Abstract
Crude glycerol is an ideal feedstock for bioproduction of 1,3-propanediol (1,3-PDO) while pure culture always shows low substrate tolerance and limited productivity. In this study, an anaerobic microbial consortium for conversion of crude glycerol was selected and its 1,3-PDO production capacity was evaluated. The consortium was obtained from anaerobic activated sludge by 19 serial transfers and mainly consisted of 94.64% Clostridiaceae and 4.47% Peptostreptococcaceae. The consortium adapted well with high glycerol concentration of 120 g/L as well as wide substrate concentration fluctuation from 15 to 80 g/L, producing 60.61 and 82.66 g/L 1,3-PDO in the batch and fed-batch fermentation, with the productivity of 3.79 and 3.06 g/(L∙h), respectively, which are among the best results published so far. Furthermore, mini consortia isolated by serial dilution exhibited similar microbial composition but gradually decreasing tolerance to crude glycerol. Four randomly selected Clostridium butyricum displayed different substrate tolerance and insufficient 1,3-PDO production capacity. This work demonstrated that the high adaptation to crude glycerol of the consortium was the collaborative effort of different individuals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel, originated from biomass such as vegetable oils and animals fats, is one of the most sustainable and environmentally safe alternatives to petroleum diesel. As the global production of biodiesel increases, the rational utilization of crude glycerol, an inherent by-product of biodiesel (10% w/w), is becoming challenging (Dobson et al. 2012; Li et al. 2013). Glycerol could be used as the carbon source for numerous microbes to produce value-added chemicals, but crude glycerol usually has a negative effect on microbial growth. Some studies showed that a lot of impurities such as salts, methanol, free fatty acids, heavy metals, colorants, and ash in crude glycerol could cause the inhibitions on cell growth and metabolism (Ayoub and Abdullah 2012). Generally, crude glycerol in market consists of about 65 ∼ 85% glycerol. As the refining process to higher purity is more complex and less cost effective, direct conversion of crude glycerol to value-added chemicals by microorganisms has become the tendency (Dobson et al. 2012; Yang et al. 2012).
1,3-Propanediol (1,3-PDO), one of the most promising bulk biochemical from glycerol, is extensively used in medicine, food, cosmetics, and other industries. The demand for 1,3-PDO is constantly increasing as the monomer of polytrimethylene terephthalate (PTT), which is expected to have a strong application potential in textile industry (Liu et al. 2010). So far, a few microorganisms, including Enterobacteriaceae, Clostridiaceae, and Lactobacillaceae have been used to convert glycerol to 1,3-PDO (Lee et al. 2015). Among these natural 1,3-PDO producers, Klebsiella pneumoniae (Liu et al. 2007; Sun et al. 2008) and Clostridium butyricum (Chatzifragkou et al. 2011a, b; Wilkens et al. 2012) have been attracted much attention owing to their competitive yield and productivity. K. pneumoniae, a facultative anaerobic bacterium, grows fast and is easy to genetically manipulate, but it is classified as opportunistic pathogen, which brings hidden trouble to practical application (Celinska 2012). Non-pathogenic C. butyricum, by contrast, is a better choice for industrialized production. However, industrial 1,3-PDO production by C. butyricum is limited due to the requirement of strictly anaerobic culture conditions and relatively slow growth rate (Willke and Vorlop 2008). Meanwhile, both of them show relatively poor performances using crude glycerol as the carbon source (Chatzifragkou et al. 2010; Mu et al. 2006). Many efforts have been made to improve bacterial adaptation to crude glycerol, such as substrate pretreatment (Zhu et al. 2013), strain selection and domestication (Ringel et al. 2012), but the 1,3-PDO production and productivity are still far away from that using pure glycerol as substrate (Samul et al. 2014).
Microbial consortium, with characteristics of robustness against environmental fluctuations and excellent performances for complex tasks (Brenner et al. 2008), has been widely used in traditional food and beverage fermentation since thousands of years ago (Navarrete-Bolaños 2012). Moreover, microbial consortium has occupied an important position in biodegradation and bioremediation, for example, wastewater treatment (Appels et al. 2008) and toxic compounds degradation (Ghosh and Philip 2004). Recently, application of microbial consortium on bioconversion of raw materials to biofuels and biochemicals, such as hydrogen, ethanol, and propionate has been paid much attention (Kleerebezem and van Loosdrecht 2007; Sabra et al. 2010; Jiang et al. 2016b). In view of utilization of crude glycerol, only a few studies have used microbial consortium as inoculum to produce 1,3-PDO (Dietz and Zeng 2014; Liu et al. 2013; Moscoviz et al. 2016; Selembo et al. 2009; Varrone et al. 2015). Dietz and Zeng (2014) selected an anaerobic consortium that could efficiently convert crude glycerol to 1,3-PDO under unsterile conditions with a simple medium, in which the final 1,3-PDO concentration reached 70 g/L with a productivity of 2.6 g/(L∙h). However, the consortium could only tolerate up to 65 g/L crude glycerol, and the change of substrate concentration in fed-batch fermentations had a serious impact on the fermentation performance. Mixed culture obtained by Moscoviz et al. (2016) achieved a high 1,3-PDO yield of 0.56–0.64 mol/mol over the range of pH, but the 1,3-PDO concentration could hardly satisfy the requirement of industrial application.
The aim of this study was to select a microbial consortium for efficient conversion of crude glycerol to 1,3-PDO. Then, batch and fed-batch fermentations were carried out to evaluate the consortium adaptation to crude glycerol. Furthermore, the community structure was simplified by serial dilution and pure culture isolation to investigate the individual contribution of the microbial consortium to adaptation to crude glycerol.
Material and methods
Inoculum
Activated sludge was obtained from an anaerobic digester for co-digestion of waste activated sludge and kitchen garbage at Dongtai Industrial Waste Treatment Co., Ltd., Dalian, China. The digestion temperature ranged from 37 to 40 °C.
Culture media
The compositions of seed and fermentation media were similar to those described by Jiang et al. (2016a), except that in seed medium, crude glycerol was used as the carbon source to a final concentration of 22 g/L with addition of 0.5 g/L L-cysteine∙HCl, and in fermentation medium, pure or crude glycerol was used as the carbon source with different concentrations according to the requirements of the experiment. The solid medium had the same components as seed medium with addition of 1.2% agar. Crude glycerol used in this study was provided by Sichuan Tianyu Oleochemical Co. Ltd., China. It contained 78% glycerol, 0.87% ash, 15 ∼ 17% moisture, a little salt (the equivalent of electrical conductivity as 0.43% sodium chloride), and the pH value was 6.91.
Cultivation conditions
Seed cultures were performed in 250-mL serum bottles containing 100 mL of the nitrogen-gassed sterilized seed medium, and incubated at 37 °C with shaking at 200 rpm for 12 ∼ 96 h. Sterile syringes were used for inoculation and sampling.
Batch and fed-batch fermentations were performed in a 5-L bioreactor (Baoxing Biotech, Shanghai, China) with 2 L working volume. The seed culture was incubated for 15 h before inoculation into the bioreactor with 10% inoculum volume. The temperature and agitation rate were maintained at 37 °C and 250 rpm. The medium pH was controlled at 7.0 by automatic addition of 5 M NaOH. An anaerobic environment in the bioreactor was produced by sparging with nitrogen gas at 0.15 vvm for 1 h before and after inoculation.
In fed-batch fermentations, three feeding strategies were adopted: continuous, two-pulse, and one-pulse feeding. By continuous feeding strategy, glycerol concentration in fermenter was maintained 15 or 40 g/L during the cultivation by manually controlling the substrate feeding rate. By two-pulse feeding strategy, glycerol was added to a preset value by twice when the substrate concentration was below 15 or 40 g/L. By one-pulse feeding strategy, glycerol was added instantaneously to 120 g/L at one time when the substrate concentration was below 15 or 40 g/L.
Isolation of mini consortia and single strains
Mini consortia were isolated by serial dilution of the original consortium with sterile saline and then incubated in the seed medium. Once substrates were totally consumed, the enriched consortia were serially transferred to the fresh seed medium with 2% inoculum size three times for the steady microbial composition. Two replicates were used for each dilution. Consortium with the highest dilution that could still convert crude glycerol was defined as the minimal consortium. Single strains from the consortium were isolated randomly and purified by streaking on solid medium. All operations were performed in an anaerobic glove box.
Identification of microbial community of the consortia and single strains
The genomic DNAs of the microbial consortium, mini consortia, and single strains were extracted using a DNA Extraction Kit (TaKaRa Bio).
For consortia, the quantity and quality of extracted DNA were checked using the Qubit2.0 DNA Assay Kit (Sangon Biotech, Shanghai, China). The V4-V5 region of the 16S rRNA gene was amplified using the universal primers 515F: 5′- GTGCCAGCMGCCGCGGTAA-3′and 907R: 5′-CCGTCAATTCMTTTRAGTTT-3′ and sequenced on Illumina Miseq sequencing platforms at Sangon Biotech (Shanghai, China). To analyze the microbial community structure, the sequences were compared with those in the SILVA 16S rRNA gene database.
For isolated strains, the 16S rRNA genes were amplified using primers 7F: 5′- CAGAGTTTGATCCTGGCT-3′ and 1540R: 5′-AGGAGGTGATCCAGCCGCA-3′ and sequenced at Sangon Biotech (Shanghai, China).
Analytics
Cell growth was monitored by measuring the optical density at 650 nm. Glycerol was determined by titration of formate produced quantitatively by oxidation of glycerol with excess sodium periodate, in which the unreacted sodium periodate was neutralized by ethylene glycol before the titration (Wang et al. 2001). Concentrations of 1,3-PDO, lactate, acetate, and butyrate were analyzed using HPLC as previously described (Jiang et al. 2016a).
Nucleotide sequence accession numbers
16S rRNA gene sequences of the consortia have been submitted to the NCBI Sequence Read Archive (SRA) under accession number SRP071045. 16S rRNA gene sequences of the isolated strains have been deposited in GenBank under accession numbers KY203639, KY203640, KY203641, and KY224082.
Results
Selection of microbial consortium
Activated sludge samples (2.0 g) were first inoculated into the seed medium with crude glycerol as the sole carbon source (22 g/L glycerol content). When the substrate was nearly consumed, the seed broth was transferred to fresh seed medium with a gradually smaller inoculation size. As shown in Fig. 1a, b, microbial consortium broadly underwent three phases during 19 serial transfers: enrichment phase (1st–4th), fluctuating phase (5th–12th), and stable phase (13th–19th). In the enrichment phase, microorganisms with high efficiency to utilize crude glycerol were enriched rapidly, while other microorganisms that could not grow or grew slowly on this substrate were eliminated. During this stage, the incubation time was shortened from 96 to 24 h, the OD650 value increased from 1.7 to 3.5, and the concentration of 1,3-PDO achieved to 11.7 g/L. In the fluctuating phase, microbial growth and metabolism were unstable. The incubation time ranged from 24 to 36 h, and the 1,3-PDO production ranged from 7.33 to 11.96 g/L. The concentrations of by-products such as lactate, acetate, and butyrate also fluctuated during this phase. After 12 serial transfers, the incubation time, the microbial growth as well as the production of 1,3-PDO, and all by-products changed little along with the transfers, which indicated that the microbial consortium became stable. After long-term domestication with crude glycerol as the sole carbon source, a stable functional microbial consortium with a high 1,3-PDO yield of 0.69 mol/mol was selected and named as C2-2M. Acetate and butyrate were the main by-products and lactate production was ceased after tenth generation.
Analysis of microbial community
Microbial community analysis was conducted to identify the composition of the selected consortium C2-2M. As shown in Table 1, Clostridiaceae and Peptostreptococcaceae were the predominant families of the consortium, which was closely related to C. butyricum with 99% similarity and Paraclostridium bifermentans with 97% similarity, and accounted for 94.65 and 4.47%, respectively. In addition, 0.84% Lactobacillaceae was also identified in the consortium, the dominant of which was closely related to Lactobacillus equicursoris (99% similarity), representing 0.79% of the total microbial community. Unclassified microorganisms accounted for 0.04% in the consortium.
Fermentation performance of microbial consortium C2-2M
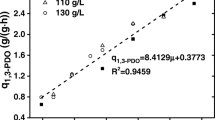
Batch fermentations were carried out with crude (40–140 g/L glycerol content) and pure glycerol (140 g/L). As shown in Fig. 2a–c, when using crude glycerol as substrate, microbial consortium C2-2M could tolerate high concentration of glycerol up to 120 g/L, producing 60.61 g/L 1,3-PDO with a productivity of 3.79 g/(L∙h) under anaerobic conditions. When glycerol concentration was increased to 140 g/L, cell growth was severely inhibited by using either crude or pure glycerol as substrate, which indicated that the inhibitory effect was mainly attributed to the high glycerol concentration. In addition, when the glycerol concentration ranged from 40 to 120 g/L, the yield and productivity of 1,3-PDO remained constant, with linear relationships between glycerol consumption and 1,3-PDO production and between fermentation time and 1,3-PDO production (Fig. 2d).
As shown in batch fermentations, the consortium growth was strongly inhibited when glycerol concentration exceeded 140 g/L (Fig. 2). To reduce substrate inhibition and further increase 1,3-PDO production, fed-batch fermentations were conducted with an initial glycerol concentration of 80 g/L. As predicted, in a fed-batch fermentation by continuous feeding, the 1,3-PDO concentration increased 33.6% (80.96 vs 60.61 g/L) compared with that in batch fermentation (Fig. 3a). At the same time, the change of residual glycerol concentrations from 15 to 40 g/L during the cultivation did not affect the consortium’s capacity for 1,3-PDO production despite some differences on biomass production and by-products distribution (Fig. 3b). In addition, similar 1,3-PDO production of 83.71 g/L was obtained with pure glycerol (Fig. 3c), which showed that microbial consortium C2-2M had a good adaptability for crude glycerol.
Fed-batch fermentations of microbial consortium C2-2M using crude (a, b, d–g) and pure (c) glycerol. Three feeding strategies were adopted including continuous, two-pulse, and one-pulse feeding. a, b Continuous feeding at the residual glycerol concentration of 15 and 40 g/L. c Continuous feeding at the residual glycerol concentration of 15 g/L using pure glycerol as substrate. d, e Two-pulse feeding when the crude glycerol concentration was below 15 and 40 g/L. f, g One-pulse feeding when the crude glycerol concentration was below 15 and 40 g/L. Values are means of two independent fermentations
As the initial substrate concentrations shifting from 40 to 120 g/L had little effect on batch fermentation performance of the consortium (Fig. 2), it was assumed that a fluctuation of crude glycerol concentration during the fed-batch fermentation could also be tolerated by the consortium. When glycerol was fed by two-pulse strategy from 15 to 80 g/L (Fig. 3d) or from 40 to 80 g/L (Fig. 3e), both 1,3-PDO concentrations and productivities maintained the same levels as that in fed-batch fermentation by continuous feeding (Fig. 3a, b), producing 82.66 and 80.97 g/L 1,3-PDO with productivities of 3.06 and 3.00 g/(L∙h).
Fed-batch fermentations with one-pulse feeding were also carried out, in which crude glycerol was added instantaneously to a final glycerol concentration of 120 g/L when residual substrate concentration was below 15 (Fig. 3f) or 40 g/L (Fig. 3g). Unfortunately, the consortium could not fully adapt to these dramatic changes in substrate concentration, resulting in lower 1,3-PDO productions (59.83 and 72.27 g/L, respectively) than that using continuous or two-pulse feeding. The results indicated that there was a limited fluctuation range of crude glycerol concentration that the consortium could adapt.
Determination of the mini consortium
To simplify the community structure of the consortium, serial dilution in sterile saline was carried out under anaerobic conditions. When incubated in seed medium, the mini consortia from 10−2 to 10−6 dilution could completely utilize crude glycerol (22 g/L glycerol content), but no glycerol was consumed in the 10−7 diluted consortium (Fig. 4a). After transferred three times in fresh medium, the mini consortia showed little differences in bacterial growth and metabolism compared with the original consortium (Fig. 4b). The 10−6 diluted consortium was regarded as the minimal consortium. Bacterial community analysis showed that Lactobacillaceae was eliminated after 10−4 dilution while Clostridiaceae and Peptostreptococcaceae preserved the original proportion during the dilution processes (Table 1).
Fermentation performances of the diluted mini consortia using crude glycerol. a Glycerol consumption during the dilution process in seed medium. b OD650 and concentrations of substrate and products in the end of third subculture. c Batch fermentations with initial glycerol concentration of 120 g/L. d Fed-batch fermentation of the minimal consortium (10−6 dilution) using high substrate concentration (the initial glycerol concentration of 80 g/L and the residual concentration at around 40 g/L). e Fed-batch fermentation of the minimal consortium (10−6 dilution) using low substrate concentration (the initial glycerol concentration of 40 g/L and the residual concentration of 15 g/L). Values are means of two independent fermentations
To evaluate tolerance of the mini consortia (10−2, 10−4, and 10−6 dilutions) to crude glycerol, batch fermentations were carried out with high initial glycerol concentration of 120 g/L (Fig. 4c). Unlike that in seed culture (Fig. 4b), the fermentation time of the 10−2 and 10−4 diluted consortium was prolonged by 3 and 7 h compared with that of the original consortium, respectively. More serious inhibition was observed in the minimal consortium (10−6 dilution), where the consortium could not consume all the glycerol in 35 h. The results showed that high tolerance to crude glycerol was the unique feature of the original consortium, which was gradually lost with the dilution.
In addition, in a fed-batch fermentation at high crude glycerol concentration, e.g., initial glycerol concentration of 80 g/L and maintaining 40 g/L during the fermentation, the minimal consortium produced only 47.94 g/L 1,3-PDO in 36 h (Fig. 4d) compared to 80.66 g/L 1,3-PDO in 27 h by the original consortium C2-2M. Interestingly, the 1,3-PDO production capacity of the minimal consortium recovered to a normal level (80.22 g/L with a productivity of 2.97 g/(L∙h)) at a low glycerol concentration, e.g., initial concentration of 40 g/L and maintaining 15 g/L during the fermentation (Fig. 4e). The results further proved that crude glycerol inhibition was the only limiting factor for poor fermentation performances of the mini consortium, excluding other possibilities such as products inhibition.
Comparison of substrate tolerance among single strains
Four single colonies, S1-S4 were randomly isolated from the original consortium. All strains were identified to be C. butyricum and the 16S rRNA sequences of C. butyricum S1-S4 showed a high similarity with gene of C. butyricum JKT37 (100, 100, 99, and 99%, respectively).
The capability of crude glycerol utilization of the four C. butyricum were evaluated with different substrate concentration. In seed cultures (22 g/L glycerol content), all four strains produced 1,3-PDO as the main product, but they had obviously different growth rates, where the fastest growth was obtained with C. butyricum S2, followed by S3, S4, and S1 (Fig. 5a). In batch fermentations with higher initial crude glycerol concentration (80 g/L glycerol content), although all strains showed similar 1,3-PDO yields, C. butyricum S3 and S4 grew much faster than S1 and especially than S2 (Fig. 5b). Moreover, similar performance was achieved by C. butyricum S3 compared with that by the original consortium at higher initial glycerol concentration of 120 g/L (Fig. S1). In fed-batch fermentations with a high substrate concentration, e.g., initial glycerol concentration of 80 g/L and maintaining 40 g/L during the fermentation, however, none of the four strains could compare with the consortium in 1,3-PDO production capacity (Fig. 5c). Even for C. butyricum S3 with similar substrate tolerance in batch fermentation, lower 1,3-PDO production (74.12 g/L) was observed after 38 h than that of the consortium C2-2M (80.04 g/L) after 27 h. The disparity occurred in the mid-to-late fermentation period in spite of similar microbial growth. The results showed that the some of the single strains (S3) had good tolerance to crude glycerol but insufficient capacity for 1,3-PDO production.
Microbial growth and 1,3-PDO production of C. butyricum S1-S4 and microbial consortium C2-2M using crude glycerol. a Seed cultures with initial glycerol concentration of 22 g/L. b Batch fermentations with initial glycerol concentration of 80 g/L. c Fed-batch fermentations with initial glycerol concentration of 80 g/L and the residual glycerol concentration of 40 g/L. Values are means of two independent fermentations
Discussion
Consortium selection and identification
Anaerobic activated sludge is characteristic of complex microbial community structure with high substrate diversity, which is a perfect choice for functional consortium selection to degrade complex substrates (Forney et al. 2001). During the serial transfers, the microbial community diversity would be reduced and functional bacteria would be enriched (Jiménez et al. 2014). In this study, fermentation performances (microbial growth and metabolism) of the consortium has undergone two stages (enrichment and fluctuation) before reaching stable state, whereas changes of the consortium population during the selection process are unknown. It is assumed that the well-defined three stages of the growth and metabolism are accompanying with the change of population composition of the consortium caused by microbial interactions such as collaboration and competition. The stable consortium obtained by sufficient serial transfer ensured the same inoculum for the later fermentation experiments.
As for community structure analysis, only a few types of bacteria were preserved after 19 generations with crude glycerol as the sole carbon source. C. butyricum is a classical 1,3-PDO producer, and its overwhelming superiority is consistent with the distribution of products (Wilkens et al. 2012). P. bifermentans (reclassification of Clostridium bifermentans (Sasi Jyothsna et al. 2016)) is also identified as a 1,3-PDO producer (Myszka et al. 2012) but the identified Lactobacillaceae family including L. equicursoris and Lactobacillus zeae is rarely reported.
High fermentation performance of microbial consortium C2-2M
In batch and fed-batch fermentations with crude glycerol, the highest 1,3-PDO concentrations of 60.61 and 82.66 g/L was achieved by the consortium, with the productivity of 3.78 and 3.06 g/(L∙h), respectively. To our knowledge, these are the best results reported for 1,3-PDO production by crude glycerol (Table 2).
In batch fermentation, natural 1,3-PDO producers were generally unable to efficiently utilize high concentration of crude glycerol. Besides substrate inhibition (Biebl 1991), accumulation of impurities in high concentration of crude glycerol would cause significant inhibitory effects on microbial growth, especially for C. butyricum (Moon et al. 2010). Dietz and Zeng (2014) reported that a selected mixed culture from biogas plants would tolerate 65 g/L initial crude glycerol. When glycerol concentration increased, microbial growth was entirely inhibited. C. butyricum CNCM 1211 reported by Himmi et al. (1999) could completely convert crude glycerol (120 g/L glycerol content) to 1,3-PDO, but the process took over 80 h. However, in this study, consortium C2-2M could efficiently convert crude glycerol with glycerol concentration of 120 g/L to 1,3-PDO in only 16 h. Fermentation performance of the consortium was hardly influenced by the glycerol concentration ranging from 40 to 120 g/L with nearly overlapping growth curves (Fig. 2b), stable 1,3-PDO yields (mean value 0.53 g/g) and productivities (mean value 3.99 g/(L∙h)) (Fig. 2d), which is vastly different from that of pure culture (Metsoviti et al. 2012).
In fed-batch fermentation, usually, a suitable feeding strategy is crucial to guarantee a high production of bio-products since microbial growth and product formation are susceptible to substrate concentration (Lee et al. 1999). The feeding process could be complicated and difficult to control precisely, resulting in decline of production and increase of operation costs (Johnson 1987). Many explorations have been made to optimize feeding strategy in 1,3-PDO production (Hao et al. 2008; Reimann and Biebl 1996; Saint-Amans et al. 1994). However, in our research, microbial consortium C2-2M maintained good robustness and stable and efficient 1,3-PDO production capacity against glycerol concentration fluctuation ranging from 15 to 80 g/L during the fermentation process, which could make it possible to simplify the fermentation process. The easy-to-control feeding process using the consortium could reduce the risk for inappropriate feeding operation, guarantee high and stable 1,3-PDO production, and decrease the operation costs, which would be of benefit especially to large-scale industrial production.
Furthermore, it was observed that the consortium could tolerate high initial glycerol concentration until 120 g/L (Fig. 2), but could not resist the substrate concentration fluctuation to 120 g/L in one-pulse fed-batch fermentation (Fig. 3f, g). This might be due to the changes in microbial community during the fed-batch fermentations at initial substrate concentration of 80 g/L, in which the bacteria resisting high concentration of crude glycerol, e.g., 120 g/L, were no longer predominant when the feeding started.
Fermentation performance degradation by serial dilution and single strain isolation
Serial dilution is an easy and efficient approach to isolate the microbial consortium with simplest community structure and target function without isolating pure strains (Wang et al. 2010; Zhang et al. 2013). The success of this approach requires that the functional strains should remain in the reactor while the non-functional strains should be screened off by the dilution (Adav et al. 2009). In our research, the tolerance to crude glycerol was decreased gradually with increasing dilution level but the high 1,3-PDO production capacity was still remained. Obviously, some functional strains contributed to high substrate tolerance were eliminated during the dilution. There are two hypotheses that could account for this phenomenon. One is that the different bacterial strains in the consortium have different tolerances to crude glycerol. Functional strains with high crude glycerol tolerance might have no growth advantage in seed culture and be reduced or eliminated during the dilution. The other possibility is that different bacteria have no significant difference in substrate tolerance but interaction exists in the consortium, for example, microbial communication. The consortium could grow in high concentration of crude glycerol better together than any isolated microorganisms (Hays et al. 2015).
To verify the above hypotheses, four pure cultures were selected randomly from the consortium. Both genetic and phenotypic diversity of the dominant species (C. butyricum) existed in the consortium. The higher the initial crude glycerol concentration, the bigger performance differences among single stains. However, none of their fermentation capacities could compare with that of the consortium. The results further proved that the high adaptation to crude glycerol is the unique feature of the consortium. In nature, division of labor is ubiquitous in a microbial community and crucial for expanded functional and metabolic capacities (Hays et al. 2015). This kind of division might also exist in microbial consortium C2-2M. Perhaps the strain S3 does not occupy a high proportion in consortium C2-2M but plays an important role in high substrate tolerance of the consortium. While the strain S2 with high abundance and fast growth shows poor performance at high crude glycerol concentration. All bacterial strains with different abundances and different crude glycerol tolerances live and work together in the consortium, make struggle against the high crude glycerol concentration and do a better job for 1,3-PDO production. During dilution or single strain isolation, this collaborative relationship was weakened or broken, resulting in poor fermentation performance compared with that of the original consortium.
In conclusion, a microbial consortium mainly containing C. butyricum for conversion of crude glycerol to 1,3-PDO was selected and its fermentation performance was explored. The consortium achieved stable after 19 serial transfers using crude glycerol as the sole carbon source. In batch and fed-batch fermentations, the consortium showed good adaptability with high initial crude glycerol concentration and strong robustness against substrate concentration fluctuation. The highest 1,3-PDO production of 82.66 g/L with a productivity of 3.06 g/(L∙h) was obtained by an easy-to-control two-pulse fed-batch fermentation. Then, fermentations by the diluted mini consortia and the isolated single strains proved that high adaptation to crude glycerol of the consortium was the collaborative effort of different individuals, where the bacterial strains with different abundances showed different growth rates and substrate tolerances. The intra- and inter-species interactions in the microbial consortium need to be further investigated in the future. This work provides an alternative for efficient bioconversion of crude glycerol to value-added chemical 1,3-PDO.
References
Adav SS, Lee DJ, Wang A, Ren N (2009) Functional consortium for hydrogen production from cellobiose: concentration-to-extinction approach. Bioresour Technol 100:2546–2550
Appels L, Baeyens J, Degrève J, Dewil R (2008) Principles and potential of the anaerobic digestion of waste-activated sludge. Prog Energ Combust 34:755–781
Ayoub M, Abdullah AZ (2012) Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew Sust Energ Rev 16:2671–2686
Biebl H (1991) Glycerol fermentation of 1,3-propanediol by Clostridium butyricum. Measurement of product inhibition by use of a pH-auxostat. Appl Microbiol Biotechnol 35:701–705
Brenner K, You L, Arnold FH (2008) Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol 26:483–489
Celinska E (2012) Klebsiella spp as a 1, 3-propanediol producer: the metabolic engineering approach. Crit Rev Biotechnol 32:274–288
Chatzifragkou A, Dietz D, Komaitis M, Zeng AP, Papanikolaou S (2010) Effect of biodiesel-derived waste glycerol impurities on biomass and 1,3-propanediol production of Clostridium butyricum VPI 1718. Biotechnol Bioeng 107:76–84
Chatzifragkou A, Aggelis G, Komaitis M, Zeng AP, Papanikolaou S (2011a) Impact of anaerobiosis strategy and bioreactor geometry on the biochemical response of Clostridium butyricum VPI 1718 during 1,3-propanediol fermentation. Bioresour Technol 102:10625–10632
Chatzifragkou A, Papanikolaou S, Dietz D, Doulgeraki AI, Nychas GJ, Zeng AP (2011b) Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl Microbiol Biotechnol 91:101–112
Dietz D, Zeng AP (2014) Efficient production of 1,3-propanediol from fermentation of crude glycerol with mixed cultures in a simple medium. Bioprocess Biosyst Eng 37:225–233
Dobson R, Gray V, Rumbold K (2012) Microbial utilization of crude glycerol for the production of value-added products. J Ind Microbiol Biot 39:217–226
Forney LJ, Liu WT, Guckert JB, Kumagai Y, Namkung E, Nishihara T, Larson RJ (2001) Structure of microbial communities in activated sludge: potential implications for assessing the biodegradability of chemicals. Ecotox Environ Safe 49:40–53
Ghosh PK, Philip L (2004) Atrazine degradation in anaerobic environment by a mixed microbial consortium. Water Res 38:2276–2283
Hao J, Lin R, Zheng Z, Sun Y, Liu D (2008) 3-Hydroxypropionaldehyde guided glycerol feeding strategy in aerobic 1,3-propanediol production by Klebsiella pneumoniae. J Ind Microbiol Biot 35:1615–1624
Hays SG, Patrick WG, Ziesack M, Oxman N, Silver PA (2015) Better together: engineering and application of microbial symbioses. Curr Opin Biotechnol 36:40–49
Himmi EH, Bories A, Barbirato F (1999) Nutrient requirements for glycerol conversion to 1,3-propanediol by Clostridium butyricum. Bioresour Technol 67:123–128
Hirschmann S, Baganz K, Koschik I, Vorlop K-D (2005) Development of an integrated bioconversion process for the production of 1,3-propanediol from raw glycerol waters. Landbauforsch Völkenrode (FAL Agricultural Research) 55:261–267
Jiang LL, Liu HF, Mu Y, Sun YQ, Xiu ZL (2016a) High tolerance to glycerol and high production of 1,3-propanediol in batch fermentations by microbial consortium from marine sludge. Eng Life Sci. doi:10.1002/elsc.201600215
Jiang LL, Zhou JJ, Wang XD, Sun YQ, Xiu ZL (2016b) Progress in chemicals production by microbial consortia. Chin J Biotechnol 32:1496–1506
Jiménez DJ, Dini-Andreote F, Van Elsas JD (2014) Metataxonomic profiling and prediction of functional behaviour of wheat straw degrading microbial consortia. Biotechnol Biofuels 7:1
Johnson A (1987) The control of fed-batch fermentation processes—a survey. Automatica 23:691–705
Kleerebezem R, van Loosdrecht MC (2007) Mixed culture biotechnology for bioenergy production. Curr Opin Biotechnol 18:207–212
Lee J, Lee SY, Park S, Middelberg APJ (1999) Control of fed-batch fermentations. Biotechnol Adv 17:29–48
Lee CS, Aroua MK, Daud WMAW, Cognet P, Pérès-Lucchese Y, Fabre PL, Reynes O, Latapie L (2015) A review: conversion of bioglycerol into 1,3-propanediol via biological and chemical method. Renew Sust Energ Rev 42:963–972
Li C, Lesnik KL, Liu H (2013) Microbial conversion of waste glycerol from biodiesel production into value-added products. Energies 6:4739–4768
Liu HJ, Zhang DJ, Xu YH, Mu Y, Sun YQ, Xiu ZL (2007) Microbial production of 1,3-propanediol from glycerol by Klebsiella pneumoniae under micro-aerobic conditions up to a pilot scale. Biotechnol Lett 29:1281–1285
Liu H, Xu Y, Zheng Z, Liu D (2010) 1,3-Propanediol and its copolymers: research, development and industrialization. Biotechnol J 5:1137–1148
Liu B, Christiansen K, Parnas R, Xu Z, Li B (2013) Optimizing the production of hydrogen and 1,3-propanediol in anaerobic fermentation of biodiesel glycerol. Int J Hydrogen Energ 38:3196–3205
Metsoviti M, Paraskevaidi K, Koutinas A, Zeng AP, Papanikolaou S (2012) Production of 1,3-propanediol, 2,3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. Process Biochem 47:1872–1882
Moon C, Ahn JH, Kim SW, Sang BI, Um Y (2010) Effect of biodiesel-derived raw glycerol on 1,3-propanediol production by different microorganisms. Appl Biochem Biotechnol 161:502–510
Moscoviz R, Trably E, Bernet N (2016) Consistent 1,3-propanediol production from glycerol in mixed culture fermentation over a wide range of pH. Biotechnol Biofuels 9:32
Mu Y, Teng H, Zhang DJ, Wang W, Xiu ZL (2006) Microbial production of 1,3-propanediol by Klebsiella pneumoniae using crude glycerol from biodiesel preparations. Biotechnol Lett 28:1755–1759
Myszka K, Leja K, Olejnik-Schmidt AK, Czaczyk K (2012) Isolation process of industrially useful Clostridium bifermentans from natural samples. J Biosci Bioeng 113:631–633
Navarrete-Bolaños JL (2012) Improving traditional fermented beverages: how to evolve from spontaneous to directed fermentation. Eng Life Sci 12:410–418
Papanikolaou S, Fick M, Aggelis G (2004) The effect of raw glycerol concentration on the production of 1,3-propanediol by Clostridium butyricum. J Chem Technol Biot 79:1189–1196
Petitdemange E, Dürr C, Andaloussi S, Raval G (1995) Fermentation of raw glycerol to 1,3-propanediol by new strains of Clostridium butyricum.. J Ind Microbiol Biot 15:498–502
Rehman A, Saman WRG, Nomura N, Sato S, Matsumura M (2008) Pre-treatment and utilization of raw glycerol from sunflower oil biodiesel for growth and 1,3-propanediol production by Clostridium butyricum. J Chem Technol Biotechnol 83:1072–1080
Reimann A, Biebl H (1996) Production of 1, 3-propanediol by Clostridium butyricum DSM 5431 and product tolerant mutants in fed-batch culture: feeding strategy for glycerol and ammonium. Biotechnol Lett 18:827–832
Ringel AK, Wilkens E, Hortig D, Willke T, Vorlop KD (2012) An improved screening method for microorganisms able to convert crude glycerol to 1,3-propanediol and to tolerate high product concentrations. Appl Microbiol Biotechnol 93:1049–1056
Sabra W, Dietz D, Tjahjasari D, Zeng AP (2010) Biosystems analysis and engineering of microbial consortia for industrial biotechnology. Eng Life Sci 10:407–421
Saint-Amans S, Perlot P, Goma G, Soucaille P (1994) High production of 1,3-propanediol from glycerol by Clostridium butyricum VPI 3266 in a simply controlled fed-batch system. Biotechnol Lett 16:831–836
Samul D, Leja K, Grajek W (2014) Impurities of crude glycerol and their effect on metabolite production. Ann Microbiol 64:891–898
Sasi Jyothsna TS, Tushar L, Sasikala C, Ramana CV (2016) Paraclostridium benzoelyticum gen. nov. sp. nov., isolated from marine sediment and reclassification of Clostridium bifermentans as Paraclostridium bifermentans comb. nov. Proposal of a new genus Paeniclostridium gen. nov. to accommodate Clostridium sordellii and Clostridium ghonii. Int J Syst Evol Microbiol 66:1268–1274
Selembo PA, Perez JM, Lloyd WA, Logan BE (2009) Enhanced hydrogen and 1,3-propanediol production from glycerol by fermentation using mixed cultures. Biotechnol Bioeng 104:1098–1106
Sun YQ, Qi WT, Teng H, Xiu ZL, Zeng AP (2008) Mathematical modeling of glycerol fermentation by Klebsiella pneumoniae: concerning both enzyme-catalytic reductive pathway and transport of glycerol and 1,3-propanediol across cell membrane. Biochem Eng J 38:22–32
Varrone C, Heggeset TM, Le SB, Haugen T, Markussen S, Skiadas IV, Gavala HN (2015) Comparison of different strategies for selection/adaptation of mixed microbial cultures able to ferment crude glycerol derived from second-generation biodiesel. Biomed Res Int:2015
Wang JF, Xiu ZL, Fan SD (2001) Determination of glycerin concentration during the fermentation of glycerin to 1, 3-propanediol. Ind Microbiol 31:33–35
Wang A, Gao L, Ren N, Xu J, Liu C, Lee DJ (2010) Enrichment strategy to select functional consortium from mixed cultures: consortium from rumen liquor for simultaneous cellulose degradation and hydrogen production. Int J Hydrogen Energ 35:13413–13418
Wilkens E, Ringel AK, Hortig D, Willke T, Vorlop KD (2012) High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a. Appl Microbiol Biotechnol 93:1057–1063
Willke T, Vorlop K (2008) Biotransformation of glycerol into 1,3-propanediol. Eur J Lipid Sci Tech 110:831–840
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol biofuels 5:1
Zhang Q, Tian M, Tang L, Li H, Li W, Zhang J, Zhang H, Mao Z (2013) Exploration of the key microbes involved in the cellulolytic activity of a microbial consortium by serial dilution. Bioresour Technol 132:395–400
Zhu C, Chen B, Fang B (2013) Pretreatment of raw glycerol with activated carbon for 1,3-propanediol production by Clostridium butyricum. Eng Life Sci 13:376–384
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21476042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 152 kb)
Rights and permissions
About this article
Cite this article
Zhou, JJ., Shen, JT., Jiang, LL. et al. Selection and characterization of an anaerobic microbial consortium with high adaptation to crude glycerol for 1,3-propanediol production. Appl Microbiol Biotechnol 101, 5985–5996 (2017). https://doi.org/10.1007/s00253-017-8311-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8311-8