Abstract
Sabinene is an important naturally occurring bicyclic monoterpene which can be used as flavorings, perfume additives, fine chemicals, and advanced biofuels. Up to now, this valuable terpene is commercially unavailable since there is no applicable manufacturing process. Microbial synthesis can be a promising route for sabinene production. In this review, we summarize knowledge about the metabolic pathway and key enzymes for sabinene biosynthesis. Recent advances that have been made in production of sabinene by microbial fermentation are highlighted. In these studies, researchers have identified the general synthetic pathway of sabinene from simple intermediate metabolites. Sabinene synthases of different origins were also cloned and characterized. Additionally, heterologous systems of the model microbes Escherichia coli and Saccharomyces cerevisiae were constructed to produce sabinene. This review also suggests new directions and attempts to gain some insights for achieving an industrial level production of sabinene. The combination of traditional molecular biology with new genome and proteome analysis tools will provide a better view of sabinene biosynthesis and a greater potential of microbial production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sabinene (C10H16, 4-methylene-1-(1-methylethyl)bicyclo[3.1.0]hexane) is a naturally occurring bicyclic unsaturated monoterpene, which exists as (+)- and (−)-enantiomers (Fig. 1). Sabinene can be emitted by a number of plants (Hakola et al. 2003) and has been found in different plant essential oils (Rossi et al. 2007). It is an important component in culinary spices for the special odor (Menon and Padmakumari 2005), as well as being used for perfume additives and fine chemicals. Sabinene possesses anti-fungal (Yamasaki et al. 2007) and anti-inflammatory (Valente et al. 2013) activities, thus playing a role in pharmaceutical industry. Due to its compact structure compared to ordinary hydrocarbons, sabinene has a high density and a high combustion heat. It shows the potential to serve as a feedstock for advanced biofuels (Peralta-Yahya et al. 2011; Renninger et al. 2008).
The chemical catalysis synthetic route of sabinene has been established by organic chemists (Urabe et al. 1997; Zaidlewicz and Gimiñska 1997). However, the complicated ring structure of this monoterpene makes it difficult to be produced by chemical processes. Sabinene could also be extracted from plants, but it requires considerable expenditure of natural resources since its content is low in plants (Woguem et al. 2013). Moreover, the occurrence of other similar terpenes makes the separation of this valuable monoterpene from natural sources complex and inefficient. Up to now, there is no commercial manufacturing method for sabinene. Therefore, the development of biosynthetic routes for the production of sabinene from renewable sugar is gaining interest. Compared with the traditional chemical synthesis and plant tissue extraction methods, microbial synthesis of sabinene offers many technical advantages, e.g., mild reaction conditions, negligible environmental pollution, and no requirement for arable land.
This review provides information on the biosynthesis and production of sabinene, which gives an alternative to conventional routes. In the following, we briefly summarize recent progresses in the metabolic pathway, key enzymes, and engineering approaches for the production of sabinene. Advancements of modern molecular biology techniques greatly enhance the engineering of microbes to produce this monoterpene. We hope that this review can give some implications to further improve current sabinene biosynthesis systems.
Metabolic pathway for sabinene biosynthesis
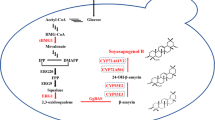
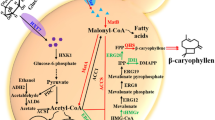
Like many other terpenoids, sabinene is synthesized from the isoprene unit. The key intermediates of this pathway are two common C5 precursors, isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP) (Guan et al. 2015). IPP and DMAPP can be produced from the mevalonate (MVA) pathway or the methylerythritol 4-phosphate (MEP) pathway (Vranová et al. 2013). The C5 backbone is responsible for biosynthesis of the C10 unit geranyl pyrophosphate (GPP), the direct precursor of sabinene. The metabolic pathway of sabinene from the general carbon source glucose is shown in Fig. 2.
Metabolic pathway for sabinene biosynthesis. Enzymes involved in the MEP pathway include the following: Dxs, 1-deoxy-D-xylulose-5-phosphate synthase; Dxr, 1-deoxy-D-xylulose 5-phosphate reductoisomerase; IspD, 4-pyrophosphocytidyl-2-methylerythritol synthase; IspE, 4-pyrophosphocytidyl-2-methylerythritol kinase; IspF, 2-methylerythritol 2,4-cyclopyrophosphate synthase; IspG, 4-hydroxy-3-methylbut-2-enyl pyrophosphate synthase; IspH, 1-hydroxy-2-methyl-butenyl 4-pyrophosphate reductase. Enzymes involved in the MVA pathway include the following: MvaE, acetyl-CoA acetyltransferase/HMG-CoA reductase; MvaS, HMG-CoA synthase; MK, MVA kinase; PMK, phosphomevalonate kinase; MVD, MVA 5-pyrophosphate decarboxylase. IPI, isopentenyl pyrophosphate isomerase; GPPS, GPP synthase; SabS, sabinene synthase
The MVA pathway starts from the central metabolite acetyl-CoA. Two molecules of acetyl-CoA are condensed by acetoacetyl-CoA thiolase (AACT) to generate acetoacetyl-CoA, and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) synthase further adds one acetyl-CoA to acetoacetyl-CoA, resulting HMG-CoA (Bach et al. 1990). Then, HMG-CoA is reduced by the enzyme HMG-CoA reductase (HMGR) to yield MVA. In certain bacteria, the AACT and HMGR are encoded by a dual-function polypeptide (Hedl et al. 2002; Yang et al. 2012). In the next steps, MVA is sequentially phosphorylated by MVA kinase (MK) and phosphomevalonate kinase (PMK), which convert MVA to MVA 5-pyrophosphate (MVAPP). Finally, MVAPP decarboxylase (MVD) catalyzes the decarboxylation of MVAPP to IPP (Pérez-Gil and Rodríguez-Concepción 2013). The MVA pathway is present in the cytosol of plants, animals, fungi, archaea, and some Gram-positive bacteria.
In the 1990s, a non-MVA pathway for the early steps of terpenoid biosynthesis was identified (Rohmer et al. 1993). Using 13C isotope-labeled substrates, the C5 framework of the isoprene unit was found to be synthesized from the condensation of pyruvate and a triose phosphate. By the joint effects of many different labs, this novel pathway was completely elucidated (Lichtenthaler et al. 1997; Rohdich et al. 2002). It starts from a transketolase-type condensation of two metabolites of glycolysis, pyruvate, and glyceraldehyde-3-phosphate, forming 1-deoxy-D-xylulose 5-phosphate (DXP), followed by the rearrangement and reduction of DXP to MEP. Since MEP and DXP are the key intermediates, it is termed as MEP pathway or DXP pathway. MEP is then converted to 2-methylerythritol-2,4-cycloyrophosphate (cMEPP) under the sequential action of three enzymes. The ring of cMEPP is reductively opened to 1-hydroxy-2-methyl-2-butenyl-4-pyrophosphate (HMBPP). Finally, HMBPP is further reduced to DMAPP by a reductase (Adam et al. 2002; Partow et al. 2012). The MEP pathway is generally found in most bacteria and chloroplasts of plants.
The two pathways are quite different in terms of precursors, reaction steps, and coenzyme consumption. Figure 3 shows the overall stoichiometries of conversing glucose to IPP/DMAPP through glycolysis (Steinbüchel 2003). MEP pathway has a higher theoretical yield with only one glucose molecule required for DMAPP/IPP synthesis compared with 1.5 glucose molecules required by the MVA pathway. In contrast, MVA pathway is more energetically favorable as it has a net gain in NAD(P)H reducing equivalents, and MEP pathway requires ATP and NAD(P)H for metabolic balance (Gruchattka et al. 2013). Therefore, MVA pathway could be coupled with other biosynthesis process for NAD(P)H recycling.
The IPP/DMAPP ratio is critical for subsequential terpenoid biosynthesis, and IPP isomerase (IPI) could catalyze the reversible inter-conversion of IPP and DMAPP (Ramos-Valdivia et al. 1997). After the synthesis of the C5 units IPP and DMAPP either by the MVA or MEP pathways, the next step for sabinene biosynthesis is chain elongation, which is called the prenyl pyrophosphate pathway. GPP is generated from the head-to-tail condensation of the IPP with DMAPP, catalyzed by GPP synthase (Jongedijk et al. 2016). GPP is the direct precursor for all monoterpenoids. GPP can be further elongated through the prenyl pyrophosphate pathway to generate farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which can be used for the synthesis of sesquiterpenoids and diterpenoids (Majdi et al. 2016).
Sabinene synthase—the key enzyme for sabinene biosynthesis
The enzymatic cyclization of the precursor GPP leading to monoterpene has been well characterized in different organisms (Degenhardt et al. 2009). Among them, sabinene synthase (SabS) catalyzes the committed step for sabinene biosynthesis through a polycyclization reaction. A potential mechanism for sabinene cyclization has been postulated and is shown in Fig. 4 (Peters and Croteau 2003). GPP is first isomerized to form 3R-linalyl pyrophosphate by the rotation of the C2-C3 single bond to the cisoid conformer. 3R-linalyl pyrophosphate promotes electrophilic attack on the C6-C7 double bond by C1, resulting in the carbocationic intermediate. Further cyclization of this key intermediate leads to the formation of sabinene (Adam and Croteau 1998; Wise et al. 1998).
A number of plants and fungi have the ability to emit sabinene. However, only nine SabSs have been cloned and characterized from plants so far (Table 1). These plant sources include common sage (Salvia officinalis), Salvia pomifera, rough lemon (Citrus jambhiri), Citrus unshiu, sitka spruce (Picea sitchensis), Thuja plicata, Hedychium coronarium, and Murraya koenigii. Amino acid sequence alignment of the nine known SabSs is shown in Fig. 5. These SabSs show 55.37% identity to each other. Similar to many other terpene cyclases, all the nine SabSs have a highly conserved prenyl pyrophosphate substrate binding site with an aspartate-rich DDXXD motif, which is responsible for the coordination of divalent cations (Starks et al. 1997). The N-terminal RR(X)8W motif essential for the enzymatic activity of many monoterpene synthases is also found in the SabSs (Bohlmann et al. 1998). In addition, a conserved GTXXEL/I sequence adjacent to DDXXD might also participate in the enzymatic catalysis process.
Amino acid sequence alignment of nine sabinene synthases. SoSabS, SabS of S. officinalis; SpSabS, SabS of S. pomifera; CjSabS, SabS of C. jambhiri; CuSabS, SabS of C. unshiu; PsSabS1, SabS of P. sitchensis susceptible to weevil, PsSabS2, SabS of P. sitchensis with weevil resistance, TpSabS, SabS of T. plicata, HcSabS, SabS of H. coronarium, MkSabS, Murraya koenigii. The conserved motifs are marked by rectangles
Although sabinene is the primary product of all the SabSs, a variety of similar monoterpenes can also be generated as the by-products during sabinene biosynthesis, which include α-pinene (Kampranis et al. 2007), myrcene (Foster et al. 2013), and 3-carene (Roach et al. 2014). It has the potential to further improve the SabSs’ selectivity towards sabinene. Protein engineering is an important means to enhance the catalytic activity and specificity of enzymes. However, the structures of these SabSs have not been resolved up to now. Advancements in understanding the reaction mechanism (Kampranis et al. 2007; Roach et al. 2014) would enable to develop mutant SabSs with improved activity and selectivity. Moreover, directed evolution which has been applied for engineering of other terpene synthases (Furubayashi et al. 2014; Tashiro et al. 2016) might also be employed to enhance the catalytic activity of SabSs.
Biotechnological production of sabinene
A variety of microorganisms have been investigated for sabinene production. These microorganisms include fungi, bacteria, and yeast. In addition, cell-free production of sabinene was also achieved with encouraging results. Recent advances of sabinene production using different systems are summarized in Table 2 and described below.
Natural producers and mutant strains
Although SabSs were mainly identified in plants, several microbial species could also produce this valuable monoterpene. For instance, an endophytic fungus, Phomopsis sp. EC-4, showed the ability to emit sabinene as a primary component of its volatile organic compounds (VOCs). Meanwhile, isopentanol, isobutanol, and phenylethanol were also detected in the mixture of volatiles. The total measurable VOC production was only 18.4 ppmv, representing a very low sabinene concentration (Singh et al. 2011). Another endophytic fungus Hypoxylon sp. CI-4 could also synthesize a number of terpenes as its VOCs. The traditional random mutation and screening method has been very effective for isolating strains which could synthesize novel compounds despite the considerable amount of time and resources it demands. Therefore, the wild-type strain was treated by two chemical mutagens, suberoylanilide hydroxamic acid and 5-azacytidine, and the resulting variants were able to produce an array of terpenes, such as sabinene, α-thujene, γ-terpinene, α-terpinolene, and β-selinene. However, sabinene was not the major component among all the VOCs (Ul-Hassan et al. 2012). To make these producing systems economically feasible, further work is required to increase the productivity and reduce the by-products. Up to now, the genetic engineering technique for these strains is not yet established. It is urgent to develop genetic modification tools for these fungi in spite of the diverse genomics and biochemical machinery (Deng et al. 2017).
Engineered strains
Escherichia coli is a good candidate for the production of value-added chemicals (Cao et al. 2015). It was extensively studied to biosynthesize terpenoids due to the easiness of genetic modifications and vast molecular resources. Reiling et al. (2004) first constructed an engineered E. coli strain for the production of sabinene. Three key enzymes in the precursor-supplying pathway including the native DXS, IPI from Haematococcus pluvialis and mutant of the native FPP synthase were co-expressed to enhance the intracellular GPP pool. A cyclase from Picea abies was further employed to generate monoterpene. Due to its poor enzymatic activity towards sabinene, the major product in this system was 3-carene while sabinene accounted for only 5.9% of the dominant products. E. coli utilizes a native MEP pathway to synthesize IPP and DMAPP. The MEP pathway has a higher theoretical yield, but it is tightly regulated by the hosts. It has been demonstrated that a heterologous MVA pathway was more effective to increase the precursor supply for the production of terpenoids in E. coli (Boronat and Rodríguez-Concepción 2015; Martin et al. 2003). To construct a heterologous system for sabinene production with high specificity, we introduced the SabS of S. pomifera into E. coli. By assembling the biosynthetic pathway using both the MEP and MVA pathways combined with the GPP synthase genes, sabinene production was achieved with a maximum titer of 82.18 mg/L under shake-flask conditions. Fed-batch fermentation of this engineered strain using the optimized culture medium and process conditions further increased sabinene production to a concentration of 2.65 g/L with a yield on glycerol of 3.49% (Zhang et al. 2014). The MVA pathway can be divided into the upper proportion (from acetyl-CoA to MVA) and the lower proportion (from MVA to IPP). By using MVA as the feeding substrate for sabinene production, sabinene titer was significantly improved to 150 mg/L by shake-flask fermentation (Liu et al. 2017). The strategy of feeding MVA did not require the upper proportion of MVA pathway. The reduction of heterologous overexpressed genes could release the burden to the host and avoid accumulation of MVA in the fermentation broth, thus leading to an enhanced sabinene production.
Sabinene production was also accomplished using the yeast Saccharomyces cerevisiae. Generally speaking, S. cerevisiae was not a good candidate for monoterpene production. The endogenous FPP synthase (encoded by Erg20) of S. cerevisiae is more favorable to synthesize FPP from IPP and DMAPP which might limit the precursor pool of monoterpene. Two of the Erg20 variants, Erg20K197G and Erg20F96W-N127W, were found to show more GPP synthase activity than FPP synthase activity. The intracellular GPP levels were greatly enhanced by the FPP synthase mutants and controlling endogenous Erg20 expression, coupled with increasing the expression of the MVA pathway (Zhao et al. 2016). The SabS from S. pomifera was also selected to be expressed in S. cerevisiae using a galactose-inducible expression vector, and 0.5 mg/L of sabinene was produced in a shake-flask culture. Erg20p was engineered into a GPP synthase and a 340-fold increase in sabinene yield was achieved, that is 17.5 mg/L (Ignea et al. 2014).
As discussed above, common strategies in engineering sabinene production are to overexpress MVA or MEP pathway enzymes along with the SabSs. Tuning of the expression levels and catalytic specificity of GPP synthase could greatly enhance sabinene production, indicating that this enzyme is a second committed step for sabinene biosynthesis in addition to the SabSs. However, overexpression of too many genes in multiple plasmids is a huge metabolic burden on the host cells, which might also decrease the cell growth rate. Chromosome integration of these genes might be a promising approach to achieve a stable and long-term sabinene-producing system (Yang et al. 2013).
Cell-free systems
In vitro biocatalytic systems have been greatly advanced along with the development of synthetic biology, which provides an alternative approach to biosynthesis of value-added products. Compared with the traditional producing systems, these cell-free systems are attractive due to their reduced complexity as well as not requiring cell viability (Billerbeck et al. 2013). In a recent study, a complex cell-free system comprising 27 enzymes was designed for the conversion of glucose into monoterpenes that generated both NAD(P)H and ATP by a modified glucose breakdown module and utilized these cofactors for building terpenes. Different monoterpenes were produced in this system by changing the terpene synthases. Among them, sabinene titer reached 15.9 g/L with a yield of 94.5% of theoretical limit, which was the highest report up to now (Korman et al. 2017). Nevertheless, cell-free systems are still at its infant stage and further research is required to reach an industrial application. Two major factors hampering cell-free biosynthesis are the costly coenzymes/cofactors and the stability of the enzymes (Ullah et al. 2016). The coenzymes/cofactors can be recycled to maintain a balance by pathway design, and immobilization strategies can be employed to develop stable and reusable enzymes.
Future perspectives: challenges and opportunities
In recent years, naturally occurring terpenoids have attracted considerable interest due to their extensive applications in a variety of fields. Many research studies focus on the applications of these terpenoids in therapeutic and medical applications (Jaeger and Cuny 2016). Monoterpenes represent a large class of terpenes that consist of two isoprene units. They can be classified into acyclic monoterpenes, monocyclic monoterpenes, and bicyclic monoterpenes (Koziol et al. 2014). Figure 6 shows the structures of several representative monoterpenes. These compounds have aroused widespread concerns for their unique characteristics. As an important monoterpene, the increasing demands of sabinene and its limited sources have led to an extensive search for efficient and economical manufacturing routes. Microorganism-based processes seem to be a reliable, economically attractive source of sabinene and can provide an efficient way for large-scale production.
However, the biosynthesis and production of sabinene still face the following challenges. Microbes can suffer from the presence of sabinene due to its anti-microbial properties (Asili et al. 2010). This would require increasing the product tolerance of the corresponding strains, and promising results have been obtained for similar monoterpene-producing strains (Brennan et al. 2015; Tomko and Dunlop 2015). In addition, two-phase fermentation could be employed to alleviate toxicity of sabinene to microorganisms by capturing the product from the culture. Up to now, there is no separation and purification technique for sabinene. Sabinene is a volatile hydrocarbon. It would be taken by the off-gas especially for aerobic fermentation. The recovery of sabinene produced by microbes could draw on the experience of the apparatus for the purification of bio-isoprene (Feher et al. 2013).
Biosynthesis offers new opportunities and good perspectives for sabinene production. Attempts at elucidating the metabolic pathway and key enzymes for sabinene biosynthesis have met with mixed success. Although the sabinene yields obtained in current studies remain low, they demonstrate the possibility of employing genetic manipulation to modify the metabolic pathway, which might result in a further improvement in sabinene productivity. This review can serve as the basis for the construction of much more robust strains for sabinene production in the future. Now, we cannot accomplish an industrial level sabinene production, but different strategies such as fermentation engineering, enzyme engineering, and cell engineering can be adopted to finally make the microbial sabinene-producing system economically feasible. Advances in genome and proteome analysis tools (Khairy et al. 2016) will also guide the engineering of sabinene production strains. Moreover, recent research progresses summarized here would contribute to the microbial production of other polycyclic terpenes. We hope this review on the biosynthesis of sabinene could provide useful information to the field of terpenes production.
References
Adam KP, Croteau R (1998) Monoterpene biosynthesis in the liverwort Conocephalum conicum: demonstration of sabinene synthase and bornyl diphosphate synthase in honour of Professor G. H. Neil Towers 75th birthday. Phytochemistry 49(2):475–480. https://doi.org/10.1016/S0031-9422(97)00741-3
Adam P, Hecht S, Eisenreich WG, Kaiser J, Grawert T, Arigoni D, Bacher A, Rohdich F (2002) Biosynthesis of terpenes: studies on 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase. Proc Natl Acad Sci U S A 99(19):12108–12113. https://doi.org/10.1073/pnas.182412599
Asili J, Emami SA, Rahimizadeh M, Fazly-Bazzaz BS, Hassanzadeh MK (2010) Chemical and antimicrobial studies of Juniperus Sabina L. and Juniperus foetidissima Willd. essential oils. J Essent Oil Bear Plants 13(1):25–36. https://doi.org/10.1080/0972060X.2010.10643787
Bach TJ, Weber T, Motel A (1990) Some properties of enzymes involved in the biosynthesis and metabolism of 3-hydroxy-3-methylglutaryl-CoA in plants. In: Towers GHN, Stafford HA (eds) Biochemistry of the mevalonic acid pathway to terpenoids. Springer US, Boston, pp 1–82. https://doi.org/10.1007/978-1-4684-8789-3_1
Billerbeck S, Härle J, Panke S (2013) The good of two worlds: increasing complexity in cell-free systems. Curr Opin Biotechnol 24(6):1037–1043. https://doi.org/10.1016/j.copbio.2013.03.007
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci U S A 95(8):4126–4133. https://doi.org/10.1073/pnas.95.8.4126
Boronat A, Rodríguez-Concepción M (2015) Terpenoid biosynthesis in prokaryotes. In: Schrader J, Bohlmann J (eds) Biotechnology of isoprenoids. Springer International Publishing, Cham, pp 3–18
Brennan TCR, Williams TC, Schulz BL, Palfreyman RW, Krömer JO, Nielsen LK (2015) Evolutionary engineering improves tolerance for replacement jet fuels in Saccharomyces cerevisiae. Appl Environ Microbiol 81(10):3316–3325. https://doi.org/10.1128/aem.04144-14
Cao Y, Niu W, Guo J, Xian M, Liu H (2015) Biotechnological production of 1,2,4-butanetriol: an efficient process to synthesize energetic material precursor from renewable biomass. Sci Rep 5(1):18149. https://doi.org/10.1038/srep18149
Degenhardt J, Köllner TG, Gershenzon J (2009) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70(15):1621–1637. https://doi.org/10.1016/j.phytochem.2009.07.030
Deng Y, Zhang X, Zhang X (2017) Recent advances in genetic modification systems for Actinobacteria. Appl Microbiol Biotechnol 101(6):2217–2226. https://doi.org/10.1007/s00253-017-8156-1
Feher FJ, Kan JK, Mcauliffe JC, Mccall TF, Rodewald S, Sabo TA, Wong TH, Ploetz CD, Pickert LJ (2013) Purification of isoprene from renewable resources. US Patent 8569562
Foster AJ, Hall DE, Mortimer L, Abercromby S, Gries R, Gries G, Bohlmann J, Russell J, Mattsson J (2013) Identification of genes in Thuja plicata foliar terpenoid defenses. Plant Physiol 161(4):1993–2004. https://doi.org/10.1104/pp.112.206383
Furubayashi M, Ikezumi M, Kajiwara J, Iwasaki M, Fujii A, Li L, Saito K, Umeno D (2014) A high-throughput colorimetric screening assay for terpene synthase activity based on substrate consumption. PLoS One 9(3):e93317. https://doi.org/10.1371/journal.pone.0093317
Gruchattka E, Hädicke O, Klamt S, Schütz V, Kayser O (2013) In silico profiling of Escherichia coli and Saccharomyces cerevisiae as terpenoid factories. Microb Cell Factories 12(1):84. https://doi.org/10.1186/1475-2859-12-84
Guan Z, Xue D, Abdallah II, Dijkshoorn L, Setroikromo R, Lv G, Quax WJ (2015) Metabolic engineering of Bacillus subtilis for terpenoid production. Appl Microbiol Biotechnol 99(22):9395–9406. https://doi.org/10.1007/s00253-015-6950-1
Hakola H, Tarvainen V, Laurila T, Hiltunen V, Hellén H, Keronen P (2003) Seasonal variation of VOC concentrations above a boreal coniferous forest. Atmos Environ 37(12):1623–1634. https://doi.org/10.1016/S1352-2310(03)00014-1
Hall DE, Robert JA, Keeling CI, Domanski D, Quesada AL, Jancsik S, Kuzyk MA, Hamberger B, Borchers CH, Bohlmann J (2011) An integrated genomic, proteomic and biochemical analysis of (+)-3-carene biosynthesis in Sitka spruce (Picea sitchensis) genotypes that are resistant or susceptible to white pine weevil. Plant J 65(6):936–948. https://doi.org/10.1111/j.1365-313X.2010.04478.x
Hedl M, Sutherlin A, Wilding EI, Mazzulla M, McDevitt D, Lane P, Burgner JW, Lehnbeuter KR, Stauffacher CV, Gwynn MN, Rodwell VW (2002) Enterococcus faecalis acetoacetyl-coenzyme A thiolase/3-hydroxy-3-methylglutaryl-coenzyme A reductase, a dual-function protein of isopentenyl diphosphate biosynthesis. J Bacteriol 184(8):2116–2122. https://doi.org/10.1128/jb.184.8.2116-2122.2002
Ignea C, Pontini M, Maffei ME, Makris AM, Kampranis SC (2014) Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth Biol 3(5):298–306. https://doi.org/10.1021/sb400115e
Jaeger R, Cuny E (2016) Terpenoids with special pharmacological significance: a review. Nat Prod Commun 11(9):1373–1390
Jongedijk E, Cankar K, Buchhaupt M, Schrader J, Bouwmeester H, Beekwilder J (2016) Biotechnological production of limonene in microorganisms. Appl Microbiol Biotechnol 100(7):2927–2938. https://doi.org/10.1007/s00253-016-7337-7
Kampranis SC, Ioannidis D, Purvis A, Mahrez W, Ninga E, Katerelos NA, Anssour S, Dunwell JM, Degenhardt J, Makris AM, Goodenough PW, Johnson CB (2007) Rational conversion of substrate and product specificity in a Salvia monoterpene synthase: structural insights into the evolution of terpene synthase function. Plant Cell 19(6):1994–2005. https://doi.org/10.1105/tpc.106.047779
Khairy H, Meinert C, Wübbeler JH, Poehlein A, Daniel R, Voigt B, Riedel K, Steinbüchel A (2016) Genome and proteome analysis of Rhodococcus erythropolis MI2: elucidation of the 4,4′-dithiodibutyric acid catabolism. PLoS One 11(12):e0167539. https://doi.org/10.1371/journal.pone.0167539
Kohzaki K, Gomi K, Yamasaki-Kokudo Y, Ozawa R, Takabayashi J, Akimitsu K (2009) Characterization of a sabinene synthase gene from rough lemon (Citrus jambhiri). J Plant Physiol 166(15):1700–1704. https://doi.org/10.1016/j.jplph.2009.04.003
Korman TP, Opgenorth PH, Bowie JU (2017) A synthetic biochemistry platform for cell free production of monoterpenes from glucose. Nat Commun 8:15526. https://doi.org/10.1038/ncomms15526
Koziol A, Stryjewska A, Librowski T, Salat K, Gawel M, Moniczewski A, Lochynski S (2014) An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini-Rev Med Chem 14(14):1156–1168
Lichtenthaler HK, Rohmer M, Schwender J (1997) Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol Plant 101(3):643–652. https://doi.org/10.1111/j.1399-3054.1997.tb01049.x
Liu H, Cheng T, Zou H, Zhang H, Xu X, Sun C, Aboulnaga E, Cheng Z, Zhao G, Xian M (2017) High titer mevalonate fermentation and its feeding as a building block for isoprenoids (isoprene and sabinene) production in engineered Escherichia coli. Process Biochem 62:1–9. https://doi.org/10.1016/j.procbio.2017.07.021
Majdi M, Ashengroph M, Abdollahi MR (2016) Sesquiterpene lactone engineering in microbial and plant platforms: parthenolide and artemisinin as case studies. Appl Microbiol Biotechnol 100(3):1041–1059. https://doi.org/10.1007/s00253-015-7128-6
Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21(7):796–802. https://doi.org/10.1038/nbt833
Meena S, Rajeev Kumar S, Dwivedi V, Singh A, Chanotiya C, Akhtar M, Kumar K, Shasany A, Nagegowda D (2017) Transcriptomic insight into terpenoid and carbazole alkaloid biosynthesis, and functional characterization of two terpene synthases in curry tree (Murraya koenigii). Sci Rep 7:44126. https://doi.org/10.1038/srep44126
Menon AN, Padmakumari K (2005) Studies on essential oil composition of cultivars of black pepper (Piper nigrum L.) - V. J Essent Oil Res 17(2):153–155. https://doi.org/10.1080/10412905.2005.9698862
Partow S, Siewers V, Daviet L, Schalk M, Nielsen J (2012) Reconstruction and evaluation of the synthetic bacterial MEP pathway in Saccharomyces cerevisiae. PLoS One 7(12):e52498. https://doi.org/10.1371/journal.pone.0052498
Peralta-Yahya PP, Ouellet M, Chan R, Mukhopadhyay A, Keasling JD, Lee TS (2011) Identification and microbial production of a terpene-based advanced biofuel. Nat Commun 2:483. https://doi.org/10.1038/ncomms1494
Pérez-Gil J, Rodríguez-Concepción M (2013) Metabolic plasticity for isoprenoid biosynthesis in bacteria. Biochem J 452(1):19–25. https://doi.org/10.1042/bj20121899
Peters RJ, Croteau RB (2003) Alternative termination chemistries utilized by monoterpene cyclases: chimeric analysis of bornyl diphosphate, 1,8-cineole, and sabinene synthases. Arch Biochem Biophys 417(2):203–211. https://doi.org/10.1016/S0003-9861(03)00347-3
Ramos-Valdivia AC, van der Heijden R, Verpoorte R (1997) Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function. Nat Prod Rep 14(6):591–603. https://doi.org/10.1039/NP9971400591
Reiling KK, Yoshikuni Y, Martin VJJ, Newman J, Bohlmann J, Keasling JD (2004) Mono and diterpene production in Escherichia coli. Biotechnol Bioeng 87(2):200–212. https://doi.org/10.1002/bit.20128
Renninger NS, Ryder JA, Fisher KJ (2008) Jet fuel compositions and methods of making and using same. US Patent 7942940
Roach CR, Hall DE, Zerbe P, Bohlmann J (2014) Plasticity and evolution of (+)-3-carene synthase and (−)-sabinene synthase functions of a sitka spruce monoterpene synthase gene family associated with weevil resistance. J Biol Chem 289(34):23859–23869. https://doi.org/10.1074/jbc.M114.571703
Rohdich F, Hecht S, Gärtner K, Adam P, Krieger C, Amslinger S, Arigoni D, Bacher A, Eisenreich W (2002) Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. Proc Natl Acad Sci U S A 99(3):1158–1163. https://doi.org/10.1073/pnas.032658999
Rohmer M, Knani M, Simonin P, Sutter B, Sahm H (1993) Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J 295(Pt 2):517–524. https://doi.org/10.1042/bj2950517
Rossi PG, Berti L, Panighi J, Luciani A, Maury J, Muselli A, Serra DR, Gonny M, Bolla JM (2007) Antibacterial action of essential oils from Corsica. J Essent Oil Res 19(2):176–182. https://doi.org/10.1080/10412905.2007.9699254
Sakai H, Ito S (2009) Recombinant sabinene synthase and use thereof. Japanese patent JP2009207402
Singh SK, Strobel GA, Knighton B, Geary B, Sears J, Ezra D (2011) An endophytic Phomopsis sp. possessing bioactivity and fuel potential with its volatile organic compounds. Microb Ecol 61(4):729–739. https://doi.org/10.1007/s00248-011-9818-7
Starks CM, Back K, Chappell J, Noel JP (1997) Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science 277(5333):1815–1820. https://doi.org/10.1126/science.277.5333.1815
Steinbüchel A (2003) Production of rubber-like polymers by microorganisms. Curr Opin Microbiol 6(3):261–270. https://doi.org/10.1016/S1369-5274(03)00061-4
Tashiro M, Kiyota H, Kawai-Noma S, Saito K, Ikeuchi M, Iijima Y, Umeno D (2016) Bacterial production of pinene by a laboratory-evolved pinene-synthase. ACS Synth Biol 5(9):1011–1020. https://doi.org/10.1021/acssynbio.6b00140
Tomko TA, Dunlop MJ (2015) Engineering improved bio-jet fuel tolerance in Escherichia coli using a transgenic library from the hydrocarbon-degrader Marinobacter aquaeolei. Biotechnol Biofuels 8(1):165. https://doi.org/10.1186/s13068-015-0347-3
Ul-Hassan SR, Strobel GA, Booth E, Knighton B, Floerchinger C, Sears J (2012) Modulation of volatile organic compound formation in the mycodiesel-producing endophyte Hypoxylon sp. CI-4. Microbiology 158(2):465–473. https://doi.org/10.1099/mic.0.054643-0
Ullah MW, Khattak WA, Ul-Islam M, Khan S, Park JK (2016) Metabolic engineering of synthetic cell-free systems: strategies and applications. Biochem Eng J 105(Part B):391–405. https://doi.org/10.1016/j.bej.2015.10.023
Urabe H, Suzuki K, Sato F (1997) Intramolecular cyclization of 2,7- or 2,8-bis-unsaturated esters mediated by (η2-propene)Ti(O-i-Pr)2. facile construction of mono- and bicyclic skeletons with stereoselective introduction of a side chain. A synthesis of d-sabinene. J Am Chem Soc 119(42):10014–10027. https://doi.org/10.1021/ja9716160
Valente J, Zuzarte M, Gonçalves MJ, Lopes MC, Cavaleiro C, Salgueiro L, Cruz MT (2013) Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem Toxicol 62:349–354. https://doi.org/10.1016/j.fct.2013.08.083
Vranová E, Coman D, Gruissem W (2013) Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol 64(1):665–700. https://doi.org/10.1146/annurev-arplant-050312-120116
Wise ML, Savage TJ, Katahira E, Croteau R (1998) Monoterpene synthases from common sage (Salvia officinalis) : cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1,8-cineole synthase, and (+)-bornyl diphosphate synthase. J Biol Chem 273(24):14891–14899. https://doi.org/10.1074/jbc.273.24.14891
Woguem V, Maggi F, Fogang HPD, Tapondjou LA, Womeni HM, Quassinti L, Bramucci M, Vitali LA, Petrelli D, Lupidi G, Papa F, Vittori S, Barboni L (2013) Antioxidant, antiproliferative and antimicrobial activities of the volatile oil from the wild pepper Piper capense used in Cameroon as a culinary spice. Nat Prod Commun 8(12):1791–1796
Yamasaki Y, Kunoh H, Yamamoto H, Akimitsu K (2007) Biological roles of monoterpene volatiles derived from rough lemon (Citrus jambhiri Lush) in citrus defense. J Gen Plant Pathol 73(3):168–179. https://doi.org/10.1007/s10327-007-0013-0
Yang J, Xian M, Su S, Zhao G, Nie Q, Jiang X, Zheng Y, Liu W (2012) Enhancing production of bio-isoprene using hybrid MVA pathway and isoprene synthase in E. coli. PLoS One 7(4):e33509. https://doi.org/10.1371/journal.pone.0033509
Yang J, Nie Q, Ren M, Feng H, Jiang X, Zheng Y, Liu M, Zhang H, Xian M (2013) Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol Biofuels 6(1):60. https://doi.org/10.1186/1754-6834-6-60
Yue Y, Yu R, Fan Y (2014) Characterization of two monoterpene synthases involved in floral scent formation in Hedychium coronarium. Planta 240(4):745–762. https://doi.org/10.1007/s00425-014-2127-x
Zaidlewicz M, Gimiñska M (1997) Syntheses with organoboranes—VIII. Transformation of (1S,6R)-(+)-2-carene into (1S,6R)-(+)-3(10)-carene and (1R,5R)-(−)-α-thujene into (1R,5R)-(+)-sabinene. Tetrahedron-Asymmetry 8(23):3847–3850. https://doi.org/10.1016/S0957-4166(97)00589-2
Zhang H, Liu Q, Cao Y, Feng X, Zheng Y, Zou H, Liu H, Yang J, Xian M (2014) Microbial production of sabinene—a new terpene-based precursor of advanced biofuel. Microb Cell Factories 13(1):20. https://doi.org/10.1186/1475-2859-13-20
Zhao J, Bao X, Li C, Shen Y, Hou J (2016) Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 100(10):4561–4571. https://doi.org/10.1007/s00253-016-7375-1
Funding
This work was sponsored by the National Natural Science Foundation of China (grant number 31200030) and Taishan Scholars Climbing Program of Shandong (grant number tspd20150210).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Cao, Y., Zhang, H., Liu, H. et al. Biosynthesis and production of sabinene: current state and perspectives. Appl Microbiol Biotechnol 102, 1535–1544 (2018). https://doi.org/10.1007/s00253-017-8695-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8695-5