Abstract
Malate synthase (Mls), a key enzyme in the glyoxylate cycle, is required for virulence in microbial pathogens. In this study, we identified the AoMls gene from the nematode-trapping fungus Arthobotrys oligospora. The gene contains 4 introns and encodes a polypeptide of 540 amino acids. To characterize the function of AoMls in A. oligospora, we disrupted it by homologous recombination, and the ΔAoMls mutants were confirmed by PCR and Southern blot analyses. The growth rate and colony morphology of the ΔAoMls mutants showed no obvious difference from the wild-type strains on potato dextrose agar (PDA) plate. However, the disruption of gene AoMls led to a significant reduction in conidiation, failure to utilize fatty acids and sodium acetate for growth, and its conidia were unable to germinate on minimal medium supplemented with sodium oleate. In addition, the trap formation was retarded in the ΔAoMls mutants, which only produced immature traps containing one or two rings. Moreover, the nematicidal activity of the ΔAoMls mutants was significantly decreased. Our results suggest that the gene AoMls plays an important role in conidiation, trap formation and pathogenicity of A. oligospora.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematophagous fungi are a heterogeneous group of organisms broadly distributed in terrestrial and aquatic ecosystems (Nordbring-Hertz et al. 2011; Zhang et al. 2011). Nematode-trapping fungi, one of the four nematophagous fungal groups (nematode-trapping fungi, endoparasitic fungi, toxin-producing fungi and opportunistic fungi), are capable of developing specific trapping devices (traps) such as adhesive networks, adhesive knobs, and constricting rings to capture nematodes and then extract nutrients from their nematode prey (Li et al. 2005; Nordbring-Hertz et al. 2011). Most nematode-trapping fungi can live as both saprophytes and parasites (Nordbring-Hertz et al. 2011; Yang et al. 2007b; Zhang et al. 2011). They play important roles in maintaining nematode population density in natural environments. The formation of traps by nematode-trapping fungi is an important indicator of their switch from the saprophytic to the predacious lifestyles (Yang et al. 2007b; Zhang et al. 2011). The trapping initiates a series of processes including adhesion, capture, penetration, and immobilization of nematodes (Nordbring-Hertz et al. 2011; Yang et al. 2007a, b). Previous studies have identified the morphological characteristics of major trapping devices, including their evolution and phylogenetic distribution (Li et al. 2005; Yang et al. 2007b; Yang et al. 2012). However, little is known about the molecular mechanism of trap formation in nematode-trapping fungi.
The glyoxylate cycle is an anaplerotic pathway of the tricarboxylic acid (TCA) cycle that allows organisms to grow on C2 compounds by bypassing the CO2-generating steps of the TCA cycle. The unique enzymes of this route are isocitrate lyase (Icl) and malate synthase (Mls; Dunn et al. 2009). Several plant and human pathogenic fungi and bacteria utilize the glyoxylate cycle during host infection (Lorenz and Fink 2001; Solomon et al. 2004; Dunn et al. 2009). For instance, the expression of gene Icl1 was upregulated significantly in conidia, appressoria, mycelia and hyphae during infection by Magnaporthe oryzae (Wang et al. 2003). Meanwhile, deletion of the M. oryzae Icl1 gene caused a reduction in appressorium formation, conidiogenesis and cuticle penetration, and an overall decrease in damage to leaves of rice and barley (Rauyaree et al. 2001; Wang et al. 2003). Moreover, expression analysis of the Mls1 gene in Stagonospora nodorum during its interaction with wheat showed an increased expression in ungerminated spores followed by a dramatic decrease in transcription after germination. Spores of an Mls1 null mutant inoculated onto wheat seedlings and leaves were unable to induce necrotic lesions on either tissue, indicating that this gene is essential for virulence of S. nodorum on wheat (Solomon et al. 2004). Recently, the glyoxylate cycle was also found to be involved in conidial germination, pathogenesis and saprobic growth of insect pathogenic fungi Metarhizium anisopliae and Beauveria bassiana (Padilla-Guerrero et al. 2011).
Arthrobotrys oligospora is one of the best-studied nematode-trapping fungi (Nordbring-Hertz 2004; Yang et al. 2011, 2013a). It captures nematodes using specialized traps (adhesive three-dimensional networks), and extracellular enzymes play a very important role during its infection against nematodes (Tunlid et al. 1994; Åhman et al. 2002; Yang et al. 2007a, 2013b). In the presence of nematodes and other inductive substances, A. oligospora enters the parasitic stage by forming traps to capture nematodes (Pramer and Stoll 1959; Nordbring-Hertz 2004; Hsueh et al. 2013). Recently, the genome of A. oligospora was sequenced, and proteomic analysis suggested that diverse cellular processes were activated during trap formation (Yang et al. 2011). Among them, two key enzymes (AoMls and AoIcl) in the glyoxylate cycle were up-regulated during A. oligospora trap formation (Yang et al. 2011), indicating that this pathway is also important for trap formation in nematode-trapping fungi. To further characterize the role of the glyoxylate cycle in A. oligospora, we disrupted the single copy gene AoMls in the glyoxylate cycle by homologous recombination, and investigated the effects of AoMls deletion on growth, conidiation, trap formation, and virulence.
Materials and methods
Strains, plasmids, and culture conditions
A. oligospora strain ATCC24927 used in this study was purchased from American Type Culture Collection (ATCC) and maintained on potato dextrose agar (PDA) at 26°. Saccharomyces cerevisiae (FY834) was used for recombinational cloning procedure and grown in YPD (10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose) or YPDA plates (YPD plus 16 g/L agar). SC-Ura plates (26.7 g/L drop-out base with glucose, 2 g/L drop-out mix synthetic minus uracil without yeast nitrogen base, 15 g/L agar) were used for selecting colonies of yeast strain FY834 harboring correctly recombined constructs (Park et al. 2011). Plasmid (pRS426; Christianson et al. 1992) was maintained in Escherichia coli strain DH5а (Takara, Shiga, Japan). Regeneration medium (PDASS, PDA supplying with 0.6 M sucrose, 0.3 g/L yeast extract, 0.3 g/L tryptone, 0.3 g/L peptone) was used for protoplast regeneration (Zhang et al. 2008), and PDASS supplemented with 200 μg/mL hygromycin B (Amresco, Solon, USA) was used for selecting transformants.
Caenorhabditis elegans (strain N2) was grown in oatmeal medium at 26 °C for 6–7 days, and the nematode extract was prepared according to our previous report (Yang et al. 2011).
Sequence and phylogenetic analysis of AoMls
The amino acid sequence of AoMsl (EGX45923) in A. oligospora was downloaded from the GenBank (http://www.ncbi.nlm.nih.gov/genbank/). The theoretical isoelectric point (pI) and molecular weight of AoMsl were calculated using the pI/MW tool (http://web.expasy.org/compute_pi/) (Gasteiger et al. 2005), and conserved functional domains were analyzed using InterProScan 4.8 (http://www.ebi.ac.uk/Tools/pfa/iprscan/) with default parameter settings (Zdobnov and Apweiler 2001). The amino acid sequence of AoMls was retrieved from National Center for Biotechnology Information (NCBI), and the sequences of Mls from different fungi were downloaded from GenBank. The amino acid sequences of Mls from different fungi were analyzed using the DNAman software package (Version 5.2.2, Lynnon Biosoft, St. Louis, Canada). A neighbor-joining tree was constructed using the Mega 5.1 software package (Tamura et al. 2011).
Quantitative PCR (qPCR) analysis
Primers were designed and synthesized based on the DNA sequences of AoMls and 18S rRNA gene (AJ001987; Table 1). A. oligospora was cultured and induced by nematode extract according to our previous report (Yang et al. 2011). Total RNA was extracted with Trizol Reagent (Invitrogen, Carlsbad, California), purified with a RNeasy Mini Kit (Qiagen, Valencia, California), and then reverse transcribed with PrimeScript RT reagent Kit (Takara, Shiga, Japan) by following the manufacturer’s instructions. qPCR was conducted according to our recent report (Yang et al. 2013a).
Deletion of the gene AoMls
The AoMls gene replacement construct was generated using a modified yeast cloning procedure (Colot et al. 2006; Park et al. 2011). Two fragments (1,833 and 1,719 bp) corresponding to the 5′ and 3′ of the gene AoMls were amplified with primer sets AoMls-5f/AoMls-5r and AoMls-3f/AoMls-3r (Table 1), respectively. Primers AoMls-5r and AoMls-3f have 50 bp tails homologous to the hygromycin cassette (hph) from the vector pCSN44 (Staben et al. 1989), whereas, the tails for AoMls-5f and AoMls-3r are homologous to the vector pRS426 (a gift from Prof. K.A. Borkovich, University of California; Christianson et al. 1992). The two fragments were co-transformed into yeast strain FY834 along with the hph cassette and gapped yeast shuttle vector (pRS426). Homologous recombination created the circular construct and the final disruption vector (pRS426-AoMls-hph) was recovered by transformation into E. coli DH5a.
Plasmid pRS426-AoMls-hph was transformed into A. oligospora by a protoplast-based protocol (Tunlid et al. 1999; Zhang et al. 2008) and the AoMls gene was deleted by double crossing-over homologous recombination. Protoplasts were prepared and transformed according to methods described previously (Tunlid et al. 1999; Zhang et al. 2008; Park et al. 2011). Hygromycin-resistant transformants were selected on PDASS containing 200 μg/mL hygromycin B (Zhang et al. 2008). The positive transformants were further confirmed by PCR and Southern blot analyses. Southern analysis was carried out according to the instructions provided by the North2South® chemiluminescent hybridization and detection kit (Pierce, Rockford, USA). The primer pair AoMls-S1/AoMls-S2 was used as Southern hybridization probe, and restriction enzyme XbaI was used to digest the genomic DNA of A. oligospora for Southern analysis.
Vegetative growth and conidia yield comparisons
The mycelial plugs of the ΔAoMls mutants and wild-type strains of the same size and age were incubated on PDA medium at 26 °C for 6–10 days, respectively, the growth rate and colony morphology were observed and determined. Similarly, the growth rate was compared in minimal medium (MM, 3.0 g/L NaNO3, 1.0 g/L K2HPO4⋅3H2O, 0.5 g/L KCl, 0.5 g/L MgSO4⋅7H2O, 0.01 g/L FeSO4⋅7H2O) supplemented with different sole carbon sources, such as glucose (0.2 %), sucrose (0.2 %), glycerol (0.2 %), sodium acetate (0.2 %), and sodium oleate (0.2 %). To assess sporulation capacity of WT and each mutant, 100 μL aliquots of conidial suspension were spread on PDA plates and incubated for 10 days at 26 °C, the conidia on 6 cm diameter disks were washed into 5 mL sterile water, followed by filtration through four layers of lens tissues to remove mycelial debris. The concentration of the conidial suspension was determined using microscopic counts on a haemocytometer (Xie et al. 2012).
To assay conidial germination rates of the strains, 50 μL aliquots of conidial suspension of ΔAoMls mutants and wild-type strains were incubated respectively on MM supplemented with different sole carbon sources, such as glucose (0.2 %), sucrose (0.2 %), glycerol (0.2 %), sodium acetate (0.2 %), and sodium oleate (0.2 %). The spores were incubated at 26 °C and the numbers of germinated conidia were determined at 4 and 12 h. The experiments were performed with three replicates.
Induction of trap formation
For analysis of trap formation in A. oligospora induced by nematodes and nematode extract, approximately 104 of conidia of wild-type strain and ΔAoMls mutant were inoculated onto PDA at 26 °C. After 4–5 days of growth, 80–100 adult nematodes or 0.2 mL nematode extract were added to the A. oligospora mycelia. After 12 h, mycelia and traps were observed at specified time intervals under light microscopy (Olympus, Tokyo, Japan). The experiments were performed with three replicates.
Virulence assays
The virulence of A. oligospora wild-type strain and ΔAoMls mutant against the nematode C. elegans were assayed as follows. Approximately 104 of conidia of the wild-type strain and ΔAoMls mutant were inoculated onto PDA at 26 °C for 4–5 days, respectively. Between 80 and 100 nematodes were added to each plate at 26 °C. At specified time intervals, the number of captured nematodes was counted using a light microscope. The bioassay was performed with three replicates.
Statistical analyses
Statistical analyses were performed using the SPSS package, version 13.0 for Windows (Chicago, Illinois, USA). Differences with P < 0.05 were regarded as statistically significant, P < 0.01 as highly statistically significant.
Results
Sequence and phylogenetic analyses of AoMls in A. oligospora
One single copy gene AoMls encoding the malate synthase Mls was identified from the A. oligospora genome, which contains four introns and encodes a polypeptide of 540 amino acids. Its predicted isoelectric point and molecular weight were 10.4 and 40.2, respectively. Interproscan analysis showed that AoMls shares a conserved Mls domain (IPR001465). The amino acid sequence of AoMls shared a high degree of identity to Mls from other filamentous fungi. For example, the AoMls shares 92.4 %, 79.6 %, 79.4 %, 79.8 %, 77.9 %, 79.2 %, and 77.6 % similarity to Mls from Dactylellina haptotyla (EPS36953), M. anisopliae (EFY97080), Trichoderma reesei (EGR47514), Neurospora crassa (XP_959453), M. oryzae (XP_003720926), Aspergillus fumigates (XP_747723), and Gibberella zeae (XP_388876), respectively. Phylogenetic analysis showed that these Mls from different fungi cluster in four clades (Fig. 1). Clade A contains Mls from Sordariomycetes, including the model fungus N. crassa, entomopathogens M. anisopliae and B. bassiana, plant pathogens Fusarium oxysporum, G. zeae, M. oryzae, and cellulolytic enzymes-producing fungus Trichoderma reesi. Clade B contains Mls from Botryotinia fuckeliana and Sclerotinia sclerotiorum, both species belong to Leotiomycetes. Clade C includes nine Mls, members of this clade come from Eurotiomycetes, including Neosartorya fischeri, Ajellomyces dermatitidis, Uncinocarpus reesii, Coccidioides immitis, Arthroderma otae, Trichophyton verrucosum, and species from Aspergillus. While AoMls has a distant evolutionary relationship with Mls from other filamentous fungi, it clusters with a homolog of Mls identified from another nematode-trapping fungus D. haptotyla (Andersson et al. 2013) into clade D, these two fungi belong to Orbiliomyces.
Phylogenetic analysis based on the deduced amino acid sequences of Mls from different filamentous fungi. The amino acid sequences of Mls proteins were aligned with Clustal X version 1.83 (Thompson et al. 1997), and MEGA 5.04 was used to construct a neighbor-joining tree (Tamura et al. 2011), including bootstrap analysis with 1,000 replicates. Numbers below nodes indicate the bootstrap value. The bar marker indicates the genetic distance, which is proportional to the number of amino acid substitutions. GenBank accession numbers are given in brackets, and the Candida dubliniensis Mls is used as an outgroup. a Sordariomycetes; b Leotiomycetes; c Eurotiomycetes; d Orbiliomyces
Expression levels of gene AoMls
The 18S rRNA gene was chosen as the endogenous control. Primer dimer formation and the integrity of amplification reactions were confirmed by agarose gel electrophoresis (1.5 % agarose) and melting curve analysis. In order to calculate the PCR efficiencies from AoMls and 18S rRNA genes, a standard dilution curve was performed in duplicates using serial dilutions of standard cDNA. The expression levels of AoMls gene increased by 2.5, 5.6, and 1.0-fold induced by nematode extract for 12, 24, and 36 h, respectively.
Screening and confirmation of the ΔAoMls mutant
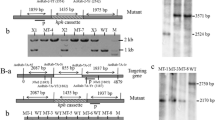
The disruption plasmid pRS426-AoMls-hph (Fig. 2a) was transformed into the protoplasts of A. oligospora, and yielded 35 transformants. Genomic DNA of these transformants was isolated and the site-specific insertion was confirmed using PCR. Compared to a 4,132-bp fragment from the wild-type strain of A. oligospora, a 4,987-bp fragment was amplified from three transformants (MT1, MT2, and MT3) using the primers AoMls-5f/AoMls-3r (Fig. 2c). In addition, these transformants were further confirmed using Southern blot analysis, the single band hybridizing to the AoMls gene probe was observed in the wild-type strains and transformants (Fig. 2b, d), and the sizes of the hybridized bands were consistent with expectation.
Deletion of AoMls gene. a The sketch map of replacement of targeting gene using homologous recombination method. b The restriction sites of XbaI and probe used for southern blot analysis were shown. c Transformants were confirmed by PCR method. W the wild-type strains, 1–3 three ΔAoMls mutants, P the positive plasmid, M marker (DL5000). d Southern analysis of the wild-type strains (WT) and ΔAoMls mutants; the genomic DNA was digested using XbaI
Effect of carbon sources on the growth and spores germination of the wild-type strain and ΔAoMls mutant
The growth rate and colony morphology of the wild-type strains and ΔAoMls mutants were compared on PDA medium, and no obvious difference in these traits was observed between them (Fig. 3a, c). However, the disruption of the AoMls gene led to a significant reduction in conidiation (Fig. 3d). To assess the roles of AoMls in the metabolism of carbon compounds or fatty acids, we tested the mycelial growth of the deletion strain on MM containing single carbon sources, such as sodium acetate, sodium oleate, glucose, sucrose, and glycerol. The mycelial growth of the wild-type strains and the mutants showed no obvious difference on the MM plate (MM supplemented with 1.8 % agar), which is consistent with their growth on PDA plate. Meanwhile, the ΔAoMls mutants showed no significant difference from the wild-type strains in liquid MM supplemented with on glucose, sucrose, and glycerol. However, the growths of ΔAoMls mutants were much slower than wild-type strains on liquid MM containing either sodium acetate or sodium oleate as the carbon source (data not shown).
Comparison on the growth, sporulation, spore germination, and nematicidal activity of the wild-type strain and ΔAoMls mutants. a The growth of fungi on PDA for 6 days at 26 °C—a the wild-type strains, b the ΔAoMls mutants. b Spore germination in MM—a germinated spore, b ungerminated spore. Bar 10 μm. c Comparison on mycelial growth on PDA plate. Gray the wild-type strains; yellow the ΔAoMls mutants. d Sporulation of the wild-type strains and ΔAoMls mutants. AO the wild-type strains; MT1, MT2, and MT3, the ΔAoMls mutants. e Comparison on the nematicidal activity. Gray the wild-type strains; white the ΔAoMls mutants. Error bars SD of the mean from three repeated assays. Statistical analyses were performed using the SPSS package, P < 0.01 versus wild-type strains; NS not significant
The conidia of the wild-type strains and ΔAoMls mutants were, respectively, incubated on the above carbon sources, and the percentage of germinating spores was determined by counting the number of spores that had produced hyphae at least the length of the spore after 4 h and 12 h. After 4 h, about 20 % of spores had germinated in the wild-type strains and mutants (Table 2, Fig. 3b). At 16 h, 92 % spores of the wild-type strains had germinated, whereas there was no significant increase in germination from the ΔAoMls mutants in MM (no carbon). The addition of either glucose or sucrose in MM had no significant effect on spore germination of the wild-type strains. In contrast, the addition of these sugars restored the germination rates of AoMls mutants to wild-type levels. The ability of the spores to germinate was also examined in the presence of the sodium acetate and sodium oleate. The germination of the wild-type strains had no effect on MM supplemented with sodium acetate, while only 10 % and 50 % of spores from mutants had germinated at 4 and 12 h under same condition, respectively. In addition, the germination of the wild-type strains and mutants was inhibited in MM supplemented with sodium oleate, with only 8 % and 47 % spores of the wild-type strains germinating at 4 and 12 h, respectively, while no increase in germination was observed for the ΔAoMls mutants at 12 h (Table 2).
Effect of ΔAoMls mutation on trap formation and nematicidal activity
Mycelial traps were observed on PDA plates about 12 h after the addition of nematodes or nematode extract. The wild-type strains produced three dimensional nets and captured nematodes (Fig. 4a, c), and all of the nematodes were captured and digested by the wild-type strains at 72 h. In contrast, the trap formation was retarded in the ΔAoMls mutants, and the mutants only produced immature traps containing one or two rings (Fig. 4b, d). Meanwhile, there were no significant differences in trap morphology between the two trap inducers (nematode and its extract) in either the wild-type strains or the mutant strains.
Comparison on trap formation and nematode capture between the wild-type strains and ΔAoMls mutants. a The wild-type strains. b The ΔAoMls mutants. c The wild-type strains induced by nematode extract and trap formation. d The ΔAoMls mutants induced by nematode extract and trap formation. The arrow showed the trapping devices. Bar 50 μm
The infection experiment was similarly initiated by adding nematodes to each plate. After the traps were formed, the nematodes were captured and gradually immobilized. As shown in Fig. 3e, the percentage of immobilized nematodes was significantly lower in the ΔAoMls mutants than in the wild-type strains. Thirty-six percent, 86 %, and 100 % nematodes were captured by the wild-type strains at 24, 48, and 72 h, respectively, while 17 %, 42 %, and 56 % nematodes were captured by the ΔAoMls mutant at those times, respectively. These results suggested that AoMls is important for the trap formation and virulence of A. oligospora.
Discussion
The glyoxylate cycle has been reported to involve in the regulation of conidial germination, utilization of fatty acids and pathogenicity of pathogenic fungi (e.g., Lorenz and Fink 2001; Solomon et al. 2004; Dunn et al. 2009; Padilla-Guerrero et al. 2011), and the key enzymes of this pathway are Mls and Icl (Dunn et al. 2009). Two hypothetical Icls (EGX47780 and EGX50704) and one Mls (EGX45923) were identified from the A. oligospora genome, and the expression levels of the encoding genes for AoIcl (EGX50704) and AoMls (EGX45923) were upregulated during A. oligospora trap formation (Yang et al. 2011), indicating that this pathway may play a role in trap formation in nematode-trapping fungi. Meanwhile, the transcriptional levels of the AoMls gene were determined in the wild-type strains by qPCR, and the result was consistent with our previous proteomic analysis. In this study, the single copy gene AoMls was selected to characterize the role of glyoxylate cycle in A. oligospora. AoMls shared a high degree of amino acid identity to Mls from other fungi, especially to Mls from another nematode-trapping fungus D. haptotyla (Andersson et al. 2013), suggested it may play a very important role in these filamentous fungi. However, the phylogenetic analysis showed AoMls to be evolutionary divergent from several other known fungal Mls (Fig. 1), consistent with the phylogenomic analysis that A. oligospora is phylogenetically distant from other filamentous fungi (Yang et al. 2011).
Although the ΔAoMls mutants cannot utilize sodium acetate and sodium oleate for growth, several carbon sources including glucose, sucrose and glycerol can restore the growth of the ΔAoMls mutants to wild-type strain levels in liquid MM. We guess the nutritional requirement of A. oligospora is not very demanding—this fungus can even grow on water agar plate, so any minor growth rate difference between the wild type and mutant strains might not be detected on MM plates. Moreover, the spore production capacity of ΔAoMls mutants decreased by 55 % (Fig. 3d) and the disruption of AoMls led to a significant reduction in germination of spores, with only 50 % spores germinated in sodium acetate medium at 12 h (Table 2) and the spores ΔAoMls mutants were unable to germinate on sodium oleate medium (Fig. 3b). Similarly, glucose, sucrose and glycerol restored the germination of spores. Our results showed that AoMls was involved in the utilization of acetate and fatty acid in A. oligospora. Similar phenomena have been reported in other pathogenic fungi. For example, the Mls1-deficient strain of S. nodorum had dramatically decreased spore germination and reduced hyphal length (Solomon et al. 2004). In the absence of any external carbon source, the ΔMls1 spores of S. nodorum were unable to germinate and consequently the mutants were non-pathogenic, while germination and pathogenicity could be restored by the addition of either glucose or sucrose, implying that S. nodorum is reliant upon the catabolism of lipids for infection (Solomon et al. 2004). This hypothesis is also supported by the observation that the ΔIcl1 mutant of Colletotrichum lagenarium failed to grow on acetate or fatty acids (Asakura et al. 2006). Recently, the deletion of GzIcl1 caused defects in growth on acetate and in perithecium (sexual fruiting body) formation but not in virulence on barley and wheat, indicating that GzIcl1 acts as the Icl of the glyoxylate cycle and is essential for self-fertility in G. zeae (Lee et al. 2009). Taken together, these results suggested that gluconeogenesis is both dependent on the glyoxylate cycle and is required for conidiation, spore germination and infection.
Previous studies have suggested that the glyoxylate cycle is likely involved in the formation of infection structures (such as appressoria) and affects the virulence of pathogenic fungi (e.g., Rauyaree et al. 2001; Wang et al. 2003; Solomon et al. 2004; Asakura et al. 2006). For example, Icl1 showed increased expression during development of infection structures and cuticle penetration, and the ΔIcl1 mutants of M. oryzae had delayed germination, infection-related development and cuticle penetration (Rauyaree et al. 2001; Wang et al. 2003). Recently, Padilla-Guerrero et al. (2011) reported that MaIcl was upregulated when M. anisopliae was grown in the presence of acetate, and MaIcl was upregulated when fungi were engulfed by insect haemocytes as well as during appressorium formation. Addition of the Icl inhibitor 3-nitroproprionate delayed conidial germination and inhibited appressorium formation. In this study, the trap formation was disturbed in the ΔAoMls mutants, and it only formed immature traps containing one or two rings (Fig. 4b, d). Mls1 is a key enzyme in the glyoxylate cycle and energy metabolism. Therefore, its deletion will impact many traits, including hyphal growth in filamentous fungi. Since trap is a specialized mycelium, it is not surprising that the ΔAoMls mutants showed deformed traps. Moreover, compared with the wild-type strains, the nematicidal activity of ΔAoMls mutants was significantly decreased (Figs. 3e and 4a). Our results thus demonstrate for the first time in nematode-trapping fungi the requirement of glyoxylate cycle for trap formation and pathogenicity.
Complicated relationships exist among different developmental and infection processes in pathogenic fungi (Jiang et al. 2013). First, all the biochemical reactions require energy. If the energy metabolism was blocked or repressed, the growth, secondary metabolite synthesis, sexual reproduction, conidiation and virulence of fungi will be all impaired (Li et al. 2012). Our previous analysis revealed that 90 genes were significantly up-regulated at the early stage of trap-formation in A. oligospora, and most of these genes were involved in translation, amino acid metabolism, carbohydrate metabolism, cell wall and membrane biogenesis (Yang et al. 2011), which suggested that there are multiple biological processes involved in trap formation. Recently, an autophagy-related gene atg8 was also suggested to be required for trap formation of A. oligospora. Disruption of the atg8 gene not only abolished the nematode-induced autophagy, but also suppressed trap formation and reduced pathogenicity for nematodes (Chen et al. 2013). Our results here elucidated the function of the gene AoMls in trap formation and pathogenicity of A. oligospora and provided a basis for further understanding the molecular mechanism of trap formation in nematode-trapping fungi.
References
Åhman J, Johansson T, Olsson M, Punt PJ, van den Hondel CA, Tunlid A (2002) Improving the pathogenicity of a nematode-trapping fungus by genetic engineering of a subtilisin with nematotoxic activity. Appl Environ Microbiol 68:3408–3415
Andersson KM, Meerupati T, Levander F, Friman E, Ahrén D, Tunlid A (2013) Proteome of the nematode-trapping cells of the fungus Monacrosporium haptotylum. Appl Environ Microbiol 79:4993–5004
Asakura M, Okuno T, Takano Y (2006) Multiple contributions of peroxisomal metabolic function to fungal pathogenicity in Colletotrichum lagenarium. Appl Environ Microbiol 72:6345–6354
Chen YL, Gao Y, Zhang KQ, Zou CG (2013) Autophagy is required for trap formation in the nematode-trapping fungus Arthrobotrys oligospora. Env Microbiol Rep 5:511–517
Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P (1992) Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119–122
Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC (2006) A highthroughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A 103:10352–10357
Dunn MF, Ramírez-Trujillo JA, Hernández-Lucas I (2009) Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 155:3166–3175
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM, Totowa NJ (eds) The proteomics protocols handbook. Humana Press, New York, pp 571–607
Hsueh YP, Mahanti P, Schroeder FC, Sternberg PW (2013) Nematode-trapping fungi eavesdrop on nematode pheromones. Curr Biol 23:83–86
Jiang DW, Zhu W, Wang YC, Sun C, Zhang KQ, Yang JK (2013) Molecular tools for functional genomics in filamentous fungi: Recent advances and new strategies. Biotechnol Adv 31:1562–1574
Lee SH, Han YK, Yun SH, Lee YW (2009) Roles of the glyoxylate and methylcitrate cycles in sexual development and virulence in the cereal pathogen Gibberella zeae. Eukaryot Cell 8:1155–1164
Li Y, Hyde KD, Jeewon R, Cai L, Vijaykrishna D, Zhang KQ (2005) Phylogenetics and evolution of nematode-trapping fungi (Orbiliales) estimated from nuclear and protein coding genes. Mycologia 97:1034–1046
Li G, Zhou X, Xu JR (2012) Genetic control of infection-related development in Magnaporthe oryzae. Curr Opin Microbiol 15:678–684
Lorenz MC, Fink GR (2001) The glyoxylate cycle is required for fungal virulence. Nature 412:83–86
Nordbring-Hertz B (2004) Morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora-an extensive plasticity of infection structures. Mycologist 18:125–133
Nordbring-Hertz B, Jansson HB, Tunlid A (2011) Nematophagous Fungi. doi:10.1002/9780470015902.a0000374.pub3
Padilla-Guerrero IE, Barelli L, González-Hernández GA, Torres-Guzmán JC, Bidochka MJ (2011) Flexible metabolism in Metarhizium anisopliae and Beauveria bassiana: role of the glyoxylate cycle during insect pathogenesis. Microbiology 157:199–208
Park G, Colot HV, Collopy PD, Krystofova S, Crew C, Ringelberg C, Litvinkova L, Altamirano L, Li L, Curilla S, Wang W, Gorrochotegui-Escalante N, Dunlap JC, Borkovich KA (2011) High-throughput production of gene replacement mutants in Neurospora crassa. Methods Mol Biol 722:179–189
Pramer D, Stoll NR (1959) Nemin: a morphogenic substance causing trap formation by predaceous fungi. Science 129:966–967
Rauyaree P, Choi W, Fang E, Blackmon B, Dean RA (2001) Genes expressed during early stages of rice infection with the rice blast fungus Magnaporthe grisea. Mol Plant Pathol 2:347–354
Solomon PS, Lee RC, Wilson TJ, Oliver RP (2004) Pathogenicity of Stagonospora nodorum requires malate synthase. Mol Microbiol 53:1065–1073
Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E (1989) Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Newsl 36:79–81
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acid Res 25:4876–4882
Tunlid A, Rosen S, Ek B, Rask L (1994) Purification and characterization of an extracellular serine protease from the nematode-trapping fungus Arthrobotrys oligospora. Microbiology 140:1687–1695
Tunlid A, Ahman J, Oliver RP (1999) Transformation of the nematode-trapping fungus Arthrobotrys oligospora. FEMS Microbiol Lett 173:111–116
Wang ZY, Thornton CR, Kershaw MJ, Debao L, Talbot NJ (2003) The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol Microbiol 47:1601–1612
Xie XQ, Li F, Ying SH, Feng MG (2012) Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS ONE 7:e30298
Yang JK, Tian BY, Liang LM, Zhang KQ (2007a) Extracellular enzymes and the pathogenesis of nematophagous fungi. Appl Microbiol Biotechnol 75:21–31
Yang Y, Yang EC, An ZQ, Liu XZ (2007b) Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on evidence from rRNA-encoding DNA and multiprotein sequences. Proc Natl Acad Sci U S A 104:8379–8384
Yang JK, Wang L, Ji XL, Feng Y, Li XM, Zou CG, Xu JP, Ren Y, Mi QL, Wu JL, Liu SQ, Liu Y, Huang XW, Wang HY, Niu XM, Li J, Liang LM, Luo YL, Ji KF, Zhou W, Yu ZF, Li GH, Liu YJ, Li L, Qiao M, Feng L, Zhang KQ (2011) Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog 7:e1002179
Yang EC, Xu LL, Yang Y, Zhang X, Xiang MC, Wang CS, An ZQ, Liu XZ (2012) Origin and evolution of carnivorism in the Ascomycota (fungi). Proc Natl Acad Sci U S A 109:10960–10965
Yang JK, Yu Y, Juan L, Zhu W, Geng ZY, Jiang DW, Wang YC, Zhang KQ (2013a) Characterization and functional analyses of the chitinase-encoding genes in the nematode-trapping fungus Arthrobotrys oligospora. Arch Microbiol 195:453–462
Yang JK, Liang LM, Li J, Zhang KQ (2013b) Nematicidal enzymes from microorganisms and their applications. Appl Microbiol Biotechnol 97:7081–7095
Zdobnov EM, Apweiler R (2001) InterProScan-an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17:847–848
Zhang L, Yang JK, Niu QH, Zhao XN, Ye FP, Liang LM, Zhang KQ (2008) Investigation on the infection mechanism of the fungus Clonostachys rosea against nematodes using the green fluorescent protein. Appl Microbiol Biotechnol 78:983–990
Zhang Y, Li GH, Zhang KQ (2011) A review on the research of nematophagous fungal species. Mycosystema 30:836–845
Acknowledgments
We are grateful to Prof. Jianping Xu of the Dept. Biology, McMaster University, for valuable comments and critical discussions. The research described here is jointly supported by the National Basic Research Program of China (2013CB127500), the National Natural Science Foundation of China (approved nos. 31272093 and 31360019), the West Light Foundation of the Chinese Academy of Sciences (to Jinkui Yang), and the China National Tobacco Corporation (110201002023). We also thank the anonymous reviewers for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xinying Zhao and Yunchuan Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, X., Wang, Y., Zhao, Y. et al. Malate synthase gene AoMls in the nematode-trapping fungus Arthrobotrys oligospora contributes to conidiation, trap formation, and pathogenicity. Appl Microbiol Biotechnol 98, 2555–2563 (2014). https://doi.org/10.1007/s00253-013-5432-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5432-6