis. Biotechnol Lett 26(8):623–627
Kim S-H, Yun J-Y, Kim S-G, Seo J-H, Park J-B (2010) Production of xylitol from D-xylose and glucose with recombinant Corynebacterium glutamicum. Enzym Microb Technol 46(5):366–371
Kim SR, Ha S-J, Wei N, Oh EJ, Jin Y-S (2012) Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol. Trends Biotechnol 30(5):274–282. https://doi.org/10.1016/j.tibtech.2012.01.005
Kim S-J, Seo S-O, Jin Y-S, Seo J-H (2013) Production of 2,3-butanediol by engineered Saccharomyes cerevisiae. Bioresour Technol 146:274–281. https://doi.org/10.1016/j.biortech.2013.07.081
Kim SJ, Seo SO, Park YC, Jin YS, Seo JH (2014) Production of 2,3-butanediol from xylose by engineered Saccharomyes cerevisiae. J Biotechnol 192(Pt B):376–382. https://doi.org/10.1016/j.jbiotec.2013.12.017
Kim S-J, Kim J-W, Lee Y-G, Park Y-C, Seo J-H (2017a) Metabolic engineering of Saccharomyes cerevisiae for 2,3-butanediol production. Appl Microbiol Biotechnol 101(6):2241–2250. https://doi.org/10.1007/s00253-017-8172-1
Kim S-J, Sim H-J, Kim J-W, Lee Y-G, Park Y-C, Seo J-H (2017b) Enhanced production of 2,3-butanediol from xylose by combinatorial engineering of xylose metabolic pathway and cofactor regeneration in pyruvate decarboxylase-deficient Saccharomyes cerevisiae. Bioresour Technol 245(Part B):1551–1557. https://doi.org/10.1016/j.biortech.2017.06.034
Kobayashi Y, Yoshida J, Iwata H, Koyama Y, Kato J, Ogihara J, Kasumi T (2013) Gene expression and function involved in polyol biosynthesis of Trichosporonoides megachiliensis under hyper-osmotic stress. J Biosci Bioeng 115(6):645–650
Kosa M, Ragauskas AJ (2011) Lipids from heterotrophic microbes: advances in metabolism research. Trends Biotechnol 29(2):53–61
Kotter P, Amore R, Hollenberg CP, Ciriacy M (1990) Isolation and characterization of the Pichia stipitis xylitol dehydrogenase gene, XYL2, and construction of a xylose-utilizing Saccharomyes cerevisiae transformant. Curr Genet 18(6):493–500
Kurosawa K, Wewetzer SJ, Sinskey AJ (2013) Engineering xylose metabolism in triacylglycerol-producing Rhodococcus opacus for lignocellulosic fuel production. Biotechnol Biofuels 6(1):134. https://doi.org/10.1186/1754-6834-6-134
Kwak S, Jin YS (2017) Production of fuels and chemicals from xylose by engineered Saccharomyes cerevisiae: a review and perspective. Microb Cell Factories 16(1):82. https://doi.org/10.1186/s12934-017-0694-9
Kwak S, Kim SR, Xu H, Zhang GC, Lane S, Kim H, Jin YS (2017) Enhanced isoprenoid production from xylose by engineered Saccharomyes cerevisiae. Biotechnol Bioeng 114(11):2581–2591. https://doi.org/10.1002/bit.26369
Kwon SG, Park SW, Oh DK (2006) Increase of xylitol productivity by cell-recycle fermentation of Candida tropicalis using submerged membrane bioreactor. J Biosci Bioeng 101(1):13–18. https://doi.org/10.1263/jbb.101.13
Lajoie CA, Kitner JB, Potochnik SJ, Townsend JM, Beatty CC, Kelly CJ (2016) Cloning, expression and characterization of xylose isomerase from the marine bacterium Fulvimarina pelagi in Escherichia coli. Biotechnol Prog 32(5):1230–1237. https://doi.org/10.1002/btpr.2309
Lane S, Dong J, Jin Y-S (2018) Value-added biotransformation of cellulosic sugars by engineered Saccharomyes cerevisiae. Bioresour Technol 260:380–394. https://doi.org/10.1016/j.biortech.2018.04.013
Lange N, Steinbuchel A (2011) Beta-carotene production by Saccharomyes cerevisiae with regard to plasmid stability and culture media. Appl Microbiol Biotechnol 91(6):1611–1622. https://doi.org/10.1007/s00253-011-3315-2
Lazar Z, Walczak E, Robak M (2011) Simultaneous production of citric acid and invertase by Yarrowia lipolytica SUC+ transformants. Bioresour Technol 102(13):6982–6989. https://doi.org/10.1016/j.biortech.2011.04.032
Lazar Z, Dulermo T, Neuvéglise C, Crutz-Le Coq A-M, Nicaud J-M (2014) Hexokinase—a limiting factor in lipid production from fructose in Yarrowia lipolytica. Metab Eng 26:89–99. https://doi.org/10.1016/j.ymben.2014.09.008
Ledesma-Amaro R, Lazar Z, Rakicka M, Guo Z, Fouchard F, Coq A-MC-L, Nicaud J-M (2016) Metabolic engineering of Yarrowia lipolytica to produce chemicals and fuels from xylose. Metab Eng 38:115–124. https://doi.org/10.1016/j.ymben.2016.07.001
Lee H (1998) The structure and function of yeast xylose (aldose) reductases. Yeast 14(11):977–984. https://doi.org/10.1002/(sici)1097-0061(199808)14:11<977::aid-yea302>3.0.co;2-j
Lee J-K, Koo B-S, Kim S-Y (2002) Fumarate-mediated inhibition of erythrose reductase, a key enzyme for erythritol production by Torula corallina. Appl Environ Microbiol 68(9):4534–4538
Lee J-K, Kim S-Y, Ryu Y-W, Seo J-H, Kim J-H (2003a) Purification and characterization of a novel erythrose reductase from Candida magnoliae. Appl Environ Microbiol 69(7):3710–3718
Lee J-K, Koo B-S, Kim S-Y (2003b) Cloning and characterization of the xyl1 gene, encoding an NADH-preferring xylose reductase from Candida parapsilosis, and its functional expression in Candida tropicalis. Appl Environ Microbiol 69(10):6179–6188
Lee JY, Kang CD, Lee SH, Park YK, Cho KM (2015) Engineering cellular redox balance in Saccharomyes cerevisiae for improved production of L-lactic acid. Biotechnol Bioeng 112(4):751–758. https://doi.org/10.1002/bit.25488
Li H, Alper HS (2016) Enabling xylose utilization in Yarrowia lipolytica for lipid production. Biotechnol J 11(9):1230–1240. https://doi.org/10.1002/biot.201600210
Li Q, Du W, Liu D (2008) Perspectives of microbial oils for biodiesel production. Appl Microbiol Biotechnol 80(5):749–756
Lian J, Zhao H (2015) Recent advances in biosynthesis of fatty acids derived products in Saccharomyes cerevisiae via enhanced supply of precursor metabolites. J Ind Microbiol Biotechnol 42(3):437–451. https://doi.org/10.1007/s10295-014-1518-0
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69(6):627–642. https://doi.org/10.1007/s00253-005-0229-x
Lin C-C, Hsieh P-C, Mau J-L, Teng D-F (2005) Construction of an intergeneric fusion from Schizosaccharomyces pombe and Lentinula edodes for xylan degradation and polyol production. Enzym Microb Technol 36(1):107–117. https://doi.org/10.1016/j.enzmictec.2004.07.007
Lin J, Li S, Sun M, Zhang C, Yang W, Zhang Z, Li X, Li S (2014) Microbial lipid production by oleaginous yeast in d-xylose solution using a two-stage culture mode. RSC Adv 4(66):34944–34949. https://doi.org/10.1039/C4RA01453G
Liu HH, Ji XJ, Huang H (2015) Biotechnological applications of Yarrowia lipolytica: past, present and future. Biotechnol Adv 33(8):1522–1546. https://doi.org/10.1016/j.biotechadv.2015.07.010
Liu Y, Koh CMJ, Yap SA, Du M, Hlaing MM, Ji L (2018) Identification of novel genes in the carotenogenic and oleaginous yeast Rhodotorula toruloides through genome-wide insertional mutagenesis. BMC Microbiol 18(1):14. https://doi.org/10.1186/s12866-018-1151-6
Livesey G (2001) Tolerance of low-digestible carbohydrates: a general view. Br J Nutr 85(S1):S7–S16
Maddox IS, Spencer K, Greenwood JM, Dawson MW, Brooks JD (1985) Production of citric acid from sugars present in wood hemicellulose using Aspergillus niger and Saccharomycopsis lipolytica. Biotechnol Lett 7(11):815–818. https://doi.org/10.1007/bf01025561
Madhavan A, Tamalampudi S, Ushida K, Kanai D, Katahira S, Srivastava A, Fukuda H, Bisaria VS, Kondo A (2008) Xylose isomerase from polycentric fungus Orpinomyces: gene sequencing, cloning, and expression in Saccharomyes cerevisiae for bioconversion of xylose to ethanol. Appl Microbiol Biotechnol 82(6):1067–1078. https://doi.org/10.1007/s00253-008-1794-6
Mäkinen K, Saag M, Isotupa K, Olak J, Nõmmela R, Söderling E, Mäkinen P-L (2005) Similarity of the effects of erythritol and xylitol on some risk factors of dental caries. Caries Res 39(3):207–215
Mamatha S, Ravi R, Venkateswaran G (2008) Medium optimization of gamma linolenic acid production in Mucor rouxii CFR-G15 using RSM. Food Bioprocess Technol 1(4):405–409
Matsushika A, Inoue H, Kodaki T, Sawayama S (2009) Ethanol production from xylose in engineered Saccharomyes cerevisiae strains: current state and perspectives. Appl Microbiol Biotechnol 84(1):37–53. https://doi.org/10.1007/s00253-009-2101-x
Matthaus F, Ketelhot M, Gatter M, Barth G (2014) Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica. Appl Environ Microbiol 80(5):1660–1669. https://doi.org/10.1128/aem.03167-13
Meijer MM, Boonstra J, Verkleij AJ, Verrips CT (1998) Glucose repression in Saccharomyes cerevisiae is related to the glucose concentration rather than the glucose flux. J Biol Chem 273(37):24102–24107
Millati R, Edebo L, Taherzadeh MJ (2005) Performance of Rhizopus, Rhizomucor, and Mucor in ethanol production from glucose, xylose, and wood hydrolyzates. Enzym Microb Technol 36(2):294–300. https://doi.org/10.1016/j.enzmictec.2004.09.007
Mirończuk AM, Biegalska A, Dobrowolski A (2017) Functional overexpression of genes involved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotechnol Biofuels 10(1):77
Misawa N, Shimada H (1997) Metabolic engineering for the production of carotenoids in non-carotenogenic bacteria and yeasts. J Biotechnol 59(3):169–181
Mohd Azhar SH, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Mohd Faik AA, Rodrigues KF (2017) Yeasts in sustainable bioethanol production: a review. Biochem Biophys Rep 10(Supplement C):52–61. https://doi.org/10.1016/j.bbrep.2017.03.003
Moliné M, Libkind D, van Broock M (2012) Production of torularhodin, torulene, and β-carotene by Rhodotorula yeasts. In: Barredo J-L (ed) Microbial carotenoids from fungi: methods and protocols. Humana, Totowa, pp 275–283
Moon H-J, Jeya M, Kim I-W, Lee J-K (2010) Biotechnological production of erythritol and its applications. Appl Microbiol Biotechnol 86(4):1017–1025. https://doi.org/10.1007/s00253-010-2496-4
Moran JW, Witter LD (1979) Effect of sugars on D-arabitol production and glucose metabolism in Saccharomyces rouxii. J Bacteriol 138(3):823–831
Moysés DN, Reis VCB, de Almeida JRM, de Moraes LMP, Torres FAG (2016) Xylose fermentation by Saccharomyes cerevisiae: challenges and prospects. Int J Mol Sci 17(3):207. https://doi.org/10.3390/ijms17030207
Nabors L, Gelardi R (2001) Alternative sweeteners: an overview, 2nd edn. Marcel Dekker, New York, pp 1–10
Nevoigt E (2008) Progress in metabolic engineering of Saccharomyes cerevisiae. Microbiol Mol Biol Rev 72(3):379–412. https://doi.org/10.1128/MMBR.00025-07
Niehus X, Crutz-Le Coq A-M, Sandoval G, Nicaud J-M, Ledesma-Amaro R (2018) Engineering Yarrowia lipolytica to enhance lipid production from lignocellulosic materials. Biotechnol Biofuels 11(1):11. https://doi.org/10.1186/s13068-018-1010-6
Nierman WC, Feldblyum TV, Laub MT, Paulsen IT, Nelson KE, Eisen J, Heidelberg JF, Alley MRK, Ohta N, Maddock JR, Potocka I, Nelson WC, Newton A, Stephens C, Phadke ND, Ely B, DeBoy RT, Dodson RJ, Durkin AS, Gwinn ML, Haft DH, Kolonay JF, Smit J, Craven MB, Khouri H, Shetty J, Berry K, Utterback T, Tran K, Wolf A, Vamathevan J, Ermolaeva M, White O, Salzberg SL, Venter JC, Shapiro L, Fraser CM (2001) Complete genome sequence of Caulobacter crescentus. Proc Natl Acad Sci U S A 98(7):4136–4141. https://doi.org/10.1073/pnas.061029298
Nunn CE, Johnsen U, Schonheit P, Fuhrer T, Sauer U, Hough DW, Danson MJ (2010) Metabolism of pentose sugars in the hyperthermophilic archaea Sulfolobus solfataricus and Sulfolobus acidocaldarius. J Biol Chem 285(44):33701–33709. https://doi.org/10.1074/jbc.M110.146332
Nyyssölä A, Pihlajaniemi A, Palva A, Von Weymarn N, Leisola M (2005) Production of xylitol from D-xylose by recombinant Lactococcus lactis. J Biotechnol 118(1):55–66
Oh D-K, Kim S-Y (1998) Increase of xylitol yield by feeding xylose and glucose in Candida tropicalis. Appl Microbiol Biotechnol 50(4):419–425. https://doi.org/10.1007/s002530051314
Oh EJ, Ha SJ, Rin Kim S, Lee WH, Galazka JM, Cate JH, Jin YS (2013) Enhanced xylitol production through simultaneous co-utilization of cellobiose and xylose by engineered Saccharomyes cerevisiae. Metab Eng 15:226–234. https://doi.org/10.1016/j.ymben.2012.09.003
Pal S, Mondal AK, Sahoo DK (2016) Molecular strategies for enhancing microbial production of xylitol. Process Biochem 51(7):809–819. https://doi.org/10.1016/j.procbio.2016.03.017
Papagianni M (2012) Metabolic engineering of lactic acid bacteria for the production of industrially important compounds. Comput Struct Biotechnol J 3:e201210003. https://doi.org/10.5936/csbj.201210003
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur J Lipid Sci Technol 113(8):1031–1051
Parajo JC, Santos V, Vazquez M (1997) Co-production of carotenoids and xylitol by Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Biotechnol Lett 19(2):139–142. https://doi.org/10.1023/a:1018356113002
Parajó JC, Santos V, Vázquez M (1998) Optimization of carotenoid production by Phaffia rhodozyma cells grown on xylose. Process Biochem 33(2):181–187. https://doi.org/10.1016/S0032-9592(97)00045-9
Park YK, Nicaud JM, Ledesma-Amaro R (2017) The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications. Trends Biotechnol 36:304–317. https://doi.org/10.1016/j.tibtech.2017.10.013
Porro D, Brambilla L, Ranzi BM, Martegani E, Alberghina L (1995) Development of metabolically engineered Saccharomyes cerevisiae cells for the production of lactic acid. Biotechnol Prog 11(3):294–298. https://doi.org/10.1021/bp00033a009
Rafiqul ISM, Sakinah AMM (2012) Design of process parameters for the production of xylose from wood sawdust. Chem Eng Res Des 90(9):1307–1312. https://doi.org/10.1016/j.cherd.2011.12.009
Rafiqul ISM, Sakinah AMM (2013) Processes for the production of xylitol—a review. Food Rev Int 29(2):127–156. https://doi.org/10.1080/87559129.2012.714434
Rangaswamy S, Agblevor F (2002) Screening of facultative anaerobic bacteria utilizing D-xylose for xylitol production. Appl Microbiol Biotechnol 60(1–2):88–93
Rao RS, Bhadra B, Shivaji S (2007) Isolation and characterization of xylitol-producing yeasts from the gut of colleopteran insects. Curr Microbiol 55(5):441–446. https://doi.org/10.1007/s00284-007-9005-8
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86(11):807–815. https://doi.org/10.1016/j.biochi.2004.09.017
Ribeiro O, Domingues L, Penttilä M, Wiebe MG (2012) Nutritional requirements and strain heterogeneity in Ashbya gossypii. J Basic Microbiol 52(5):582–589. https://doi.org/10.1002/jobm.201100383
Rodriguez GM, Hussain MS, Gambill L, Gao D, Yaguchi A, Blenner M (2016) Engineering xylose utilization in Yarrowia lipolytica by understanding its cryptic xylose pathway. Biotechnol Biofuels 9(1):149. https://doi.org/10.1186/s13068-016-0562-6
Ruan Z, Zanotti M, Wang X, Ducey C, Liu Y (2012) Evaluation of lipid accumulation from lignocellulosic sugars by Mortierella isabellina for biodiesel production. Bioresour Technol 110:198–205
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454:841–845. https://doi.org/10.1038/nature07190
Rupilius W, Ahmad S (2007) Palm oil and palm kernel oil as raw materials for basic oleochemicals and biodiesel. Eur J Lipid Sci Technol 109(4):433–439. https://doi.org/10.1002/ejlt.200600291
Ryu S, Hipp J, Trinh CT (2015) Activating and elucidating metabolism of complex sugars in Yarrowia lipolytica. Appl Environ Microbiol 82(4):1334–1345. https://doi.org/10.1128/aem.03582-15
Rywińska A, Rymowicz W, Żarowska B, Skrzypiński A (2010) Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J Microbiol Biotechnol 26(7):1217–1224. https://doi.org/10.1007/s11274-009-0291-0
Rzechonek DA, Dobrowolski A, Rymowicz W, Mirończuk AM (2018) Recent advances in biological production of erythritol. Crit Rev Biotechnol 38(4):620–633. https://doi.org/10.1080/07388551.2017.1380598
Saha BC, Bothast RJ (1997) Microbial production of xylitol fuels and chemicals from biomass. ACS Symposium Series, vol 666. American Chemical Society, pp 307–319
Saha BC, Sakakibara Y, Cotta MA (2007) Production of d-arabitol by a newly isolated Zygosaccharomyces rouxii. J Ind Microbiol Biotechnol 34(7):519–523. https://doi.org/10.1007/s10295-007-0211-y
Saloheimo A, Rauta J, Stasyk OV, Sibirny AA, Penttila M, Ruohonen L (2007) Xylose transport studies with xylose-utilizing Saccharomyes cerevisiae strains expressing heterologous and homologous permeases. Appl Microbiol Biotechnol 74(5):1041–1052. https://doi.org/10.1007/s00253-006-0747-1
Salusjärvi L, Kankainen M, Soliymani R, Pitkänen J-P, Penttilä M, Ruohonen L (2008) Regulation of xylose metabolism in recombinant Saccharomyes cerevisiae. Microb Cell Factories 7(1):18. https://doi.org/10.1186/1475-2859-7-18
Sampaio FC, Silveira WB, Chaves-Alves VM, Passos FML, Coelho JLC (2003) Screening of filamentous fungi for production of xylitol from D-xylose. Braz J Microbiol 34:321–324
Sampaio FC, Chaves-Alves VM, Converti A, Lopes Passos FM, Cavalcante Coelho JL (2008) Influence of cultivation conditions on xylose-to-xylitol bioconversion by a new isolate of Debaryomyces hansenii. Bioresour Technol 99(3):502–508. https://doi.org/10.1016/j.biortech.2007.01.017
Sarthy AV, McConaughy BL, Lobo Z, Sundstrom JA, Furlong CE, Hall BD (1987) Expression of the Escherichia coli xylose isomerase gene in Saccharomyes cerevisiae. Appl Environ Microbiol 53(9):1996–2000
Sasaki M, Jojima T, Inui M, Yukawa H (2010) Xylitol production by recombinant Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 86(4):1057–1066
Schellenberg GD, Sarthy A, Larson AE, Backer MP, Crabb JW, Lidstrom M, Hall BD, Furlong CE (1984) Xylose isomerase from Escherichia coli. Characterization of the protein and the structural gene. J Biol Chem 259(11):6826–6832
Schneider H, Lee H, Barbosa Mde F, Kubicek CP, James AP (1989) Physiological properties of a mutant of Pachysolen tannophilus deficient in NADPH-dependent d-xylose reductase. Appl Environ Microbiol 55(11):2877–2881
Seip J, Jackson R, He H, Zhu Q, Hong SP (2013) Snf1 is a regulator of lipid accumulation in Yarrowia lipolytica. Appl Environ Microbiol 79(23):7360–7370. https://doi.org/10.1128/aem.02079-13
Shi S, Zhao H (2017) Metabolic engineering of oleaginous yeasts for production of fuels and chemicals. Front Microbiol 8:2185. https://doi.org/10.3389/fmicb.2017.02185
Shi S, Chen Y, Siewers V, Nielsen J (2014) Improving production of malonyl coenzyme A-derived metabolites by abolishing Snf1-dependent regulation of Acc1. MBio 5(3):e01130–e01114. https://doi.org/10.1128/mBio.01130-14
Shiba Y, Paradise EM, Kirby J, Ro D-K, Keasling JD (2007) Engineering of the pyruvate dehydrogenase bypass in Saccharomyes cerevisiae for high-level production of isoprenoids. Metab Eng 9(2):160–168. https://doi.org/10.1016/j.ymben.2006.10.005
Skory CD (2003) Lactic acid production by Saccharomyes cerevisiae expressing a Rhizopus oryzae lactate dehydrogenase gene. J Ind Microbiol Biotechnol 30(1):22–27. https://doi.org/10.1007/s10295-002-0004-2
Spencer J, Sallans H (1956) Production of polyhydric alcohols by osmophilic yeasts. Can J Microbiol 2(2):72–79
Stephen Dahms A (1974) 3-Deoxy-D-pentulosonic acid aldolase and its role in a new pathway of D-xylose degradation. Biochem Biophys Res Commun 60(4):1433–1439. https://doi.org/10.1016/0006-291X(74)90358-1
Stephens C, Christen B, Fuchs T, Sundaram V, Watanabe K, Jenal U (2007) Genetic analysis of a novel pathway for D-xylose metabolism in Caulobacter crescentus. J Bacteriol 189(5):2181–2185. https://doi.org/10.1128/jb.01438-06
Stincone A, Prigione A, Cramer T, Wamelink MMC, Campbell K, Cheung E, Olin-Sandoval V, Grüning N-M, Krüger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser M (2015) The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev 90(3):927–963. https://doi.org/10.1111/brv.12140
Suryadi H, Katsuragi T, Yoshida N, Suzuki S, Tani Y (2000) Polyol production by culture of methanol-utilizing yeast. J Biosci Bioeng 89(3):236–240
Suzuki T, S-i Y, Kinoshita Y, Yamada H, Hatsu M, Takamizawa K, Kawai K (1999) Expression of xyrA gene encoding for D-xylose reductase of Candida tropicalis and production of xylitol in Escherichia coli. J Biosci Bioeng 87(3):280–284
Tai Y-S, Xiong M, Jambunathan P, Wang J, Wang J, Stapleton C, Zhang K (2016) Engineering nonphosphorylative metabolism to generate lignocellulose-derived products. Nat Chem Biol 12:247–253. https://doi.org/10.1038/nchembio.2020 https://www.nature.com/articles/nchembio.2020#supplementary-information
Tippmann S, Chen Y, Siewers V, Nielsen J (2013) From flavors and pharmaceuticals to advanced biofuels: production of isoprenoids in Saccharomyes cerevisiae. Biotechnol J 8(12):1435–1444. https://doi.org/10.1002/biot.201300028
Toivari MH, Salusjärvi L, Ruohonen L, Penttilä M (2004) Endogenous xylose pathway in Saccharomyes cerevisiae. Appl Environ Microbiol 70(6):3681–3686. https://doi.org/10.1128/AEM.70.6.3681-3686.2004
Toivola A, Yarrow D, van den Bosch E, van Dijken JP, Scheffers WA (1984) Alcoholic fermentation of d-xylose by yeasts. Appl Environ Microbiol 47(6):1221–1223
Turner TL, Zhang GC, Kim SR, Subramaniam V, Steffen D, Skory CD, Jang JY, Yu BJ, Jin YS (2015) Lactic acid production from xylose by engineered Saccharomyes cerevisiae without PDC or ADH deletion. Appl Microbiol Biotechnol 99(19):8023–8033. https://doi.org/10.1007/s00253-015-6701-3
Turner TL, Kim E, Hwang C, Zhang G-C, Liu J-J, Jin Y-S (2017) Conversion of lactose and whey into lactic acid by engineered yeast. J Dairy Sci 100(1):124–128. https://doi.org/10.3168/jds.2016-11784
Umemoto Y, Shibata T, Araki T (2012) D-xylose isomerase from a marine bacterium, Vibrio sp. strain XY-214, and D-xylulose production from β-1,3-xylan. Mar Biotechnol 14(1):10–20. https://doi.org/10.1007/s10126-011-9380-9
Upton DJ, McQueen-Mason SJ, Wood AJ (2017) An accurate description of Aspergillus niger organic acid batch fermentation through dynamic metabolic modelling. Biotechnol Biofuels 10:258. https://doi.org/10.1186/s13068-017-0950-6
Van Eck JH, Prior BA, Brandt EV (1993) The water relations of growth and polyhydroxy alcohol production by ascomycetous yeasts. Microbiol 139(5):1047–1054. https://doi.org/10.1099/00221287-139-5-1047
van Zyl C, Prior BA, Kilian SG, Kock JL (1989) D-xylose utilization by Saccharomyes cerevisiae. J Gen Microbiol 135(11):2791–2798. https://doi.org/10.1099/00221287-135-11-2791
Vandeska E, Amartey S, Kuzmanova S, Jeffries T (1995) Effects of environmental conditions on production of xylitol by Candida boidinii. World J Microbiol Biotechnol 11(2):213–218. https://doi.org/10.1007/bf00704652
Veiga-da-Cunha M, Santos H, Van Schaftingen E (1993) Pathway and regulation of erythritol formation in Leuconostoc oenos. J Bacteriol 175(13):3941–3948
Walfridsson M, Bao X, Anderlund M, Lilius G, Bulow L, Hahn-Hagerdal B (1996) Ethanolic fermentation of xylose with Saccharomyes cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active xylose (glucose) isomerase. Appl Environ Microbiol 62(12):4648–4651
Walfridsson M, Anderlund M, Bao X, Hahn-Hägerdal B (1997) Expression of different levels of enzymes from the Pichia stipitis XYL1 and XYL2 genes in Saccharomyes cerevisiae and its effects on product formation during xylose utilisation. Appl Microbiol Biotechnol 48(2):218–224
Waltermann M, Steinbuchel A (2005) Neutral lipid bodies in prokaryotes: recent insights into structure, formation, and relationship to eukaryotic lipid depots. J Bacteriol 187(11):3607–3619. https://doi.org/10.1128/jb.187.11.3607-3619.2005
Wang TH, Zhong YH, Huang W, Liu T, You YW (2005) Antisense inhibition of xylitol dehydrogenase gene, xdh1 from Trichoderma reesei. Lett Appl Microbiol 40(6):424–429. https://doi.org/10.1111/j.1472-765X.2005.01685.x
Wang Y, Zhang S, Zhu Z, Shen H, Lin X, Jin X, Jiao X, Zhao ZK (2018) Systems analysis of phosphate-limitation-induced lipid accumulation by the oleaginous yeast Rhodosporidium toruloides. Biotechnol Biofuels 11(1):148. https://doi.org/10.1186/s13068-018-1134-8
Weete JD (2012) Lipid biochemistry of fungi and other organisms. Media Plenum, New York
Weimberg R (1961) Pentose oxidation by Pseudomonas fragi. J Biol Chem 236:629–635
Werpy T, Petersen G, Aden A, Bozell J, Holladay J, White J, Manheim A, Eliot D, Lasure L, Jones S (2004) Top value added chemicals from biomass. Volume 1—results of screening for potential candidates from sugars and synthesis gas. Department of Energy Washington DC
Weyda I, Lubeck M, Ahring BK, Lubeck PS (2014) Point mutation of the xylose reductase (XR) gene reduces xylitol accumulation and increases citric acid production in Aspergillus carbonarius. J Ind Microbiol Biotechnol 41(4):733–739. https://doi.org/10.1007/s10295-014-1415-6
Whitworth DA, Ratledge C (1977) Phosphoketolase in Rhodotorula graminis and other yeasts. Microbiology 102(2):397–401. https://doi.org/10.1099/00221287-102-2-397
Wilson BL, Mortlock RP (1973) Regulation of D-xylose and D-arabitol catabolism by Aerobacter aerogenes. J Bacteriol 113(3):1404–1411
Xiong X, Wang X, Chen S (2012) Engineering of a xylose metabolic pathway in Rhodococcus strains. Appl Environ Microbiol 78(16):5483–5491. https://doi.org/10.1128/AEM.08022-11
Xu H, Kim S, Sorek H, Lee Y, Jeong D, Kim J, Oh EJ, Yun EJ, Wemmer DE, Kim KH, Kim SR, Jin Y-S (2016) PHO13 deletion-induced transcriptional activation prevents sedoheptulose accumulation during xylose metabolism in engineered Saccharomyes cerevisiae. Metab Eng 34:88–96. https://doi.org/10.1016/j.ymben.2015.12.007
Xue Z, Sharpe PL, Hong SP, Yadav NS, Xie D, Short DR, Damude HG, Rupert RA, Seip JE, Wang J, Pollak DW, Bostick MW, Bosak MD, Macool DJ, Hollerbach DH, Zhang H, Arcilla DM, Bledsoe SA, Croker K, McCord EF, Tyreus BD, Jackson EN, Zhu Q (2013) Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat Biotechnol 31(8):734–740. https://doi.org/10.1038/nbt.2622
Yaguchi A, Spagnuolo M, Blenner M (2018) Engineering yeast for utilization of alternative feedstocks. Curr Opin Biotechnol 53:122–129. https://doi.org/10.1016/j.copbio.2017.12.003
Yamada R, Yamauchi A, Kashihara T, Ogino H (2017) Evaluation of lipid production from xylose and glucose/xylose mixed sugar in various oleaginous yeasts and improvement of lipid production by UV mutagenesis. Biochem Eng J 128:76–82. https://doi.org/10.1016/j.bej.2017.09.010
Yang X, Lai Z, Lai C, Zhu M, Li S, Wang J, Wang X (2013) Efficient production of l-lactic acid by an engineered Thermoanaerobacterium aotearoense with broad substrate specificity. Biotechnol Biofuels 6(1):124. https://doi.org/10.1186/1754-6834-6-124
Yoshitake J, Ishizaki H, Shimamura M, Imai T (1973) Xylitol production by an Enterobacter species. Agric Biol Chem 37(10):2261–2267
Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56(1):17–34. https://doi.org/10.1007/s002530100624
Zha J, Li B-Z, Shen M-H, Hu M-L, Song H, Yuan Y-J (2013) Optimization of CDT-1 and XYL1 expression for balanced co-production of ethanol and xylitol from cellobiose and xylose by engineered Saccharomyes cerevisiae. PLoS One 8(7):e68317
Zhang M, Eddy C, Deanda K, Finkelstein M, Picataggio S (1995) Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis. Science 267(5195):240–243. https://doi.org/10.1126/science.267.5195.240
Zhang Z, Yeung WK, Huang Y, Chen ZY (2002) Effect of squalene and shark liver oil on serum cholesterol level in hamsters. Int J Food Sci Nutr 53(5):411–418. https://doi.org/10.1080/0963748021000044750
Zhang B, Li L, Zhang J, Gao X, Wang D, Hong J (2013) Improving ethanol and xylitol fermentation at elevated temperature through substitution of xylose reductase in Kluyveromyces marxianus. J Ind Microbiol Biotechnol 40(3–4):305–316
Zhang J, Zhang B, Wang D, Gao X, Hong J (2014) Xylitol production at high temperature by engineered Kluyveromyces marxianus. Bioresour Technol 152:192–201
Zhang J, Zhang B, Wang D, Gao X, Hong J (2015) Improving xylitol production at elevated temperature with engineered Kluyveromyces marxianus through over-expressing transporters. Bioresour Technol 175:642–645
Zhang S, Ito M, Skerker JM, Arkin AP, Rao CV (2016a) Metabolic engineering of the oleaginous yeast Rhodosporidium toruloides IFO0880 for lipid overproduction during high-density fermentation. Appl Microbiol Biotechnol 100(21):9393–9405. https://doi.org/10.1007/s00253-016-7815-y
Zhang S, Skerker JM, Rutter CD, Maurer MJ, Arkin AP, Rao CV (2016b) Engineering Rhodosporidium toruloides for increased lipid production. Biotechnol Bioeng 113(5):1056–1066. https://doi.org/10.1002/bit.25864
Zheng Y, Yu X, Zeng J, Chen S (2012) Feasibility of filamentous fungi for biofuel production using hydrolysate from dilute sulfuric acid pretreatment of wheat straw. Biotechnol Biofuels 5(1):50. https://doi.org/10.1186/1754-6834-5-50
Zhou J, Wu K, Rao CV (2016) Evolutionary engineering of Geobacillus thermoglucosidasius for improved ethanol production. Biotechnol Bioeng 113(10):2156–2167. https://doi.org/10.1002/bit.25983
Funding
This material is based upon the work supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number(s) DE-SC0018420.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jagtap, S.S., Rao, C.V. Microbial conversion of xylose into useful bioproducts. Appl Microbiol Biotechnol 102, 9015–9036 (2018). https://doi.org/10.1007/s00253-018-9294-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9294-9