Abstract
Rab GTPases are the largest group of the small GTPases family, which play a pivotal role in the secretion of proteins. Arthrobotrys oligospora is a representative nematode-trapping fungus that can produce adhesive networks to capture nematodes. In this study, the roles of two Rab GTPases AoRab-7A and AoRab-2 were characterized by gene knockout in the fungus A. oligospora. The disruption of AoRab-7A hindered the mycelial growth in different media, the conidiation of ΔAoRab-7A transformants was almost abolished, and the transcription of four sporulation-related genes (AbaA, FluG, Hyp1, and VosA) was downregulated compared to the wild-type strain (WT). Furthermore, the tolerance of the ΔAoRab-7A mutants to sodium dodecyl sulfate (SDS) and H2O2 was also significantly reduced compared to the WT, and the transcription of several genes related to environmental resistance, such as genes for catalase and trehalose synthase, was downregulated. Similarly, the extracellular proteolytic activity was decreased. Importantly, the ΔAoRab-7A mutants were unable to produce traps and capture nematodes. However, the disruption of gene AoRab-2 only affected the conidiation slightly but non-significantly, while other phenotypic traits were unaffected. Moreover, the gene AoRab-7A was also involved in the autophagy induced by nitrogen deprivation in A. oligospora. Our results revealed for the first time that the Rab GTPases are involved in the regulation of mycelial growth, conidiation, trap formation, stress resistance, and pathogenicity in the nematode-trapping fungus A. oligospora.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematophagous fungi include nematode-trapping fungi (NTFs), endoparasitic fungi, toxin-producing fungi, and opportunistic fungi. They have the capacity to capture, parasitize, or paralyze nematodes (Nordbring-Hertz et al. 2011). Among them, NTFs are characterized by developing specific trapping devices (traps), such as adhesive networks, adhesive knobs, and constricting rings to capture and then extract nutrients from nematodes (Li et al. 2005; Nordbring-Hertz et al. 2011). Aside from being weapons for capturing nematodes, traps are an important indicator of their switch from the saprophytic to the predacious lifestyles (Su et al. 2017). They play important roles in controlling nematode population density in diverse natural environments and are of great importance for improving agricultural production. Previous studies have identified the morphological characteristics of major trapping devices, including their evolution, phylogenetic distribution, and their underlying genomic controls (Li et al. 2005; Fekete et al. 2008; Yang et al. 2012; Su et al. 2017). However, little is known about the signaling pathways that regulate trap formation in nematode-trapping fungi.
Rab GTPases are a family of small, conserved proteins that cycle between GTP-bound (active) and GDP-bound (inactive) conformations and act as switches in the signaling hub of molecular circuits (Takai et al. 2001). They can be activated by extracellular stimuli and in turn regulate a diversity of downstream cytoplasmic signaling cascades (Mitin et al. 2005). In the past 20 years, they have been extensively studied in model eukaryotic cells and shown to be master regulators of membrane trafficking, responsible for many essential processes including exocytosis, endocytosis, and cellular differentiation (Tian et al. 2014). Among them, Ypt1 and Sec4 were the first functionally identified and characterized Rab genes in the exocytic pathway of the budding yeast Saccharomyces cerevisiae (Segev et al. 1988). Rab proteins exist in all eukaryotic cells (Takai et al. 2001), and their involvements in regulating the development, protein secretion, and virulence of fungi have attracted wide attentions (Punt et al. 2001; Tian et al. 2014). For instance, the Rab GTPase CLPT1 is essential for the differentiation of infectious structures in the bean pathogen Colletotrichum lindemuthianum (Siriputthaiwan et al. 2005) and hyphal development in fungi, such as Aspergillus niger (Punt et al. 2001), Aspergillus fumigatus (Powers-Fletcher et al. 2013), and Botrytis cinerea (Zhang et al. 2014). Deletion of Rab genes resulted in reduced sporulation (Zhang et al. 2014) and abnormal conidia size and shape compared to wild type (Powers-Fletcher et al. 2013; Liu et al. 2015). Furthermore, Rab GTPases could also regulate the secretory pathway in model organisms (Punt et al. 2001). For instance, deletion of Rab genes of Aspergillus nidulans resulted in decreases in extracellular levels of the major protein secretion, suggesting that it impairs secretion (Pantazopoulou and Peñalva 2011). Moreover, several studies indicated that Rab GTPases were the key regulators of virulence in pathogenic fungi (Powers-Fletcher et al. 2013).
Little is known about the roles of small GTPases in the NTFs. In 2005, the gene expression in trap cells and vegetative hyphae were compared to the nematode-trapping fungus Monacrosporium haptotylum, and several putative homologs for small GTPases were differentially expressed in knobs versus mycelium (Ahrén et al. 2005), suggesting that small GTPases may play a role in the trap formation. Arthrobotrys oligospora is a model species to investigate the interactions between nematodes and fungi. Strains of this species can produce adhesive networks to capture nematodes (Nordbring-Hertz et al. 2011). Recently, the genome of A. oligospora was sequenced (Yang et al. 2011), and 10 putative Rab genes were found in the genome of A. oligospora. In order to understand whether Rab GTPases involve in the regulation of trap formation and pathogenicity, two orthologous proteins of Rab GTPases Rab-7A and Rab-2 were retrieved from the fungus A. oligospora, and their roles in A. oligospora were identified by constructing the deletion mutants of AoRab-7A and AoRab-2. The phenotypic properties of these mutants were compared with the wild-type strain (WT). Moreover, the transcriptional level of several genes related to phenotypic properties, such as conidiation and stress resistance, were determined in the WT and mutants by real-time PCR (RT-PCR). Our results suggested that two Rab GTPases play different roles in regulating the hyphal growth, conidiation, multi-stress tolerance, and virulence in A. oligospora.
Materials and methods
Fungal strains and culture conditions
The fungus A. oligospora Fres. (ATCC24927) was cultured on potato dextrose agar (PDA) at 28 °C in the dark for 7 days. Liquid TG (1% tryptone and 1% glucose) medium was used to cultivate the mycelia for DNA extraction. The nematode Caenorhabditis elegans was maintained on oatmeal water (OA) medium at 26 °C and separated using the Baerman funnel technique (Gray 1984).
Sequence and phylogenetic analysis of AoRab-7A and AoRab-2
The small GTPases Rab-7A and Rab-2 in Neurospora crassa and A. fumigatus were used to search for homologs encoded in the genome of A. oligospora, and the orthologous proteins AoRab-7A (AOL_s00054g446) and AoRab-2 (AOL_s00054g360) were identified, and their amino acid sequences were downloaded from the GenBank. The biochemical properties and conserved domains were analyzed according to previous reports (Gasteiger et al. 2005; Jones et al. 2014). The orthologous proteins of GTPases AoRab-7A and AoRab-2 from other filamentous fungi were downloaded from GenBank, and a neighbor-joining tree was constructed using the Mega 5.1 software package (Tamura et al. 2011).
Deletion of the genes AoRab-7A and AoRab-2
Genomic DNA of the fungus A. oligospora was extracted using the CTAB (cetyl trimethylammonium bromide) procedure. The replacement constructs of genes AoRab-7A and AoRab-2 were generated using a modified yeast cloning procedure, respectively (Colot et al. 2006; Zhao et al. 2014). Briefly, the 5′ and 3′ flanking sequences of target genes and hph cassette (Staben et al. 1989) were respectively amplified, and then, the three DNA fragments and a pRS426 backbone (Christianson et al. 1992) (digested by EcoRI and XhoI) were transformed into the yeast S. cerevisiae strain FY834 (Winston et al. 1995) via electroporation. The final disruption vectors (pRS426-AoRab-7A-hph and pRS426-AoRab-2-hph) were recovered by transformation into Escherichia coli DH5a (Takara, Shiga, Japan). Transformation and screening were performed according to previous reports (Tunlid et al. 1999; Zhang et al. 2008; Zhao et al. 2014). Transformants were further confirmed by PCR amplification using primers Yf and Yr for each gene (Supplementary Table S1). Furthermore, the genomic DNA of mutants for each gene was extracted for Southern blot analysis. Southern analysis was carried out according to our recent reports (Zhao et al. 2014; Jiang et al. 2017). Primers T5 and T3 corresponding to the individual primers for the different genes listed in Supplemental Table S1 were used to prepare Southern hybridization probes, and the genomic DNA of A. oligospora and mutants was digested using restriction enzymes NheI and PvuII for Southern analysis.
Comparison of growth rates, conidia yield of the WT, and mutants on different media
The WT and ΔAoRab-7A and ΔAoRab-2 mutants were incubated on PDA medium at 26 °C for 5 days, respectively; the growth rate and colony morphology were observed and determined. Similarly, the growth rate and colony morphology were compared to TYGA (10 g/L tryptone, 5 g/L yeast extract, 10 g/L glucose, 5 g/L molasses, 20 g/L agar) and TG plates (10 g/L tryptone, 10 g/L glucose, 20 g/L agar). The WT and mutants were incubated on CMY medium (20 g/L maizena, 20 g/L agar, 5 g/L yeast extract) at 26 °C for 15 days, and the conidia yield was determined according to previous reports (Luo et al. 2012; Wang et al. 2014).
Stress tolerance test
To determine whether genes AoRab-7A and AoRab-2 are required for salt, oxidative, or detergent stress tolerance, the mycelial plugs of each strain was inoculated onto TG plates supplemented with different concentrations of NaCl (0.1–0.3 M), H2O2 (5–15 mM), and SDS (sodium dodecyl sulfate) (0.02–0.04%) and incubated at 28 °C for 5 days, respectively. The growth rate and colony morphology were observed and determined.
Protease activity assays
The WT and mutants were incubated on LMZ medium (Zhao et al. 2004) at 26 °C, respectively. The fermentation liquid was collected after culturing for 6 days, and proteolytic activity was assayed on casein plate using the method described by a previous report (Zhao et al. 2004).
Trap formation and pathogenicity of A. oligospora against nematodes
The WT and mutants were incubated on corn meal agar (CMA) medium at 28 °C for 3–4 days, and about 200 nematodes (C. elegans) were added into the middle of each plate to induce the trap formation (Chen et al. 2013). At specified time intervals, the traps and captured nematodes were counted respectively under a light microscope (BX51, Olympus, Tokyo, Japan) at 12, 24, 36, and 48 h (Jiang et al. 2017).
Real-time PCR (RT-PCR) analysis
Agar disks containing mycelium of a strain (either WT or mutants) were placed centrally onto a TYGA plate, followed by 3, 4, 5, and 6 days incubation at 28 °C. At specific time intervals, hyphae were collected and stored at − 80 °C for RNA extraction. Total RNAs were isolated from all samples per analyzed strain with a RNA Extraction Kit (Axygen, Jiangsu, China) and reversely transcribed into complementary DNAs (cDNAs) with a FastQuant RT Kit (with gDNA, Takara, Shiga, Japan). Each cDNA was used as a template to determine transcriptional levels of candidate genes associated with phenotypes, such as conidiation and stress resistance, via RT-PCR with specific paired primers (Supplementary Table S2) using β-tubulin as an internal standard. The relative transcript level of each gene was computed as the ratio of its transcript in each deletion mutant over that in WT on a given day using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Autophagy detection
Autophagy induced in the WT and the ΔAoRab-7A mutants by nitrogen deprivation was analyzed and observed according to our recent report (Chen et al. 2013).
Statistical analysis
Each experiment was performed with three biological replicates, and data from experiments are expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test is indicated when used; values of P < 0.05 were considered significant (Farnesi et al. 2015; Jiang et al. 2017). All statistical analyses were made using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California, USA).
Results
Sequence and phylogenetic analyses of Rab GTPases AoRab-7A and AoRab-2 in A. oligospora
The cDNA sequences of genes AoRab-7A and AoRab-2 were amplified from the fungus A. oligospora, respectively. The gene AoRab-7A has an open reading frame (ORF) of 1290 bp interrupted by four introns (338, 147, 64, and 90 bp, respectively). Similarly, gene AoRab-2 has an ORF of 906 bp, also interrupted by four introns (77, 64, 78, and 54 bp, respectively). AoRab-7A encodes a polypeptide of 216 amino acid residues (aa) which has a predicted isoelectric point (pI) and molecular weight (MW) of 5.07 and 24.0 kDa, respectively, while AoRab-2 encodes a polypeptide of 210 aa, with a predicted pI and MW of 7.63 and 23.4 kDa, respectively. AoRab-7A and AoRab-2 have no signal peptides, but both shared a conserved P-loop as present in nucleoside triphosphate hydrolases (IPR027417) and a small GTP-binding protein domain (IPR005225).
The homologous proteins of Rab-7A and Rab-2 in different fungi were downloaded from GenBank and aligned using the DNAman software (version 5.2.2, LynnonBiosoft, St. Louis, Canada). AoRab-7A is highly conserved among filamentous fungi. For example, it shares 96.6 and 94% sequence identities to homologs from the two NTFs Dactylellina haptotyla (Meerupati et al. 2013) and Drechslerella stenobrocha (Liu et al. 2014), respectively. Similarly, it also shares a high degree of similarity (80.7–86.8%) to orthologous proteins from other filamentous fungi (Supplementary Table S3). AoRab-2 shares 75.3% identity to the homolog from D. haptotyla and 51.7–57.7% identities to orthologous proteins from other filamentous fungi (Supplementary Table S3). Moreover, the homologs of Rab-7A and Rab-2 in various filamentous fungi contain several conserved motifs, such as WDTAGQE, GNKXD, and ETSAK (Supplementary Fig. S1). Based on results in previous studies, DTAG in WDTAGQ likely interacts with G-phosphate of GTP, NKXD in GNKXD is the guanine specificity region, and ETSA in ETSAK interacts with the D residue in the NKVD. These motifs are collectively involved in nucleotide binding and hydrolysis (Takai et al. 2001; Rajan et al. 2015).
The phylogenetic tree of small GTPases Rab-7A and Rab-2 in filamentous fungi was constructed based on their amino acid sequences, and the orthologous proteins of Rab-7A and Rab-2 were separated into two clades (A and B, Supplementary Fig. S2). The homologs of Rab-7A (clade A) can be divided into two sub-clades (I and II), with Rab-7A from three species of NTFs A. oligospora, D. haptotyla, and D. stenobrocha clustered in A-II, and homologs of Rab-7A from other filamentous fungi, such as A. nidulans, N. crassa, and Trichoderma atroviride clustered in A-I. Similarly, homologous proteins of Rab-2 from different fungi also can be divided into two sub-clades (I and II), and Rab-2 from A. oligospora and D. haptotyla were clustered in B-I, while homologs of Rab-2 from other fungi were clustered in B-II.
Screening and confirmation of the ΔAoRab-7A and ΔAoRab-2 mutants
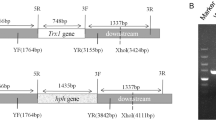
The plasmids pRS426-AoRab-7A-hph and pRS426-AoRab-2-hph were transformed into protoplasts of A. oligospora, respectively, and transformants were selected on PDASS plates containing 200 μg/mL hygromycin B (Zhang et al. 2008; Zhao et al. 2014). Genomic DNA of these transformants was isolated, and the site-specific insertion was confirmed using the PCR method. Compared to the 989-bp and 1306-bp fragments from the WTs, fragments of 1886 and 2036 bp were amplified from the ΔAoRab-2 (Fig. 1A-b) and ΔAoRab-7A mutants (Fig. 1 B-b), respectively, using primers AoRab-2-Yf/Yr and AoRab-7A-Yf/Yr (Supplementary Table S1). Finally, two mutants of gene AoRab-2 (MT-4 and MT-7) were obtained and three of the total five mutants of gene AoRab-7A (MT-1, MT-3, and MT-8) were selected for further study, respectively. In addition, these transformants were further confirmed using Southern blot analysis, a single band hybridizing to the AoRab-7A and AoRab-2 gene probes was observed in the WT and transformants (Fig. 1A-c, B-c), and the sizes of the hybridized bands were consistent with our expectation.
Knockout and verification of genes AoRab-2 and AoRab-7A in A. oligospora. A Verification of the knockout of gene Rab-2 using PCR method and Southern blot. A-a The diagrammatic sketch of homologous recombination of gene AoRab-2. Primers AoRab-2-5f/AoRab-2-5r and AoRab-2-3f/AoRab-2-3r were used for amplification of the homologous flanks of the target gene, and AoRab-2-Yf/AoRab-2-Yr (Supplementary Table S1) were used for verification of the transformants, letter p indicates the site of the Southern blot probe, and PvuII was the restriction enzyme used for Southern blotting analysis. A-b The gene AoRab-2 mutants were confirmed by the PCR method using genomic DNAs and primers AoRab-2-Yf/AoRab-2-Yr for amplification. MT-4 and MT-7 mean the positive knocked-out transformants of interest, and WT means the wild-type strain, while X1, X2, and X3 mean the heterozygotic transformants with a wild-type gene copy and a hph-replaced copy. M means the DNA marker. A-c Southern blotting analysis of the WT and the ΔAoRab-2 mutants. The genomic DNA was loaded onto a gel after it was cut by PvuII, respectively. B Verification of the knockout of gene AoRab-7A using PCR method and Southern blot. B-a The diagrammatic sketch of homologous recombination of gene AoRab-7A. Primers AoRab-7A-5f/AoRab-7A-5r and AoRab-7A-3f/AoRab-7A-3r were used for amplification of the homologous flanks of the target gene, and AoRab-7A-Yf/AoRab-7A-Yr were used for verification of the transformants, letter p indicates the site of the Southern blot probe, and NheI was the restriction enzyme used for Southern blotting analysis. B-b the gene AoRab-7A mutants were confirmed by the PCR method using genomic DNAs and primers AoRab-7A-Yf/AoRab-7A-Yr for amplification. MT-1, MT-3, MT-5, MT-6, and MT-8 mean the positive transformants. B-c Southern blotting analysis of the WT and the three selected ΔAoRab-7A mutants. The genomic DNA was loaded onto a gel after it was cut by NheI, respectively
Influence of genes AoRab-7A and AoRab-2 on the growth and conidiation in A. oligospora
Compared with the WT, the three gene AoRab-7A deletion mutants (ΔAoRab-7A mutants) showed different degrees of growth defects on PDA, TYGA, and TG media (Fig. 2A, B). After 5 days of incubation on these media, the three different ΔAoRab-7A colonies were smaller than the WT on all three media, and the three different ΔAoRab-7A colonies were less cottony and thicker than those of the WT and also of the two different ΔAoRab-2 transformants on TYGA plates. Similarly, the aerial hyphae of the ΔAoRab-7A clones were sparser than that of the WT (Fig. 2A, B). In contrast, the mycelial growth and colony sizes of the two ΔAoRab-2 mutants exhibited no difference from the WT on different agar media (Fig. 2A, B).
Comparison of the growth, conidiation, and the transcript levels of sporulation-related genes between the WT and mutants. A Comparison of mycelial growth of the WT and mutants on PDA, TYGA, and TG plates. B The colony morphology of the WT and mutants incubated on TYGA for 5 days at 28 °C. C Sporulation of the WT and mutants on CMY medium. An asterisk (*) indicates significant differences between the ΔAoRab-7A mutants and the WT (P < 0.05), and ns indicates no difference between the ΔAoRab-2 mutants and the WT. D The relative transcript levels (RTLs) of sporulation-related genes in the ΔAoRab-2 mutants (values shown are averaged from the individual data obtained for strains MT-4 and MT-7 as shown in Supplementary Fig. S3) were compared to the WT at different time points. An asterisk indicates significant differences between the ΔAoRab-2 mutants and the WT (P < 0.05). E The RTLs of sporulation-related genes in the ΔAoRab-7A mutants (values shown are averaged from the individual data obtained for strains MT-1, MT-3 and MT-8 as shown in Supplementary Fig. S3) were compared to the WT at different time points. An asterisk indicates significant differences between the ΔAoRab-7A mutants and the WT (P < 0.05)
The disruption of gene AoRab-2 led to a non-significant reduction in conidiation. Specifically, the spore number of the WT was 7.26 × 105/mL after culturing in CMY at 28 °C for 14 days, while 5.73–6.33 × 105/mL spores were found in the two ΔAoRab-2 mutants under the same conditions (Fig. 2C). Overall, compared to the WT, the spore production capacity of the two ΔAoRab-2 mutants decreased by 12.8–21.0%. However, the three ΔAoRab-7A mutants produced very few conidia on CMY plates (Fig. 2C).
Nine sporulation-related genes in A. oligospora were retrieved based on searches using homologous genes in the model fungus A. nidulans, including AbaA, FlbC, FluG, Hyp1, NsdD, Sep2, VeA, VelB, and VosA (Supplementary Table S2) (Krijgsheld et al. 2013). The transcriptional levels of these genes were determined in the WT, the three ΔAoRab-7A mutants, and the two ΔAoRab-2 mutants by RT-PCR after they were cultured on TYGA for 3, 4, 5, and 6 days (Fig. 2D, E; Supplementary Fig. S3), respectively. Our results showed that the transcriptional levels of seven genes had no obvious change in the ΔAoRab-2 mutants compared to the WT, including AbaA, FluG, NsdD, Sep2, VeA, VelB, and VosA, but the expression of FlbC was upregulated in the ΔAoRab-2 mutants during the culture time from 4 to 6 days. Similarly, the transcription of Hyp1 was also gradually upregulated with the extension of culture time (Fig. 2D; Supplementary Fig. S3). While the transcription of four genes (FlbC, NsdD, Sep2, and VelB) showed no obvious change in the ΔAoRab-7A mutants compared to the WT, that of VeA was significantly upregulated in the ΔAoRab-7A mutants, and the expression of four genes (AbaA, FluG, Hyp1, and VosA) was downregulated. The most affected was the transcription of gene Hyp1. Compared to the ΔAoRab-2 mutants, the transcription of gene VeA was upregulated significantly in the ΔAoRab-7A mutants, and the transcription of four genes (AbaA, FlbC, FluG, and Hyp1) was downregulated significantly in the ΔAoRab-7A mutants (Fig. 2E; Supplementary Fig. S3).
AoRab-2 and AoRab-7A are involved in regulating stress resistance in A. oligospora
The WT and mutants were compared for their responses to three chemicals during incubation on TG plates at 28 °C. The tested chemicals included an oxidant (H2O2), a cell wall perturbing agent (SDS) and an osmotic salt (NaCl). When the WT and mutants were incubated on TG media supplemented with different concentrations of NaCl for 6 days, neither ΔAoRab-7A nor ΔAoRab-2 mutants showed a significant change in sensitivity to NaCl compared to the WT (Supplementary Fig. S4). While the oxidant and the cell wall perturbing agent inhibited the growth of the ΔAoRab-7A mutants significantly, there was no influence on the growth of the ΔAoRab-2 mutants (Figs. 3A and 4A).
Comparison of the stress tolerance to SDS and the transcript levels of cell wall synthesis-related genes in the WT and mutants. A The colony morphology of the WT and mutants incubated on TG medium supplemented with different concentrations of SDS. B The colony diameter of the WT and mutants incubated on TG medium supplemented with different concentrations of SDS. An asterisk indicates significant differences between the ΔAoRab-7A mutants and the WT (P < 0.05). C The RTLs of cell wall synthesis-related genes in the ΔAoRab-2 mutants (values shown are averaged from the individual data obtained for strains MT-4 and MT-7 as shown in Supplementary Fig. S5) were compared to the WT at different time points. An asterisk indicates significant differences between the ΔAoRab-2 mutants and the WT (P < 0.05). D The RTLs of cell wall synthesis-related genes in the ΔAoRab-7A mutants (values shown are averaged from the individual data obtained for strains MT-1, MT-3, and MT-8 as shown in Supplementary Fig. S5) were compared to the WT at different time points. An asterisk indicates significant differences between the ΔAoRab-7A mutants and the WT (P < 0.05)
Comparison of the stress tolerance to oxidant H2O2 and the transcript levels of H2O2-degrading related genes of the WT and mutants. A The colony morphology of the WT and mutants incubated on TG medium supplemented with different concentrations of H2O2. B The colony diameter of the WT and mutants incubated on TG medium supplemented with different concentrations of H2O2. An asterisk indicates significant differences between the ΔAoRab-7A mutants and the WT (P < 0.05). C The RTLs of H2O2-degrading-related genes in the ΔAoRab-2 mutants (values shown are averaged from the individual data obtained for strains MT-4 and MT-7 as shown in Supplementary Fig. S6) were compared to the WT at different time points. An asterisk indicates significant differences between the ΔAoRab-2 mutants and the WT (P < 0.05). D The RTLs of H2O2-degrading-related genes in the ΔAoRab-7A mutants (values shown are averaged from the individual data obtained for strains MT-1, MT-3, and MT-8 as shown in Supplementary Fig. S6) were compared to the WT at different time points. An asterisk indicates significant differences between the ΔAoRab-7A mutants and the WT (P < 0.05)
The growths of the WT and of the ΔAoRab-7A and ΔAoRab-2 mutants were all inhibited significantly in TG plates supplemented with 0.02% SDS. The most significant inhibition was observed in the ΔAoRab-7A mutants where little growth was observed at 0.02% SDS and no growth was observed at 0.04% SDS (Fig. 3A, B). Five putative genes involved in cell wall synthesis and trehalose synthase (Trs) (Yang et al. 2011) were selected, and their transcriptional levels were determined in the WT and mutants by RT-PCR, including genes for chitin synthase (Chs), hexokinase (Hex), glucosamine-fructose-6-phosphate aminotransferase (Gfpa), β-glucosidase (Glu), and 1,3-β-glucan synthase (Gls). The expression of these genes was upregulated slightly in the ΔAoRab-2 mutants at the fourth and the fifth days of incubation compared to the WT (Fig. 3C; Supplementary Fig. S5). Three genes (Chs, Hex, and Gls) were upregulated in the ΔAoRab-7A mutants, especially the transcription of gene Gls was increased by threefold compared to the WT. However, the transcription of genes Gfpa and Glu showed no change (Fig. 3D; Supplementary Fig. S5). Interestingly, while the transcription of Trs was upregulated slightly in the ΔAoRab-2 mutants, its expression was inhibited significantly in the ΔAoRab-7A mutants.
Similar to the observations above, the growths of the WT and of the ΔAoRab-7A and ΔAoRab-2 strains were inhibited in TG plates supplemented with 5 mM H2O2. The three ΔAoRab-7A mutants were more sensitive to H2O2 than the WT and ΔAoRab-2 strains, and the growth of them was inhibited completely when the concentration of H2O2 was increased to 15 mM (Fig. 4A, B). Nine putative proteins involved in H2O2 metabolism and antioxidation, including catalase (Cat), glutathione reductase (Glr), glutathione S-transferase (Glt), glutathione synthase (Gls), glutathione dehydrogenase (Gld), peroxiredoxin (Pero), peroxidase (Per), thioredoxin reductase (Thr), and thioredoxin (Thi), were selected for analysis by RT-PCR. The analyses showed that the transcription of these genes had no obvious change in the ΔAoRab-2 mutants, except Thr that showed upregulation on the third and the fourth days of incubation (Fig. 4C; Supplementary Fig. S6). Compared to the WT, the transcription of three genes (Glt, Thr, and Thi) was upregulated in the ΔAoRab-7A mutants, with genes Thr and Thi showing upregulation over twofold from 4 to 6 days. In contrast, the transcription of the CAT gene was downregulated significantly from 4 to 6 days of incubation (Fig. 4D; Supplementary Fig. S6).
AoRab-2 and AoRab-7A are involved in production of serine proteases
Extracellular serine proteases were closely related to the virulence and pathogenicity in the NTFs (Yang et al. 2005). A. oligospora can produce serine proteases to immobilize the nematodes and degrade the proteinous components of nematode cuticle, such as PII (Tunlid et al. 1994; Yang et al. 2013). In this study, we found that the fermentation broth of the WT and the ΔAoRab-7A and ΔAoRab-2 mutants exhibited different proteolytic activities. The disruption of AoRab-2 had little influence on the proteolytic activity compared to the WT, but the deletion of AoRab-7A resulted in the significant reduction of proteolytic activity (Fig. 5A). Twenty-four serine proteases were identified in the genome of A. oligospora (Yang et al. 2011). In order to learn which ones contributed to the change of proteolytic activity in the ΔAoRab-7A and ΔAoRab-2 mutants, the transcriptional levels of seven serine protease genes belonging to different subfamilies, including genes for the proteinase K (ProK), class I, and OSP kexin families (Yang et al. 2011), were determined in the WT and the ΔAoRab-7A and ΔAoRab-2 mutants, respectively.
Extracellular proteolytic activity and the RTLs of genes for serine proteases in the WT and mutants. A Comparison of the extracellular proteolytic activity of the WT and mutants. The transparent zone produced due to the degradation of casein added to the medium in the plate. B The RTLs of serine protease genes in the ΔAoRab-2 mutants (values shown are averaged from the individual data obtained for strains MT-4 and MT-7 as shown in Supplementary Fig. S7) were compared to the WT at different time points. An asterisk indicates significant differences between the ΔAoRab-2 mutants and the WT (P < 0.05). C The RTLs of serine protease genes in the ΔAoRab-7A mutants (values shown are averaged from the individual data obtained for strains MT-1, MT-3, and MT-8 as shown in Supplementary Fig. S7) were compared to the WT at different time points. An asterisk indicates significant differences between the ΔAoRab-7A mutants and the WT (P < 0.05)
Compared with the WT, the transcription of seven proteases showed no obvious change in the ΔAoRab-2 mutants, but the transcription of three other proteases (112g42, 75g8, and 188g273) was upregulated slightly on the fourth and the fifth days of incubation (Fig. 5B; Supplementary Fig. S7). The transcription of most of these proteases changed significantly in the ΔAoRab-7A mutants. For example, the transcription of proteases 112g42 and 78g136 was upregulated dramatically, with that of protease 112g42 increased over fivefold. In contrast, the transcription of proteases 75g8, 188g273, and 54g992 was downregulated significantly, with that of 54g992 decreased by more than tenfold (Fig. 5C; Supplementary Fig. S7).
AoRab-7A and AoRab-2 are involved in regulating trap formation and nematocidal activity in A. oligospora
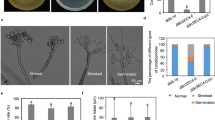
Trap formation was observed on CMA plates after the addition of nematodes for 12 h (Fig. 6A). The WT strain produced immature traps, which only contained one or two circles at 12 h. At 24 h, the fungus formed mature three dimensional nets and began to capture nematodes. At 36 h, all of the nematodes were captured. At 48 h, the majority nematodes were digested by the WT strain (Fig. 6A). In contrast, trap formation was not observed in the ΔAoRab-7A mutants after the nematodes were added into the CMA plates (Fig. 6A). Different from ΔAoRab-7A mutants, ΔAoRab-2 mutants formed traps when induced by nematodes, and there was no significant difference in morphology and number of traps between the ΔAoRab-2 mutants and the WT strain (Fig. 6B).
Comparison of the trap formation and nematicidal activity of the WT and mutants. A The trap formation of the WT and mutants induced by nematodes at different time points. B The percentage of captured nematodes by the WT and mutants at different time points. An asterisk indicates significant differences between the ΔAoRab-7A mutants and the WT (P < 0.05)
Nematodes were captured and infected by the WT when they were added to each CMA plate. After the traps were formed at 24 h, the nematodes were captured and gradually immobilized (Fig. 6A). As shown in Fig. 6, the percentages of immobilized nematodes were not different between the WT and the ΔAoRab-2 mutants: 49.1, 78.5, and 88.6% nematodes were captured by the WT at 24, 36, and 48 h (Fig. 6B), respectively, while 45.7, 75.8, and 84.4% nematodes were captured by the ΔAoRab-2 mutants at same times (Fig. 6B), respectively. However, none of the nematodes were captured by the ΔAoRab-7A mutants.
Autophagy analysis of the WT and mutant ΔAoRab-7A
The mycelia of the WT and the ΔAoRab-7A mutants were stained with the fluorescent dye mono-dansyl cadaverine (MDC) (Sigma, St. Louis, MO) for 30 min (Contento et al. 2005), and mycelia were observed by fluorescence microscopy (Nikon, Tokyo, Japan). Strong fluorescence was observed in the cytoplasm and vacuoles of the WT strain in nitrogen-deprived minimal medium (Supplementary Fig. S8). In contrast, weak fluorescence was observed in the ΔAoRab-7A mutants (see as an example mutant MT-8 in Fig. S8) under the same conditions, suggesting that the gene AoRab-7A is required for autophagy induced by nitrogen deprivation in A. oligospora.
Discussion
The functions of Rab GTPases in filamentous fungi are not well understood, but they have been implicated in the regulation of hyphal growth, conidiation, pathogenicity, autophagy, effector delivery, and secretion (Stenmark and Olkkonen 2001; Pantazopoulou and Peñalva 2011; Liu et al. 2015). In this study, two Rab GTPases AoRab-7A and AoRab-2 were identified from the nematode-trapping fungus A. oligospora. These two proteins shared partial conserved motifs (Supplementary Fig. S1), and they were clustered with homologs from other NTFs, respectively. As expected, they showed a distant evolutionary relationship with orthologous proteins from other model filamentous fungi, such as A. nidulans and N. crassa (Supplementary Fig. S2). Furthermore, the phenotypic traits of Rab GTPase-deleted strains were comprehensively compared to the WT. Our results showed that AoRab-7A was involved in the regulation of multiple biological processes, while the deletion of AoRab-2 had no obvious influence on the growth and other phenotypes. Moreover, the transcriptional levels of several genes involved in conidiation, stress tolerance, and protease production showed significantly changes in the ΔAoRab-7A mutants, which suggested that AoRab-7A might involve in the regulation of these genes.
Recently, the function of several Rab GTPases had been revealed in a subset of filamentous fungi (e.g., Powers-Fletcher et al. 2013; Tian et al. 2014; Zhang et al. 2014). For example, mutation of a Rab GTPase gene Clpt1 resulted in a severe reduction of pathogenicity in C. lindemuthianum and the mutant was unable to penetrate the host cells (Siriputthaiwan et al. 2005). Our results showed that mutants with a deletion of gene AoRab-7A were unable to form traps and lost the ability to capture and infect nematodes. Furthermore, our result is also similar to that of the Rab-7 mutants of Fusarium graminearum that were defective in plant infection and failed to produce scab symptoms on inoculated kernels (Zheng et al. 2015). In contrast, ΔAoRab-2 mutants produced traps, and its infection capacity was not significantly different from the WT.
In addition, AoRab-7A regulates the asexual reproduction of A. oligospora. The conidiation of ΔAoRab-7A mutants were almost abolished, similar to that of the Ypt7 mutant in Magnaporthe oryzae (Liu et al. 2015) and the Rab-7 mutant in F. graminearum (Zheng et al. 2015). In contrast, the ΔAoRab-2 mutants showed no obvious difference in conidiation and stress tolerance when compared to the WT similar to that of the Rab-2 mutant in F. graminearum (Zheng et al. 2015). Compared to the WT and the ΔAoRab-2 mutants, the transcription of sporulation gene VeA was upregulated significantly in the ΔAoRab-7A mutants, while the transcription of four other genes (AbaA, FlbC, FluG, and Hyp1) was significantly downregulated in the ΔAoRab-7A mutants (Fig. 2D, E). AbaA, FlbC, and FluG are the key genes regulating A. nidulans conidiation (Park and Yu 2012). Specifically, AbaA is an essential component for proper differentiation and function of phialides, and the loss of gene AbaA results in the formation of aberrant conidiophores exhibiting reiterated cylinder-like terminal cells lacking spores (Sewall et al. 1990; Tao and Yu 2011).
Previous studies indicated that autophagy may be involved in the storage of nitrogenous compounds in conidia during asexual development in filamentous fungi (Kikuma et al. 2007). Recently, a homolog of the small Ras-like GTPase Ypt7 in S. cerevisiae was characterized in M. oryzae. The ΔMoYpt7 mutant exhibited defects in mycelial growth and conidia production. The conidia of the ΔMoYpt7 mutant were malformed and defective in the formation of appressoria. Furthermore, the ΔMoYpt7 mutant showed impairment in autophagy, breached cell wall integrity, and higher sensitivity to both calcium and heavy metal stress (Liu et al. 2015). In this study, we also found that AoRab-7A might involve in autophagy induced by nitrogen deprivation (Supplementary Fig. S8). Moreover, the deletion of gene Atg8 (essential for autophagic pathway in fungi) in A. oligospora resulted in the inability of nematode-induced autophagy and suppressed trap formation (Chen et al. 2013). These results suggested that autophagy is crucial for trap formation in A. oligospora during infection of nematodes. However, the mechanism of autophagy regulating the trap formation in A. oligospora was unknown.
The growth of the ΔAoRab-7A mutants were inhibited under H2O2 and SDS stresses, suggesting that this gene plays an important role in response to oxidative and detergent stress tolerance. Surprisingly, three genes (Chs, Hex, and Gls) related to cell wall biosynthesis were upregulated in the ΔAoRab-7A mutants, while the gene Trs was downregulated; the downregulation of Trs made that the fungus produces less trehalose to resist multiple stresses, such as SDS (Liu et al. 2011). Meanwhile, detoxification of H2O2 is a fundamental aspect of the cellular antioxidation response in which catalase and peroxidases enzymes play a major role, producing H2O and O2 (Angelova et al. 2005); the downregulation of Cat made that the ΔAoRab-7A mutants became more sensitive to H2O2 while the transcription of Thi and Thr was upregulated, consistent with their roles in inhibiting the catalase activity (Thön et al. 2007). Previous reports have highlighted the key role played by sulphydryl groups (–SH) in the response to oxidative stress and, in particular, the role of the glutathione/glutaredoxin and thioredoxin systems in maintaining the redox homeostasis of the cell (Grant 2001).
Pathogenic fungi often secrete a series of virulence factors (for example, serine proteases) into the extracellular environment to facilitate infection and allow the pathogen to assimilate essential nutrients from the host (Conesa et al. 2001; Espino et al. 2010). Extracellular secretion is dependent on vesicle transport and Rab GTPases are well-established regulators of this process (Novick and Zerial 1997). The extracellular proteolytic activity of ΔAoRab-7A mutants was reduced significantly, and the transcription of several serine protease genes, such as 75g8, 188g273, and 54g992, was downregulated significantly. Among them, the products of 75g8 and 188g273 belong to the ProK family, which plays important roles during the evolution of pathogenicity, and the product of 75g8 shares a high degree of similarity to serine protease PII, an enzyme known to be involved in nematode immobilization and degradation (Tunlid et al. 1994). While the product of 54g992 belongs to class I subtilisin (also named pyrolisin), they can degrade themselves in a process termed autoproteolysis (Li et al. 2017). Moreover, the transcription of 112g42 and 78g136 was upregulated dramatically, of which the product of the gene 112g42 also belongs to the ProK family, while that of 78g136 belongs to the kexin family, which plays a significant role in post-translational modification in eukaryotes (Li et al. 2017).
Although the small GTPases, AoRab-7A and AoRab-2, have several conserved motifs, such as WDTAGQE, GNK, and ETSAK (Supplementary Fig. S1), they played different roles in mycelial growth, conidiation, trap formation, stress resistance, and virulence in A. oligospora. The ΔAoRab-7A mutants showed remarkable differences in the abovementioned phenotypic characteristics from the WT, while the ΔAoRab-2 mutants showed similar phenotypes to the WT. The observed differences between these two genes may be caused by the different pathways that these two genes regulate in membrane trafficking. Previous researches had shown that Rab-7A localizes to the vacuolar membranes (Abenza et al. 2012), suggesting that Rab-7A may have a role in vacuolar fusion and Rab-7A act as a key to lysosome biogenesis (Bucci et al. 2000). Rab-2 is a major participant in ER-to-Golgi membrane trafficking, but there is another Rab associated with the Golgi complex (Cheung et al. 2002; Zheng et al. 2015). This redundancy could help explain why the deleted AoRab-2 gene did not affect the phenotype of A. oligospora and other fungi. Moreover, the homologs of Rab-7A are more conserved than Rab-2 in filamentous fungi (Supplementary Table S3), which suggested that the function of Rab-7A is more conserved in the evolution of these fungi.
Based on our experimental results and previous reports, we propose the regulation pattern of AoRab-7A on phenotypic characteristics in A. oligospora (Fig. 7). AoRab-7A is a multifunctional regulator involved in the regulation of growth, conidiation, stress resistance, serine protease production, and trap formation in the fungus A. oligospora. In other words, AoRab-7A serves as a critical virulence determinant in A. oligospora, via regulating protein secretion and/or trap formation. However, its detailed regulation mechanism is still unclear and the functions of other Rab GTPases in A. oligospora are also needed in order to have a more complete understanding. In summary, the functions of two Rab GTPases AoRab-7A and AoRab-2 were characterized for the first time on the typical nematode-trapping fungus A. oligospora. Our results provided a basis for further understanding the signaling mechanism of mycelial differentiation, conidiation, trap formation, and pathogenicity in NTFs.
References
Abenza JF, Galindo A, Pinar M, Pantazopoulou A, de los Ríos V, Peñalva MA (2012) Endosomal maturation by Rab conversion in Aspergillus nidulans is coupled to dynein-mediated basipetal movement. Mol Biol Cell 23:1889–1901. https://doi.org/10.1091/mbc.E11-11-0925
Ahrén D, Tholander M, Fekete C, Rajashekar B, Friman E, Johansson T, Tunlid A (2005) Comparison of gene expression in trap cells and vegetative hyphae of the nematophagous fungus Monacrosporium haptotylum. Microbiology 151:789–803. https://doi.org/10.1099/mic.0.27485-0
Angelova MB, Pashova SB, Spasova BK, Vassilev SV, Slokoska LS (2005) Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat. Mycol Res 109:150–158. https://doi.org/10.1017/S0953756204001352
Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B (2000) Rab7: a key to lysosome biogenesis. Mol Biol Cell 11:467–480. https://doi.org/10.1091/mbc.11.2.467
Chen YL, Gao Y, Zhang KQ, Zou CG (2013) Autophagy is required for trap formation in the nematode-trapping fungus Arthrobotrys oligospora. Environ Microbiol Rep 5:511–517. https://doi.org/10.1111/1758-2229.12054
Cheung AY, Chen CYH, Glaven RH, De Graaf BH, Vidali L, Hepler PK, Wu HM (2002) Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14:945–962. https://doi.org/10.1105/tpc.000836
Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P (1992) Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119–122. https://doi.org/10.1016/0378-1119(92)90454-W
Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss R, Borkovich KA, Dunlap JC (2006) A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A 103:10352–10357. https://doi.org/10.1073/pnas.0601456103
Conesa A, Punt PJ, van Luijk N, van den Hondel CA (2001) The secretion pathway in filamentous fungi: a biotechnological view. Fungal Genet Biol 33:155–171. https://doi.org/10.1006/fgbi.2001.1276
Contento AL, Xiong Y, Bassham DC (2005) Visualization of autophagy in Arabidopsis using the fluorescent dye monodansylcadaverine and a GFP-AtATG8e fusion protein. Plant J 42:598–608. https://doi.org/10.1111/j.1365-313X.2005.02396.x
Espino JJ, Gutiérrez-Sánchez G, Brito N, Shah P, Orlando R, González C (2010) The Botrytis cinerea early secretome. Proteomics 10:3020–3034. https://doi.org/10.1002/pmic.201000037
Farnesi LC, Menna-Barreto RFS, Martins AJ, Valle D, Rezende GL (2015) Physical features and chitin content of eggs from the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus: connection with distinct levels of resistance to desiccation. J Insect Physiol 83:43–52. https://doi.org/10.1016/j.jinsphys.2015.10.006
Fekete C, Tholander M, Rajashekar B, Ahrén D, Friman E, Johansson T, Tunlid A (2008) Paralysis of nematodes: shifts in the transcriptome of the nematode-trapping fungus Monacrosporium haptotylum during infection of Caenorhabditis elegans. Environ Microbiol 10:364–375. https://doi.org/10.1111/j.1462-2920.2007.01457.x
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins M, Appel R, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker J (ed) The proteomics protocols handbook. Humana Press, Totowa, pp 571–607. https://doi.org/10.1385/1-59259-890-0:571
Grant CM (2001) Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol Microbiol 39:533–541. https://doi.org/10.1046/j.1365-2958.2001.02283.x
Gray NF (1984) Ecology of nematophagous fungi: comparison of the soil sprinkling method with the Baermann funnel technique in the isolation of endoparasites. Soil Biol Biochem 16:81–83. https://doi.org/10.1016/0038-0717(84)90131-7
Jiang D, Zhou J, Bai G, Xing X, Tang L, Yang X, Li J, Zhang KQ, Yang JK (2017) Random mutagenesis analysis and identification of a novel C2H2-type transcription factor from the nematode-trapping fungus Arthrobotrys oligospora. Sci Rep 7:5640. https://doi.org/10.1038/s41598-017-06075-5
Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, Mcwillian H, Maslen J, Mitchell A, Nuka G, Pesseat S (2014) InterProScan 5: genome-scale protein function classification. Bioinformatics 30:1236–1240. https://doi.org/10.1093/bioinformatics/btu031
Kikuma T, Arioka M, Kitamoto K (2007) Autophagy during conidiation and conidial germination in filamentous fungi. Autophagy 3:128–129. https://doi.org/10.4161/auto.3560
Krijgsheld P, Bleichrodt RV, Van Veluw GJ, Wang F, Müller WH, Dijksterhuis J, Wösten HAB (2013) Development in Aspergillus. Stud Mycol 74:1–29. https://doi.org/10.3114/sim0006
Li Y, Hyde KD, Jeewon R, Cai L, Vijaykrishna D, Zhang K (2005) Phylogenetics and evolution of nematode-trapping fungi (Orbiliales) estimated from nuclear and protein coding genes. Mycologia 97:1034–1046. https://doi.org/10.1080/15572536.2006.11832753
Li J, Gu F, Wu R, Yang J, Zhang KQ (2017) Phylogenomic evolutionary surveys of subtilase superfamily genes in fungi. Sci Rep 7:45456. https://doi.org/10.1038/srep45456
Liu Q, Ying SH, Feng MG (2011) Characterization of Beauveria bassiana neutral trehalase (BbNTH1) and recognition of crucial stress-responsive elements to control its expression in response to multiple stresses. Microbiol Res 166:282–293. https://doi.org/10.1016/j.micres.2010.04.001
Liu K, Zhang W, Lai Y, Xiang M, Wang X, Zhang X, Liu X (2014) Drechslerella stenobrocha genome illustrates the mechanism of constricting rings and the origin of nematode predation in fungi. BMC Genomics 15:114. https://doi.org/10.1186/1471-2164-15-114
Liu XH, Chen SM, Gao HM, Ning GA, Shi HB, Wang Y, Dong B, Qi YY, Zhang DM, Lu GD, Wang DH, Zhou J, Lin FC (2015) The small GTPase MoYpt7 is required for membrane fusion in autophagy and pathogenicity of Magnaporthe oryzae. Environ Microbiol 17:4495–4510. https://doi.org/10.1111/1462-2920.12903
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Luo X, Keyhani NO, Yu X, He Z, Luo Z, Pei Y, Zhang Y (2012) The MAP kinase Bbslt2 controls growth, conidiation, cell wall integrity, and virulence in the insect pathogenic fungus Beauveria bassiana. Fungal Genet Biol 49:544–555. https://doi.org/10.1016/j.fgb.2012.05.002
Meerupati T, Andersson KM, Friman E, Kumar D, Tunlid A, Ahrén D (2013) Genomic mechanisms accounting for the adaptation to parasitism in nematode-trapping fungi. PLoS Genet 9:e1003909. https://doi.org/10.1371/journal.pgen.1003909
Mitin N, Rossman KL, Der CJ (2005) Signaling interplay in Ras superfamily function. Curr Biol 15:R563–R574. https://doi.org/10.1016/j.cub.2005.07.010
Nordbring-Hertz B, Jansson H-B, Tunlid A (2011) Nematophagous fungi. In: Encyclopedia of life sciences. John Wiley & Sons, Chichester, pp 1–13. https://doi.org/10.1002/9780470015902.a0000374.pub3
Novick P, Zerial M (1997) The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol 9:496–504. https://doi.org/10.1016/S0955-0674(97)80025-7
Pantazopoulou A, Peñalva MA (2011) Characterization of Aspergillus nidulans RabC/Rab6. Traffic 12:386–406. https://doi.org/10.1111/j.1600-0854.2011.01164.x
Park HS, Yu JH (2012) Genetic control of asexual sporulation in filamentous fungi. Curr Opin Microbiol 15:669–677. https://doi.org/10.1016/j.mib.2012.09.006
Powers-Fletcher MV, Feng X, Krishnan K, Askew DS (2013) Deletion of the sec4 homolog srgA from Aspergillus fumigatus is associated with an impaired stress response, attenuated virulence and phenotypic heterogeneity. PLoS One 8:e66741. https://doi.org/10.1371/journal.pone.0066741
Punt PJ, Seiboth B, Weenink XO, van Zeijl C, Lenders M, Konetschny C, Ram AFJ, Montijn R, Kubicek CP, van den Hondel CAMJJ (2001) Identification and characterization of a family of secretion-related small GTPase-encoding genes from the filamentous fungus Aspergillus niger: a putative SEC4 homologue is not essential for growth. Mol Microbiol 41:513–525. https://doi.org/10.1046/j.1365-2958.2001.02541.x
Rajan N, Agarwal P, Patel K, Sanadhya P, Khedia J, Agarwal PK (2015) Molecular characterization and identification of target protein of an important vesicle trafficking gene AlRab7 from a salt excreting halophyte Aeluropus lagopoides. DNA Cell Biol 34:83–91. https://doi.org/10.1089/dna.2014.2592
Segev N, Mulholland J, Botstein D (1988) The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell 52:915–924. https://doi.org/10.1016/0092-8674(88)90433-3
Sewall TC, Mims CW, Timberlake WE (1990) AbaA controls phialide differentiation in Aspergillus nidulans. Plant Cell 2:731–739. https://doi.org/10.2307/3869172
Siriputthaiwan P, Jauneau A, Herbert C, Garcin D, Dumas B (2005) Functional analysis of CLPT1, a Rab/GTPase required for protein secretion and pathogenesis in the plant fungal pathogen Colletotrichum lindemuthianum. J Cell Sci 118:323–329. https://doi.org/10.1242/jcs.01616
Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E (1989) Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Rep 36:79–81. https://doi.org/10.4148/1941-4765.1519
Stenmark H, Olkkonen VM (2001) The Rab GTPase family. Genome Biol 2:3007–3001. https://doi.org/10.1186/gb-2001-2-5-reviews3007
Su H, Zhao Y, Zhou J, Feng H, Jiang D, Zhang KQ, Yang JQ (2017) Trapping devices of nematode-trapping fungi: formation, evolution, and genomic perspectives. Biol Rev 92:357–368. https://doi.org/10.1111/brv.12233
Takai Y, Sasaki T, Matozaki T (2001) Small GTP-binding proteins. Physiol Rev 81:153–208. https://doi.org/10.1016/S0074-7696(08)61861-6
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Tao L, Yu JH (2011) AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology 157:313–326. https://doi.org/10.1099/mic.0.044271-0
Thön M, Al-Abdallah Q, Hortschansky P, Brakhage AA (2007) The thioredoxin system of the filamentous fungus Aspergillus nidulans: impact on development and oxidative stress response. J Biol Chem 282:27259–27269. https://doi.org/10.1074/jbc.M704298200
Tian S, Zhang Z, Qin G (2014) Function of Rab GTPases in regulating the development, protein secretion and virulence of fungi. In: Prusky D, Gullino ML (eds) Post-harvest pathology. Springer, Cham, pp 3–9. https://doi.org/10.1007/978-3-319-07701-7_1
Tunlid A, Rosén S, Ek BO, Rask L (1994) Purification and characterization of an extracellular serine protease from the nematode-trapping fungus Arthrobotrys oligospora. Microbiology 140:1687–1695. https://doi.org/10.1099/13500872-140-7-1687
Tunlid A, Åhman J, Oliver RP (1999) Transformation of the nematode-trapping fungus Arthrobotrys oligospora. FEMS Microbiol Lett 173:111–116. https://doi.org/10.1111/j.1574-6968.1999.tb13491.x
Wang XX, He PH, Feng MG, Ying SH (2014) BbSNF1 contributes to cell differentiation, extracellular acidification, and virulence in Beauveria bassiana, a filamentous entomopathogenic fungus. Appl Microbiol Biotechnol 98:8657–8673. https://doi.org/10.1007/s00253-014-5907-0
Winston F, Dollard C, Ricupero-Hovasse SL (1995) Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53–55. https://doi.org/10.1002/yea.320110107
Yang JK, Huang X, Tian B, Wang M, Niu Q, Zhang KQ (2005) Isolation and characterization of a serine protease from the nematophagous fungus, Lecanicillium psalliotae, displaying nematicidal activity. Biotechnol Lett 27:1123–1128. https://doi.org/10.1007/s10529-005-8461-0
Yang JK, Wang L, Ji XL, Feng Y, Li XM, Zou CG, Xu JP, Ren Y, Mi QL, Wu JL, Liu SQ, Liu Y, Huang XW, Niu XM, Li J, Liang LM, Luo YL, Ji KF, Zhou W, Yu ZF, Li GH, Liu YJ, Li L, Qiao M, Feng L, Zhang KQ (2011) Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog 7:e1002179. https://doi.org/10.1371/journal.ppat.1002179
Yang E, Xu L, Yang Y, Zhang X, Xiang M, Wang C, An ZQ, Liu XZ (2012) Origin and evolution of carnivorism in the Ascomycota (fungi). Proc Natl Acad Sci U S A 109:10960–10965. https://doi.org/10.1073/pnas.1120915109
Yang JK, Liang L, Li J, Zhang KQ (2013) Nematicidal enzymes from microorganisms and their applications. Appl Microbiol Biotechnol 97:7081–7095. https://doi.org/10.1007/s00253-013-5045-0
Zhang L, Yang JK, Niu Q, Zhao X, Ye F, Liang L, Zhang KQ (2008) Investigation on the infection mechanism of the fungus Clonostachys rosea against nematodes using the green fluorescent protein. Appl Microbiol Biotechnol 78:983–990. https://doi.org/10.1007/s00253-008-1392-7
Zhang Z, Qin G, Li B, Tian S (2014) Knocking out Bcsas1 in Botrytis cinerea impacts growth, development, and secretion of extracellular proteins, which decreases virulence. Mol Plant-Microbe Interact 27:590–600. https://doi.org/10.1094/MPMI-10-13-0314-R
Zhao ML, Mo MH, Zhang KQ (2004) Characterization of a neutral serine protease and its full-length cDNA from the nematode-trapping fungus Arthrobotrys oligospora. Mycologia 96:16–22. https://doi.org/10.1080/15572536.2005.11832991
Zhao XY, Wang Y, Zhao Y, Huang Y, Zhang KQ, Yang JK (2014) Malate synthase gene AoMls in the nematode-trapping fungus Arthrobotrys oligospora contributes to conidiation, trap formation, and pathogenicity. Appl Microbiol Biotechnol 98:2555–2563. https://doi.org/10.1007/s00253-013-5432-6
Zheng H, Zheng W, Wu C, Yang J, Xi Y, Xie Q, Zhao X, Deng XL, Lu GD, Li GP, Ebbole D, Zhou J, Wang ZH (2015) Rab GTPases are essential for membrane trafficking-dependent growth and pathogenicity in Fusarium graminearum. Environ Microbiol 17:4580–4599. https://doi.org/10.1111/1462-2920.12982
Acknowledgements
We are grateful to Prof. Jianping Xu of the Dept. of Biology, McMaster University, for his valuable comments and critical discussions.
Funding
The research described here is jointly supported by the NSFC-Yunnan Joint Fund (U1402265), the National Basic Research Program of China (2013CB127503), the National Natural Science Foundation of China (approved nos. 31272093, 31360019, and 31560025), the Program for Excellent Young Talents of Yunnan University (to Jinkui Yang), and the General Program of the Applied Basic Research Programs of Yunnan Province (approval no. 2016FB044).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 1662 kb).
Rights and permissions
About this article
Cite this article
Yang, X., Ma, N., Yang, L. et al. Two Rab GTPases play different roles in conidiation, trap formation, stress resistance, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Appl Microbiol Biotechnol 102, 4601–4613 (2018). https://doi.org/10.1007/s00253-018-8929-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8929-1