Abstract
In this study, two NH4+-N and S2− removal strains, namely, Kosakonia oryzae (FB2-3) and Acinetobacter baumannii (L5-4), were isolated from the packing materials in a long-running biotrickling filter (BTF). The removal capacities of combined FB2-3 and L5-4 (FB2-3 + L5-4) toward 100 mg L−1 of NH4+-N and 200 mg L−1 of S2− reached 97.31 ± 1.62% and 98.57 ± 1.12% under the optimal conditions (32.0 °C and initial pH = 7.0), which were higher than those of single strain. Then, FB2-3 and L5-4 liquid inoculums were prepared, and their concentrations respectively reached 1.56 × 109 CFU mL−1 and 1.05 × 109 CFU mL−1 by adding different resuspension solutions and protective agents after 12-week storage at 25 °C. Finally, pilot-scale BTF test showed that NH3 and H2S in the real exhaust gases from a pharmaceutical factory were effectively removed with removal rates > 87% and maximum elimination capacities were reached 136 g (NH3) m−3 h−1 and 176 g (H2S) m−3 h−1 at 18 °C-34 °C and pH 4.0–7.0 in the BTF loaded with bamboo charcoal packing materials co-immobilized with FB2-3 and L5-4. After co-immobilization of FB2-3 and L5-4, in the bamboo charcoal packing materials, the new microbial diversity composition contained the dominant genera of Acinetobacter, Mycobacterium, Kosakonia, and Sulfobacillus was formed, and the diversity of entire bacterial community was decreased, compared to the control. These results indicate that FB2-3 and L5-4 have potential to be developed into liquid ready-to-use inoculums for effectively removing NH3 and H2S from exhaust gases in BTF.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Odor pollution produced during agricultural and industrial activities from landfills, livestock and poultry farms, composting plants, wastewater treatment plants, pharmaceutical factories, and other pollution sources is a severe problem all around the world (Guo and Gao 2021, Wu et al. 2018). The main components of most odorous gases, H2S and NH3, commonly coexist, and they are released in large quantity with unpleasant smell, thus mainly contributing to the odor values in many emission sources (Huan et al. 2021a, b). Both NH3 and H2S are irritating gases. NH3 is a colorless, strongly odorous, toxic, reactive, and corrosive gas with a low odor threshold (about 4 ppmv) (Morral et al. 2021). H2S is a toxic, flammable, and colorless gas with an unpleasant smell, similar to that of rotten eggs with a low odor threshold (about 0.5 ppb) (García-Pérez et al. 2020; Ren et al. 2019). Both these two odorous gases can negatively affect human health and the environment (Sironi et al. 2010). Therefore, simultaneous removal of H2S and NH3 is of great significance for reducing odor pollution.
Nowadays, in order to reduce or eliminate air pollution caused by odorous gases, many technologies have been successfully developed. Among them, the common odor treatment methods can be classified into three categories including chemical (such as chemical scrubbers, thermal oxidation, catalytic oxidation, and ozonation), physical (condensation, adsorption, water scrubbers), and biological (such as biofilters, bioscrubbers, and biotrickling filter (BTFs)) technologies (Ferdowsi et al. 2017; Morral et al. 2022). Biological technologies with the advantages of high efficiency, environment friendliness, relatively low cost, convenient maintenance and management, and no secondary pollution have been regarded as the more feasible methods for treatment of low and moderate concentrations of waste gases (Ryu et al. 2011). As the representative biological technology, BTFs with the advantages of controllable operation conditions (including pH value, salt concentration, and circulating liquid nutrients), low cost, and low-pressure drop during long-term operation can intensively treat acidic degradation products of VOCs and acidic or alkaline odorous gases, have been regarded as the more feasible methods for treatment of low and moderate concentrations of waste gases (De Vela et al. 2021; Lebrero et al. 2012).
Since the microorganisms in the biofilm formed on the surface of packing materials are the major catalysts of biodegradation and gas deodorization, they can also affect gas removal efficiencies of BTFs; thus, they are regarded as the engine of the biotreatment process (Barbusinski et al. 2017; Rybarczyk et al. 2019). Moreover, microbial species composition is the most important controlled parameter for biofilm in BTFs (Rybarczyk et al. 2019; Schiavon et al. 2016). Biofilm formed by inoculated activated sludge is frequently used in BTF systems; however, the long period of acclimation ranging from several weeks to months, that is one of the drawbacks when using them as the inoculum (Watsuntorn et al. 2020). To date, in BTF, formed biofilm by inoculated specific strains including Paracoccus versutus MAL 1HM19 (Watsuntorn et al. 2020), Paracoccus pantotrophus NTV02 (Juntranapaporn et al. 2019), Acinetobacter sp. and Alcaligenes faecalis (Potivichayanon et al. 2006), nitrifying bacteria (Xue et al. 2010), and sulfide-oxidizing bacteria(Chen et al. 2014), with the easy-to-cultivate property and good removal performance, has also proven to be feasible method for treatment of odor pollution. Therefore, it is meaningful to develop new specific bacterial inoculum with high efficiency and good environmental adaptation for treatment of odorous pollution by BTFs.
As an important heterotrophic NH4+-N and S2− removal strain, Acinetobacter sp. can convert NH4+-N to N2 through heterotrophic nitrification and aerobic denitrification and utilize NH4+-N by assimilation (An et al. 2020; Yang et al. 2019). Moreover, as the sulfide-oxidizing bacteria, this genus can convert S2− compounds into sulfate (Haosagul et al. 2020). Acinetobacter has been reported to be enriched in a pilot-scale biofilter after treatment with odorous gases (main components are NH3 and H2S at an inlet concentration of 200 to 210 ppmV) emitted from tanneries (removal efficacy of both NH3 and H2S was about 90–99%) (Gandu et al. 2021). Similarly, Acinetobacter towneri (MF765755) and sulfide-oxidizing bacteria have been found to play a key role in biogas H2S removal efficiency and system stability (Haosagul et al. 2020). Therefore, Acinetobacter sp. can be considered an effective inoculum for removal of NH3 and H2S in BTFs.
In this study, the NH4+-N and S2− removal strains named Acinetobacter baumannii and Kosakonia oryzae were isolated from long-term running BTFs in a pharmaceutical factory. The aim of this work is (1) to examine the effects of initial pH and culture temperature on the NH4+-N and S2− removal capacities of isolated strains; (2) to prepare liquid inoculum for NH4+-N and S2− removal; and (3) to investigate the NH3 and H2S removal efficiencies of liquid ready-to-use inoculums in the pilot-scale BTFs and explore the effects of these liquid inoculums on microbial diversity of packing materials.
Materials and methods
Materials, strains, and media
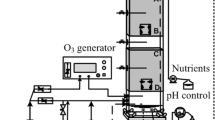
The odorous gas treatment system in a pharmaceutical factory (Changsha, Hunan, China) combines a washing tower (Fig. 1Aa), a BTF (Fig. 1Ab), a UV-photolysis equipment (Fig. 1Ac), and an air-exhausting equipment (Fig. 1Ad) (continuously running for 18 months). The packing material sample was collected from the long-term running BTF and used for bacterial screening. NH4Cl (analytical grade) and Na2S·9H2O (analytical grade) were purchased from Sinopharm Chemical Reagent Co. LTD (Shanghai, China).
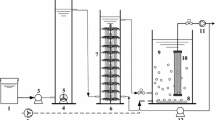
Schematic diagrams of odorous gas treatment system in a pharmaceutical factory (A) and the pilot-scale BTFs in this study (B). 1, Factory exhaust gas inlet port. 2, Dewatering port. 3, Supplementary water inlet. 4, Alkaline washing circulating liquid tank. 5, Circulating liquid inlet port, pH detection port. 6, Sample feeding port. 7, Filler layer. 8, Spray port. 9, Biotrickling filter circulating liquid tank. 10, UV-photolysis. 11, Draught fan. 12, Air-exhausting pipe. 13, H2S, NH3, temperature detection port
The screened Kosakonia oryzae FB2-3 was preserved at China Center for Type Culture Collection (CCTCC M 2,020,508) and Acinetobacter baumannii L5-4 was preserved at College of Life Science and Technology, Huazhong Agricultural University (Wuhan, Hubei, China). Luria–Bertani (LB) medium (containing10.0 g L−1 tryptone, 5.0 g L−1 yeast extract, and 10.0 g L−1 NaCl; pH 7.0–7.4) was used for bacterial culture. Heterotrophic nitrification medium (containing trisodium citrate 5.0 g L−1, (NH4)2SO4 0.5 g L−1, and vickers salt solution 50 mL L−1; pH 7.2 ~ pH7.4) was used for the determination of bacterial NH3 removal capacity. Sulfur-removing strain medium (NH4Cl 0.4 g L−1, MgCl2 0.2 g L−1, KH2PO4 2 g L−1, Na2CO3 0.4 g L−1, and Na2S·9H2O 0.75 g L−1; pH 7.2 ~ 7.4) was used for determination of bacterial H2S removal capacity. The Na2S·9H2O was dissolved into double distilled water (ddH2O) and passed through a sterile disposable syringe filter (0.22 μm, Millipore, Shanghai, China) before use. Nutrient solution containing glucose 8.0 g L−1, K2HPO4 2.0 g L−1, KH2PO4 2.0 g L−1, NH4Cl 0.4 g L−1, MgCl2·6H2O 0.4 g L−1, and CaCl2·2H2O 0.05 g L−1 (pH7.0 ~ 7.2) was used for bacterial activation and loading process in pilot-scale test.
Isolation, screening, and identification of NH4 +-N and S2− removal strains
For enrichment culture, 10 g packing materials sample (obtained from a long-term running BTFs in a pharmaceutical factory) was added into 90 mL sterile water with 20 sterilized glass beads and shaken at 30 °C at 200 r min−1 for 1 h. Subsequently, the suspension was inoculated into LB medium at 5% inoculum amount and cultured at 30 °C and shaken at 200 r min−1 for 24 h. The single colony bacteria were obtained by dilution coating method for the preliminary screening of NH4+-N and S2− removal strains. Then, the NH4+-N and S2− removal capacities of these stains were determined for secondary screening.
The colony morphology of FB2-3 and L5-4 single colonies on LB agar plate was observed. The 16S rRNA sequences of FB2-3 and L5-4 strains and those of their similar strains obtained from EzBioCloud’s Identify service (http://www.ezbiocloud.net/) (Jeon et al. 2014) were used to identify the bacteria. The phylogenetic tree was constructed by using the construct/test neighbor-joining tree programs of MEGA6 software (Tamura et al. 2013).
Determination of NH4 +-N and S2− removal capacities of screened strains
NH4+-N concentration of the supernatant was determined according to Water Quality-Determination of Ammonium-Nessler’s Reagent Colorimetric Method (the State Standard of the People’s Republic of China, GB/T 7479–87) with some modified (detail see supporting materials). S2− concentration in the supernatant was determined according to Water Quality-Determination of Sulfide-Methylene Blue Spectrophotometric Method (the State Standard of the People’s Republic of China, GB/T 16,489–1996) with some modified (detail see supporting materials). The NH4+-N or S2− removal capacity (RC) of screened strain was calculated as follows.
where C0 is NH4+-N or S2− concentration of initial medium (mg L−1); D0 is the corresponding dilution ratio during determination process; Ct is NH4+-N or S2− concentration of the supernatant after treatment with bacteria (mg L−1); and Dt is the corresponding dilution ratio during determination process. The data presented were the average of at least three assays.
NH4 +-N and S2− removal curves and specific rate of consumption
To evaluate the bacterial growth tolerance, the single colony was added into LB medium and cultured overnight. Then, the bacterial suspension was inoculated into LB medium at 1.0% (vol./vol.) under different stress conditions including pH (from 3.0 to 12.0) and culture temperature (from 10 to 45 °C). After 24-h culture, the OD600 nm of the bacterial suspension was determined.
To examine NH4+-N and S2− removal capacity and specific consumption rate, the single colony was added to LB medium and cultured overnight. Then, the bacterial suspension was inoculated into heterotrophic nitrification medium or sulfur-removing strain medium at 1.0% (vol./vol.). NH4+-N and S2− removal capacities of the bacterial suspensions and concentration of cells (N) under different incubation time were monitored.
where N is the concentration of cells (g L−1), C was the concentration of NH4+-N or S2− (mg L−1), and t is cultured time (h) (Arranz and Peinado 2017).
Preparation of liquid inoculum
The single colony was added 5.0 mL LB medium and cultured overnight. Then, the bacterial suspension was inoculated into 1.0 L LB medium at 1.0% (vol./vol.) at 28 °C. After 16-h culture, the bacterial suspension was centrifuged at 5000 rpm for 5 min twice. The bacterial precipitate was resuspended in 6 different solutions (0.01 M, 0.10 M, and 0.20 M phosphate buffer (PB); 0.05 × LB, 0.1 × LB, and 0.2 × LB) and in different protective agents (1.0%, 3.0%, and 5.0% of trehalose, sucrose, sorbitol, PEG 6000, and glycerinum) with the resuspension solution volume of 1.0 L. Initial bacterial concentration (C0) was approximately 2.16 × 109 CFU mL−1 for FB2-3 and 2.34 × 109 CFU mL−1 for L5-4. After 12-week storage at 4 or 25 °C, the bacterial concentration (Ct) of the liquid inoculums was measured by dilution coating method. Finally, survival rates (A) were calculated according to the following formula.
where C0 and V0 are initial bacterial concentration (CFU mL−1) and initial volume (mL) of liquid inoculums; Ct and Vt are bacterial concentration (CFU mL−1) and volume (mL) of liquid inoculums after storage. The data presented were the average of at least three assays. Liquid inoculum was used directly after preparation and storage.
Pilot-scale BTF tests
The pilot-scale test was carried out at Hangzhou, China; two BTFs with same size were parallel connected (Fig. 1B). One BTF was inoculated with liquid inoculums (BTF_2), and the other BTF without inoculation served as a control (BTF_1). The operation parameters were as follows: two layers of packing materials (bamboo charcoal, purchased from Tianjin Grace Environment Co., LTD, Tianjin, China, size 30 ~ 50 mm), total loading height of 1.5 m, total loading volume of 0.75 m3, air flow of 300 m3 h−1, empty bed residence time (EBRT) of 18 s, the volume of circulating spray liquid of 0.5 m3, and uninterrupted circulating liquid spray flow of 500 L h−1. Every BTF was equipped with a circulating fluid tank containing approximately 400 L loading nutrient solution. The 4.0 L FB2-3 and 4.0 L L5-4 were inoculated into circulating fluid tank for bacterial activization and loading process; in contrast, sterile water was added into the circulating fluid tank of control BTFs. After 14-day circulating spray, the loading nutrient solution was replaced with water. Then, the exhaust gas containing NH3 and H2S passed through BTFs, and pH of circulating liquid was monitored by a FE-20 type pH meter (Mettler Toledo Instruments Co., LTD, Switzerland). Temperature was measured by a AS887 type double channel thermocouple thermometer (Smart Sensor Group Co. LTD, Hong Kong, China). NH3 and H2S concentration was monitored by a MX-6 iBrid type compound gas detector (Industrial Scientific, USA). Pilot-scale test was performed for 84 days. Finally, removal efficiency (RE), inlet load (IL), and elimination capacity (EC) were calculated according to the following formulas.
where Cinlet is the inlet NH3 or H2S concentration (mg (m3)−1); Coutlet is the outlet NH3 or H2S concentration (mg (m3)−1); Q is air flow (m3 h−1), and V is the total loading volume (m3) of packing materials (Fernández et al. 2014).
Bacterial community sequencing and analysis
A total of 200 g bamboo charcoal packing materials (lower layer) were collected for bacterial community analysis after the BTFs tests running for 30 days. Bacterial genomic DNA in bamboo charcoal packing materials was extracted by QIAamp ® Fast DNA Stool Mini Kit (Qiagen, Germany). The hypervariable V3-V4 region of the 16S rRNA gene from bamboo charcoal packing materials was subjected to high-throughput sequencing using the primers 338 F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806 R (5′-GGACTACHVGGGTWTCTAAT-3′) on an Illumina MiSeq platform, and subsequently analyzed by Personalbio Co., Ltd. (Shanghai, China). Each sample was tested in three parallel assays.
Data analysis
Statistical analysis was carried out using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA). The statistical significance was evaluated through Student’s t-test. P < 0.05 was considered statistically significant.
Results and discussion
Identification of NH4 +-N and S2− removal strains
The packing materials from the long-term running BTFs were used for isolating strains. As shown in Tables S1 and S2, FB2-3 and L5-4 strains exhibited the highest NH4+-N and S2− removal capacity. Both FB2-3 and L5-4 were determined to be gram-negative, non-spore-forming, and rod-shaped bacteria. After culturing on LB agar plates at 28 °C for 48 h, FB2-3 colonies were yellowish, round, and smooth with a bulged surface (Fig. S1A), whereas L5-4 colonies were white, round, and smooth with a bulged surface (Fig. S1B). As shown in Fig. 2, the phylogenetic tree showed that partial 16S rRNA gene sequences of strain FB2-3 (GenBank accession No. MW812239.1) and L5-4 (GenBank accession No. MZ935668) exhibited the highest similarities to the 16S rRNA gene of Kosakonia oryzae Ola 51(T) (GenBank accession No. CP014007) (99.86%) and Acinetobacter baumannii ATCC 19,606(T) (GenBank accession No. ACQB01000091) (99.73%). Therefore, morphologically and phylogenetically, FB2-3 and L5-4 were identified as Kosakonia oryzae strain and Acinetobacter baumannii strain, respectively.
Acinetobacter baumannii have been recognized as an environmental pollutant degrading bacterial agent (Zhang et al. 2021). Kosakonia oryzae have been recognized as a plant endophytic and growth-promoting strain (Preyanga et al. 2021) and have the biodegradation potential for pollutants (Dash and Osborne 2020). To the best of our knowledge, there has been no report on NH4+-N or S2− removal capacity of Kosakonia sp. Therefore, FB2-3 and L5-4 with highest NH4+-N or S2− removal capacity were selected for further investigation.
Effects of initial pH and culture temperature on NH4 +-N and S2− removal capacities for isolated bacteria
Previously, the autotrophic BTF inoculum Rhodococcus sp. zw11 showed growth tolerance at pH 5.5 to 6.5 and 20 to 28 °C (Zhang et al. 2009). The acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater showed good adaptabilities toward pH 4.5 to 10.0 and 20 to 40 °C (Yang et al. 2019). By contrast, FB2-3 and L5-4 exhibited good growth tolerance (bacterial suspension OD600 nm > 0.6) at pH 4.0–9.0 and pH 4.0–10.0 (Fig. S2A), 10–37 °C and 15–45 °C (Fig. S2B) in LB medium after 24-h culture, respectively, indicating both FB2-3 and L5-4 have good adaptabilities to the application environment.
Previously, combination used Enterobacter sp. Z1 and Klebsiella sp. Z2 exhibited remarkable abilities for simultaneous nitrogen removal (Zhang et al. 2019). Moreover, the Acinetobacter sp. MU1_03 and Alcaligenes faecalis MU2_03 exhibited more than 91% of hydrogen sulfide removal efficiency while a mixture of the two strains was capable of 98% hydrogen sulfide removal in the bioscrubber systems (Potivichayanon et al. 2006). To explore the possibility of combining these two strains, effects of pH and culture temperature on NH4+-N and S2− removal capacities and specific consumption rates under the optimal conditions were measured. Interestingly, under the same conditions of initial pH and culture temperature, FB2-3 + L5-4 exhibited higher NH4+-N and S2− removal capacities than single strain (FB2-3 or L5-4) (Fig. 3). The highest NH4+-N and S2− removal rates reached 96.46 ± 1.68% and 96.62 ± 1.61% at initial pH 7.0, respectively (Fig. 3A and B). The highest NH4+-N and S2− removal rates reached 97.31 ± 1.62% and 98.57 ± 1.42% at 32.0 °C, respectively (Fig. 3C and D).
Specific substrate consumption rate for isolated bacteria
As shown in Fig. 4, under the optimal conditions (pH 7.0 and 32.0 °C), FB2-3 + L5-4 exhibited higher maximum specific NH3 and H2S consumption rates (243.86 mgsub gDW−1 h−1 and 509.87 mgsub gDW−1 h−1) than single strain FB2-3 (217.57 mgsub gDW−1 h−1 and 277.55 mgsub gDW−1 h−1) or L5-4 (166.21 mgsub gDW−1 h−1 and 474.02 mgsub gDW−1 h−1). Similarly, Wang et al. (2019) reported that Acinetobacter sp. JQ1004 exhibited the maximum specific degradation rate of ammonium occurred at the substrate concentration of 13.94 mg L−1 with a value of 3.97 g gDCW−1 day−1 (165.41 mgsub gDW−1 h−1). Moreover, Cho K.S. et al. reported the heterotrophic bacteria Xanthomonas sp. strain DY44 exhibited the relatively lower maximum specific H2S removal rate of 3.92 mmol gdry cells−1 h−1 (133.28 mgsub gDW−1 h−1) at pH 7 and 30 °C (Cho et al. 1992). However, Lee et al. (2006) reported the chemoautotrophic bacteria Acidithiobacillus thiooxidans AZ1 exhibited a relatively higher maximum specific sulfur oxidation rate of 21.2 g gDCW−1 day−1 (883.33 mgsub gDW−1 h−1), which was observed at pH 1.5.
These results indicated the simultaneous application of these two bacteria has better odorous gas removal effects than the use of one bacterium alone. As a result, we combined FB2-3 and L5-4 to prepare liquid ready-to-use inoculums and further applied in BTFs.
Preparation of FB2-3 and L5-4 liquid inoculums
Since the microbial communities inhabiting the packing materials play a crucial role in the biodegradation of the pollutants (Wu et al. 2018, 2020), the development of new bacterial agents is of great significance for increasing the removal efficiencies of odorous gases in BTFs. Nowadays, the major commercial bacterial agents usually are prepared by using spray-drying method with main bacteria being spore-forming bacteria, since the dormant spore has the advantages of high resistance to high temperature, hunger, dryness, radiation, and others (Yánez-Mendizábal et al. 2012). In contrast, the drying survival rates of the non-spore-forming strains, especially thin cell-wall gram-negative bacteria including FB2-3 and L5-4, are relatively low; thus, they are not suitable for spray-drying (Wu et al. 2021; Yánez-Mendizábal et al. 2012). In this study, we developed the liquid bacterial inoculum preparation methods specifically for FB2-3 and L5-4 bacterial agents.
As shown in Fig. 5A, the addition of 1.0% polyethylene glycol (PEG) as the protective agent significantly increased the survival rate of FB2-3 during the bacterial agent preparation and storage, which is consistent with the previous reports (Muangchinda et al. 2020; Torres et al. 2003). During the preparation of FB2-3 liquid inoculum, water and 1.0% of PEG were selected as the resuspension solution and the protective agent, respectively, and the storage temperature was set as 25 °C. The survival rate of FB2-3 was 72.13 ± 2.01% with bacterial concentration of 1.56 × 109 CFU mL−1 after 12-week storage.
Effects of different resuspension solutions and protective agents on survival rates for FB2-3 (A) and L5-4 (B) in liquid inoculums after 12-week storage at 25 °C or 4 °C. a, Statistically significant compared with the control (water as the resuspension solution) after storage at 25 °C (P < 0.05). b, Statistically significant compared with the control (water as the resuspension solution) after storage at 4 °C (P < 0.05). c, Statistically significant compared with the control (water and 0.01 M PB respectively serving as the resuspension solution of FB2-3 and L5-4, with no protective agent) after storage at 25 °C (P < 0.05). d, Statistically significant compared with the control (water and 0.01 M PB respectively serving as resuspension solution of FB2-3 and L5-4, with no protective agent) after storage at 4 °C (P < 0.05)
As shown in Fig. 5B, the addition of 0.01 M PB and 1.0% trehalose as the resuspension liquid and the protective agent significantly improved the survival rate of L5-4 during the bacterial agent preparation and storage, which was in agreement with previous findings by Torres et al. (2003) and Sui et al. (2015). Therefore, 0.01 M PB and 1.0% trehalose were selected for preparation of L5-4 liquid inoculum. The survival rates of L5-4 were 44.94 ± 1.24% and 76.46 ± 4.67% after being stored at 25 °C and 4 °C for 12 weeks, respectively.
Considering the cost, the storage temperature was selected as 25 °C, and the bacterial concentration was 1.05 × 109 CFU mL−1 after 12-week storage. However, the survival rate of L5-4 in liquid inoculum was lower than that of FB2-3 under 25 °C storage conditions; thus, the addition of PB and trehalose needs to be further optimized in the future study.
Pilot-scale BTF tests
In the long-term running BTF_0, the NH3 and H2S removal capacities ranged from 45.2 to 100.0% and from 55.4 to 100% (Fig. 6A) at the NH3 inlet concentration of 6.8 ~ 228.9 mg (m3)−1 and H2S inlet concentration of 3.5 ~ 242.0 mg (m3)−1 (Fig. S3A). The daily average environment temperature was from 27.0 to 36.0 °C and running pH was from 5.3 to 7.2. The maximum NH3 and H2S EC of the BTF_0 were 52.3 g (NH3) m−3 h−1 and 58.4 g (H2S) m−3 h−1 with 57.1% and 60.3% removal efficiency, respectively. The critical NH3 and H2S elimination capacities (ECcrit) of the BTF_0 were 18.2 g (NH3) m−3 h−1 and 16.4 g (H2S) m−3 h−1 with > 95% removal efficiency, respectively (Fig. 6C and D).
For pilot-scale BTF tests, in the control BTF_1, both the NH3 and H2S removal capacities were < 45.0% (Fig. S4) at NH3 inlet concentration ranging from 0.8 to 345.7 mg (m3)−1 and H2S inlet concentration from 1.6 to 413.4 mg (m3)−1 (Fig. S3B), respectively. Both the maximum NH3 and H2S ECs of BTF_1 were < 10.0%. Interestingly, after 8-day inoculation with FB2-3 and L5-4 liquid inoculums, NH3 and H2S removal capacities in BTF_2 respectively ranged from 87.8 to 100.0% and from 79.1 to 94.35% (Fig. 6B) at the NH3 inlet concentration of 0.9 ~ 380.0 mg (m3)−1 and H2S inlet concentration of 1.5 ~ 474.0 mg (m3)−1 (Fig. S3C). The daily average environment temperature was from 18.8 to 34.0 °C, and running pH was from 4.3 to 7.0. The maximum NH3 and H2S ECs of the BTF_2 were 136 g (NH3) m−3 h−1 and 176 g (H2S) m−3 h−1 with 89.5% and 93.0% removal efficiency, respectively. The critical NH3 and H2S elimination capacities (ECcrit) of the BTF_2 were 40.0 g (NH3) m−3 h−1 and 71.4 g (H2S) m−3 h−1 with > 95% removal efficiency, respectively (Fig. 6C and D). By contrast, the maximum NH3 and H2S EC were ranged from 38.7 to 241 g (NH3) m−3 h−1 and 4.6 to 370 g (H2S) m−3 h−1 after inoculated with Acinetobacter sp., Alcaligenes faecalis, Paracoccus pantotrophus, Paracoccus versutus, nitrifying bacteria, sulfide-oxidizing bacteria, or activated sludge (Table 1) (Aroca et al. 2007; Chen et al. 2014, 2019; Huan et al. 2021a; Juntranapaporn et al. 2019; Potivichayanon et al. 2006; Watsuntorn et al. 2020; Xue et al. 2010). The results indicated that the BTF inoculated with FB2-3 and L5-4 liquid inoculums exhibited excellent NH3 and H2S removal capacities.
Bacterial community analysis of bamboo charcoal packing materials after running
Biofilm formed on the packing materials is the key factor affecting the odorous gas removal from BTFs (Mudliar et al. 2010; Wu et al. 2020). As shown in Figure S5, biofilm formation on bamboo charcoal was clearly observed by naked eye and Scanning Electron Microscopy (SEM) after inoculated with FB2-3 and L5-4 liquid inoculums and running for 84 days. Since the high-throughput sequencing is frequently used method to examine the microbial communities, especially the unculturable strains, in the biofilm on the surface of packing materials in BTFs (Wu et al. 2018), the microbial communities of packing materials were analyzed by high-throughput sequencing in this study. A total of 1,749,828 (range from 91,702 to 129,928) valid sequences were extracted from the twelve samples (Table S3). In total, 52 to 2053 operational taxonomic units (OTUs) were detected from the twelve samples at the genus level. The biodiversity of bamboo charcoal packing material samples inoculated with the liquid inoculums was significantly decreased, relative to that of bamboo charcoal packing material samples without inoculation (Fig. S6), indicating that addition of the liquid inoculums could decrease the biodiversity of the microbial community (Sun et al. 2020).
This study explored the similarities and differences in the bacterial communities among the bamboo charcoal packing material samples at the genus levels after BTF running. In the long-term running BTFs, the dominant bacteria (BPM_0) in the packing materials were Sulfobacillus (50.33 ± 8.42%), Mycobacterium (7.45 ± 1.31%), Alicyclobacillus (5.40%), Novosphingobium (4.57 ± 1.86%), and Azospirillum (1.45 ± 0.59%). In BTF_1, the dominant bacteria in the bamboo charcoal packing materials without liquid inoculum inoculation (BPM_1) were Brochothrix (20.94 ± 3.08%), Bacillus (1.66 ± 2.62%), Lactobacillus (10.76 ± 6.51%), Aquabacterium (6.54 ± 2.90%), and Serratia (6.80 ± 0.68%), whereas the dominant bacteria in the bamboo charcoal packing materials (BPM_2) inoculated with the FB2-3 and L5-4 liquid inoculums were Acinetobacter (63.73 ± 6.32%), Mycobacterium (17.84 ± 5.93%), Kosakonia (4.25 ± 1.78%), and Sulfobacillus (9.54 ± 1.21%) (Fig. 7A). Acinetobacter genera have been reported to be enriched in a pilot-scale biofilter (Gandu et al. 2021) and have the ability to remove H2S (Potivichayanon et al. 2006). Mycobacterium and Sulfobacillus genera were likely the players for H2S oxidation, based on their capability of the oxidation of sulfur compounds (Bu et al. 2022). At present, there is no relevant report on the removal of NH3 or H2S by Kosakonia genera. In addition, as shown in Table 1, the composition of BTF_2 microflora is different from that reported previously.
Further principal coordinates analysis (PCoA) based on weighted UniFrac distances (Fig. 7B) showed that the bamboo charcoal packing material samples exhibited high similarities in bacterial community composition after liquid inoculum inoculation in different pilot-scale tests, whereas the bamboo charcoal packing materials samples with or without inoculation and packing materials samples from the long-term running BTFs exhibited differences. Moreover, the comparison of bacterial communities at the genus level showed that the relative abundances of Acinetobacter and Kosakonia were significantly increased in bamboo charcoal packing materials inoculated with the liquid inoculums in BTF_2 (Fig. S7). However, both the heterotrophic bacteria Kosakonia and Acinetobacter are not the abundant genus in the long-term running BTFs_0. This result might be due to the growth rate of some dominant autotrophic bacteria in BTFs_0 is relatively lower than that of heterotrophic bacteria under the screening condition. Nevertheless, after inoculation with easily cultivated liquid inoculums, the new microbial community composed of Kosakonia and Acinetobacter and other genera were formed in the packaging materials also exhibited effectively treat NH3 and H2S exhaust gases in BTFs.
Conclusion
In the present study, the NH4+-N and S2− removal strains FB2-3 and L5-4 were successfully isolated from the packing materials in the long-running BTFs. Combined utilization of FB2-3 and L5-4 exhibited high NH4+-N and S2− removal capacities of 97.31 ± 1.62% with maximum specific NH3 consumption rate 243.86 mgsub gDW−1 h−1 and 98.57 ± 1.12% with maximum specific H2S consumption rate 509.87 mgsub gDW−1 h−1 and good tolerance to pH, temperature, and salt. Water and 0.01 M PB were selected as the resuspension solution, and 1.0% of PEG and 1.0% of trehalose as the protective agent respectively for preparation of FB2-3 and L5-4 liquid inoculums at 25 °C. The pilot-scale test of BTF inoculated with FB2-3 and L5-4 showed that NH3 and H2S could be effectively removed (removal rates > 87%, maximum NH3 and H2S ECs were reached 136 g (NH3) m−3 h−1 and 176 g (H2S) m−3 h−1 with 89.5% and 93.0% removal efficiency, respectively) from the exhaust gases, and that the relative abundances of genera Acinetobacter (L5-4) and Kosakonia (FB2-3) were significantly increased in the packing materials, whereas the diversity of entire bacterial communities was decreased, indicating that these two strains have the potential to be developed into the liquid ready-to-use inoculums for effectively removing NH3 and H2S from BTFs.
Data Availability
Not applicable.
Abbreviations
- BTF:
-
Biotrickling filter
- NH3 :
-
Ammonia
- H2S:
-
Hydrogen sulfide
- VOCs:
-
Volatile organic chemicals
- BTF_0:
-
The long-term running BTFs in a pharmaceutical factory
- BTF_1:
-
The BTFs without inoculated bacterial agent
- BTF_2:
-
The BTFs inoculated bacterial agent
- BPM_0:
-
The bamboo charcoal packing materials samples from BTF_0
- BPM_1:
-
The bamboo charcoal packing materials samples from BTF_1
- BPM_2:
-
The bamboo charcoal packing materials samples from BTF_2
- EC:
-
Elimination capacity
- ECmax:
-
Maximum elimination capacity
- ECcrit:
-
Critical elimination capacity
- IL:
-
Inlet load
References
An Q, Zhou Y, Zhao B, Huang XL (2020) Efficient ammonium removal through heterotrophic nitrification-aerobic denitrification by Acinetobacter baumannii strain AL-6 in the presence of Cr(VI). J Biosci Bioeng 130:622–629. https://doi.org/10.1016/j.jbiosc.2020.07.010
Aroca G, Urrutia H, Núñez D, Oyarzún P, Arancibia A, Guerrero K (2007) Comparison on the removal of hydrogen sulfide in biotrickling filters inoculated with Thiobacillusthioparus and Acidithiobacillusthiooxidans. Electron J Biotechn 10:514–520. https://doi.org/10.2225/vol10-issue4-fulltext-6
Arranz FJ, Peinado JM (2017) A mesoscopic stochastic model for the specific consumption rate in substrate-limited microbial growth. PLoS ONE 12:e0171717. https://doi.org/10.1371/journal.pone.0171717
Barbusinski K, Kalema K, Kasperczyk D, Urbaniec K, Kozik V (2017) Biological methods for odor treatment - a review. J Clean Prod 152:223–241. https://doi.org/10.1016/j.jclepro.2017.03.093
Bu H, Carvalho G, Huang C, Sharma KR, Yuan Z, Song Y, Bond P, Keller J, Yu M, Jiang G (2022) Evaluation of continuous and intermittent trickling strategies for the removal of hydrogen sulfide in a biotrickling filter. Chemosphere 291:132723. https://doi.org/10.1016/j.chemosphere.2021.132723
Chen Y, Fan Z, Ma L, Yin J, Luo M, Cai W (2014) Performance of three pilot-scale immobilized-cell biotrickling filters for removal of hydrogen sulfide from a contaminated air steam. Saudi J Biol Sci 21:450–456. https://doi.org/10.1016/j.sjbs.2014.05.008
Chen Y, Xie L, Cai W, Wu J (2019) Pilot-scale study using biotrickling filter to remove H2S from sewage lift station: experiment and CFD simulation. Biochem Eng J 144:177–184. https://doi.org/10.1016/j.bej.2019.02.003
Cho KS, Hirai M, Shoda M (1992) Degradation of hydrogen sulfide by Xanthomonas sp. strain DY44 isolated from peat. Appl Environ Microbiol 58:1183–1189. https://doi.org/10.1128/aem.58.4.1183-1189.1992
Dash DM, Osborne WJ (2020) Rapid biodegradation and biofilm-mediated bioremoval of organophosphorus pesticides using an indigenous Kosakoniaoryzae strain -VITPSCQ3 in a vertical-flow packed bed biofilm bioreactor. Ecotoxicol Environ Saf 192:110290. https://doi.org/10.1016/j.ecoenv.2020.110290
De Vela RJ, Wigley K, Baronian K, Gostomski PA (2021) Effect of metabolic uncouplers on the performance of toluene-degrading biotrickling filter. Environ Sci Pollut Res Int 28:41881–41895. https://doi.org/10.1007/s11356-021-13708-w
Ferdowsi M, Ramirez AA, Jones JP, Heitz Me (2017) Elimination of mass transfer and kinetic limited organic pollutants in biofilters: a review. Int Biodeter Biodegr 119, 336e348. https://doi.org/10.1016/j.ibiod.2016.10.015.
Fernández M, Ramírez M, Gómez JM, Cantero D (2014) Biogas biodesulfurization in an anoxic biotrickling filter packed with open-pore polyurethane foam. J Hazard Mater 264:529–535. https://doi.org/10.1016/j.jhazmat.2013.10.046
Gandu B, Palanivel S, Juntupally S, Arelli V, Begum S, Anupoju GR (2021) Removal of NH3 and H2S from odor causing tannery emissions using biological filters: impact of operational strategy on the performance of a pilot-scale bio-filter. J Environ Sci Health A Tox Hazard Subst Environ Eng 56:625–634. https://doi.org/10.1080/10934529.2021.1903283
García-Pérez T, Hernández-Jiménez S, Revah S (2020) Operational parameters in H(2)S biofiltration under extreme acid conditions: performance, biomass control, and CO(2) consumption. Environ Sci Pollut Res Int 27:4502–4508. https://doi.org/10.1007/s11356-019-06789-1
Guo J, Gao Q (2021) Enhancement of ethylbenzene removal from contaminated gas and corresponding mechanisms in biotrickling filters by a biosurfactant from piggery wastewater. J Environ Manage 277:111411. https://doi.org/10.1016/j.jenvman.2020.111411
Haosagul S, Prommeenate P, Hobbs G, Pisutpaisal N (2020) Sulfide-oxidizing bacteria community in full-scale bioscrubber treating H2S in biogas from swine anaerobic digester. Renew Energ 150:973–980. https://doi.org/10.1016/j.renene.2019.11.139
Huan C, Fang J, Tong X, Zeng Y, Liu Y, Jiang X, Ji G, Xu L, Lyu Q, Yan Z (2021a) Simultaneous elimination of H2S and NH3 in a biotrickling filter packed with polyhedral spheres and best efficiency in compost deodorization. J Clean Prod 284:124708. https://doi.org/10.1016/j.jclepro.2020.124708
Huan C, Lyu Q, Tong X, Li H, Zeng Y, Liu Y, Jiang X, Ji G, Xu L, Yan Z (2021b) Analyses of deodorization performance of mixotrophic biotrickling filter reactor using different industrial and agricultural wastes as packing material. J Hazard Mater 420:126608. https://doi.org/10.1016/j.jhazmat.2021.126608
Jeon YS, Lee K, Park SC, Kim BS, Cho YJ, Ha SM, Chun J (2014) EzEditor: a versatile sequence alignment editor for both rRNA- and protein-coding genes. Int J Syst Evol Microbiol 64:689–691. https://doi.org/10.1099/ijs.0.059360-0
Juntranapaporn J, Vikromvarasiri N, Soralump C, Pisutpaisal N (2019) Hydrogen sulfide removal from biogas in biotrickling filter system inoculated with Paracoccuspantotrophus. Int J Hydrogen Energ 44:29554–29560. https://doi.org/10.1016/j.ijhydene.2019.03.069
Lebrero R, Estrada JM, Muñoz R, Quijano G (2012) Toluene mass transfer characterization in a biotrickling filter. Biochem Eng J 60:44–49. https://doi.org/10.1016/j.bej.2011.09.017
Lee EY, Lee NY, Cho KS, Ryu HW (2006) Removal of hydrogen sulfide by sulfate-resistant Acidithiobacillusthiooxidans AZ11. J Biosci Bioeng 101:309–314. https://doi.org/10.1263/jbb.101.309
Morral E, Gabriel D, Dorado AD, Gamisans X (2021) A review of biotechnologies for the abatement of ammonia emissions. Chemosphere 273:128606. https://doi.org/10.1016/j.chemosphere.2020.128606
Morral E, Dorado AD, Gamisans X (2022) A novel bioscrubber for the treatment of high loads of ammonia from polluted gas. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-19065-6
Muangchinda C, Srisuwankarn P, Boubpha S, Chavanich S, Pinyakong O (2020) The effect of bioaugmentation with Exiguobacterium sp. AO-11 on crude oil removal and the bacterial community in sediment microcosms, and the development of a liquid ready-to-use inoculum. Chemosphere 250:126303. https://doi.org/10.1016/j.chemosphere.2020.126303
Mudliar S, Giri B, Padoley K, Satpute D, Dixit R, Bhatt P, Pandey R, Juwarkar A, Vaidya A (2010) Bioreactors for treatment of VOCs and odours - a review. J Environ Manage 91:1039–1054. https://doi.org/10.1016/j.jenvman.2010.01.006
Potivichayanon S, Pokethitiyook P, Kruatrachue M (2006) Hydrogen sulfide removal by a novel fixed-film bioscrubber system. Process Biochem 41:708–715. https://doi.org/10.1016/j.procbio.2005.09.006
Preyanga R, Anandham R, Krishnamoorthy R, Senthilkumar M, Gopal NO, Vellaikumar A, Meena S (2021) Groundnut (Arachishypogaea) nodule Rhizobium and passenger endophytic bacterial cultivable diversity and their impact on plant growth promotion. Rhizosphere 17:100309. https://doi.org/10.1016/j.rhisph.2021.100309
Ren B, Zhao Y, Lyczko N, Nzihou A (2019) Current status and outlook of odor removal technologies in wastewater treatment plant. Waste Biomass Valori 10:1443–1458. https://doi.org/10.1007/s12649-018-0384-9
Rybarczyk P, Szulczyński B, Gębicki J, Hupka J (2019) Treatment of malodorous air in biotrickling filters: a review. Biochem Eng J 141:146–162. https://doi.org/10.1016/j.bej.2018.10.014
Ryu HW, Cho KS, Lee TH (2011) Reduction of ammonia and volatile organic compounds from food waste-composting facilities using a novel anti-clogging biofilter system. Bioresour Technol 102:4654–4660. https://doi.org/10.1016/j.biortech.2011.01.021
Schiavon M, Ragazzi M, Rada EC, Torretta V (2016) Air pollution control through biotrickling filters: a review considering operational aspects and expected performance. Crit Rev Biotechnol 36:1143–1155. https://doi.org/10.3109/07388551.2015.1100586
Sironi S, Capelli L, Centola P, Rosso RD, Pierucci S (2010) Odour impact assessment by means of dynamic olfactometry, dispersion modelling and social participation. Atmos Environ 44:354–360. https://doi.org/10.1016/j.atmosenv.2009.10.029
Sui Y, Wisniewski M, Droby S, Liu J (2015) Responses of yeast biocontrol agents to environmental stress. Appl Environ Microbiol 81:2968–2975. https://doi.org/10.1128/aem.04203-14
Sun X, Chen L, Liu C, Xu Y, Ma W, Ni H (2020) Biodegradation of CP/TCP by a constructed microbial consortium after comparative bacterial community analysis of long-term CP domesticated activated sludge. J Environ Sci Health B 55:898–908. https://doi.org/10.1080/03601234.2020.1794453
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Torres R, Usall J, Teixidó N, Abadias M, Viñas I (2003) Liquid formulation of the biocontrol agent Candida sake by modifying water activity or adding protectants. J Appl Microbiol 94:330–339. https://doi.org/10.1046/j.1365-2672.2003.01843.x
Wang X, Wang W, Zhang Y, Zhang J, Li J, Wang S, Chen G (2019) Isolation and characterization of Acinetobacter sp. JQ1004 and evaluation of its inhibitory kinetics by free ammonia. Desalin Water Treat 147:316–325. https://doi.org/10.5004/dwt.2019.23757
Watsuntorn W, Khanongnuch R, Chulalaksananukul W, Rene ER, Lens PNL (2020) Resilient performance of an anoxic biotrickling filter for hydrogen sulphide removal from a biogas mimic: steady, transient state and neural network evaluation. J Clean Prod 249:119351. https://doi.org/10.1016/j.jclepro.2019.119351
Wu H, Yan H, Quan Y, Zhao H, Jiang N, Yin C (2018) Recent progress and perspectives in biotrickling filters for VOCs and odorous gases treatment. J Environ Manage 222:409–419. https://doi.org/10.1016/j.jenvman.2018.06.001
Wu H, Yang M, Tsui T-H, Yin Z, Yin C (2020) Comparative evaluation on the utilization of applied electrical potential in a conductive granule packed biotrickling filter for continuous abatement of xylene: performance, limitation, and microbial community. J Environ Manage 274:111145. https://doi.org/10.1016/j.jenvman.2020.111145
Wu P, Wang Z, Zhu Q, Xie Z, Mei Y, Liang Y, Chen Z (2021) Stress preadaptation and overexpression of rpoS and hfq genes increase stress resistance of Pseudomonas fluorescens ATCC13525. Microbiol Res 250:126804. https://doi.org/10.1016/j.micres.2021.126804
Xue N, Wang Q, Wu C, Zhang L, Xie W (2010) Enhanced removal of NH3 during composting by a biotrickling filter inoculated with nitrifying bacteria. Biochem Eng J 51:86–93. https://doi.org/10.1016/j.bej.2010.05.007
Yánez-Mendizábal V, Viñas I, Usall J, Torres R, Solsona C, Abadias M, Teixidó N (2012) Formulation development of the biocontrol agent Bacillus subtilis strain CPA-8 by spray-drying. J Appl Microbiol 112:954–965. https://doi.org/10.1111/j.1365-2672.2012.05258.x
Yang J-R, Wang Y, Chen H, Lyu Y-K (2019) Ammonium removal characteristics of an acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater capable of heterotrophic nitrification-aerobic denitrification. Bioresource Technol 274:56–64. https://doi.org/10.1016/j.biortech.2018.10.052
Zhang L, Wang Q, Tian S, Wang X, Xie W, Sun P (2009) Biodegradation of hydrogen sulphide by inoculated Rhodococcus sp.zw11 in a pilot-scale biotrickling filter. Int J Environ Pollut 37:450–465. https://doi.org/10.1504/IJEP.2009.026061
Zhang X, Kong D, Liu X, Xie H, Lou X, Zeng C (2021) Combined microbial degradation of crude oil under alkaline conditions by Acinetobacter baumannii and Talaromyces sp. Chemosphere 273:129666. https://doi.org/10.1016/j.chemosphere.2021.129666
Zhang Y, Xu Z, Li J, Liu D, Yuan Y, Chen Z, Wang G (2019) Cooperation between two strains of Enterobacter and Klebsiella in the simultaneous nitrogen removal and phosphate accumulation processes. Bioresour Technol 291:121854. https://doi.org/10.1016/j.biortech.2019.121854
Acknowledgements
Great gratitude goes to linguistics Prof. Ping Liu from Huazhong Agricultural University, Wuhan, China, for her work at English editing and language polishing.
Funding
The work was supported by the Fundamental Research Funds for the Central Universities of China (Grant No. 2662021FW006) and Key Research and Development Program of Hubei Province, China (Grant No. 2020BAB095).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Investigation, formal analysis, and writing—original draft were performed by QZ. PW, BC, QW, FC, HW, and XS contributed to methodology, resources, and data curation. LX and YM contributed to co-supervision and methodology. The supervision, project administration, funding acquisition, and writing—review and editing were ensured by ZC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Gerald Thouand
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Q., Wu, P., Chen, B. et al. Improving NH3 and H2S removal efficiency with pilot-scale biotrickling filter by co-immobilizing Kosakonia oryzae FB2-3 and Acinetobacter baumannii L5-4. Environ Sci Pollut Res 30, 33181–33194 (2023). https://doi.org/10.1007/s11356-022-24426-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24426-2