Abstract
The Pal/Rim pathway essential for fungal adaptation to ambient pH has been unexplored in Beauveria bassiana, a classic fungal entomopathogen. Here, we show the characterized Pal pathway comprising transcription factor PacC and upstream six Pal partners (PalA/B/C/F/H/I) in B. bassiana. Their coding genes were all transcribed most abundantly in standard wild-type culture under the alkaline condition of pH 9. Deletion of pacC or each pal gene resulted in a significant delay of culture acidification in a minimal broth (initial pH = 7.3). This delay concurred with altered accumulation levels of intra/extracellular organic acids and drastically depressed expression of some enzyme genes required for the syntheses of oxalic and lactic acids. Our deletion mutants except ΔpalI showed growth defects and maximal sensitivity to NaCl, KCl, LiCl, or sorbitol at pH 9, an alkaline condition leading to fragmented vacuoles in their hyphal cells exposed to osmotic stress. In these mutants, conidiation was significantly facilitated at pH 3 more than at pH 7 but suppressed slightly at pH 9. Mild virulence defects also occurred in the absence of pacC or any pal gene. These changes were restored by targeted gene complementation. Taken together, PacC and Pal partners regulate the growth, conidiation, and osmotolerance of B. bassiana in a pH-dependent manner, highlighting their vitality for the fungal pH response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ambient pH is an environmental stimulus to induce an array of physiological and cellular events in microorganisms. Fungal adaptation to ambient pH relies on homeostasis of intracellular pH and proper expression of permeases, secreted proteases, toxins, and antibiotics (Peñalva and Arst 2002; Peñalva et al. 2008). The Rim101/Pal pathway is known to dominate fungal response to ambient pH (Tilburn et al. 1995; Peñalva and Arst 2004). This pathway relies on one or two steps of activation of transcription factor (TF) Rim101 in Saccharomyces cerevisiae (Lamb and Mitchell 2003) or PacC in Aspergilli (Mingot et al. 1999; Diez et al. 2002). The activated TF with the C-terminus being cleaved for proteolysis may activate genes expressed under alkaline conditions but represses those expressed under acidic conditions (Peñalva et al. 2008). These responsive genes are involved in various cellular events, such as growth, ion tolerance, cell differentiation, cell wall remodeling, secondary metabolism, and host infection (Nobile et al. 2008; Zou et al. 2010; Merhej et al. 2011; Cupertino et al. 2012; Cornet and Gaillardin 2014; Huang et al. 2014; O’Meara et al. 2014).
The pH-responsive pathway has been intensively studied in model fungi, such as Aspergillus nidulans, and summarized in a revised model (Peñalva et al. 2014). Six Pal proteins (PalA/B/C/F/H/I) upstream of PacC are involved in the pH signal transduction under neutral-to-alkaline conditions (Arst et al. 1994; Negrete-Urtasun et al. 1999). These proteins are conserved in yeasts and filamentous fungi (Fonseca-García et al. 2012; Daval et al. 2013; Trushina et al. 2013; Cornet and Gaillardin 2014). Of those, PalH and PalI harboring multiple transmembrane domains act as putative pH sensors (Arst and Peñalva 2003), and PalI may also assist PalH localization to plasma membrane (Calcagno-Pizarelli et al. 2007). PalF is an arrestin-related protein binding to the PalH C-terminus and hence can be phosphorylated and ubiquitinated in a PalH-dependent manner (Herranz et al. 2005; Hervás-Aguilar et al. 2010). The ubiquitinated PalF can recruit a key endosomal sorting complex required for transcript (ESCRT) I component (Vps23) to the plasma membrane for the recruitment of PalC and Pal A through a Bro1 domain (Galindo et al. 2012). The activation of PacC by proteolysis requires interactions of Vps32 and Vps20 (two ESCRT-III components) with PalA and of Vps24 with PalB (Vincent et al. 2003; Selvig and Alspaugh 2011; Cornet and Gaillardin 2014), a calpain family cysteine protease that is localized to the plasma membrane like PalA and PalC in an alkaline pH-dependent manner (Lucena-Agell et al. 2015). Upon activation, PacC moves into nuclei and binds to GCCARG-containing sequences (i.e., PacC sites) in the promoter regions of target genes for the regulation of their expression (Espeso et al. 1997; Mingot et al. 2001; Fernández-Martínez et al. 2003).
The cellular response to environmental pH is of special importance for filamentous fungal insect pathogens, such as Beauveria bassiana and Metarhizium anisopliae sensu lato, because they infect wide spectra of host insects via cuticular penetration and have been widely applied for insect pest control (Wang and Feng 2014). The biological control potential of a fungal insecticide is largely dependent on the secretion of cuticle-degrading enzymes for the penetration, such as proteases, chitinases, glycosidases, and lipases whose activities are greatly affected by ambient pH (St Leger et al. 1997). These enzymes require an appropriate pH range to function properly and hence to ensure hyphal penetration through the host cuticle for entry into the host hemocoel, where multicellular hyphae turn into unicellular blastospores (hyphal bodies) for rapid propagation by budding until the host is mycotized to death. The rapid propagation is a result of their resisting osmotic stress in the hemocoel and evading the host defense immunity (Lewis et al. 2009; Chen et al. 2014). Ambient pH also affects the activities of some enzymes associated with acidification or alkalization of host tissues in the infection course of plant pathogenic fungi (Prusky and Yacoby 2003). In M. anisopliae, cuticle-degrading enzymes have been shown to be expressed in a pH-dependent manner (St Leger et al. 1998); secreted alkaline proteases are also expressed in a microenvironment with a suitable pH level adjusted by produced ammonia (St Leger et al. 1999). In B. bassiana, oxalic acid has been shown to act as a pH-dependent virulence factor in pathogenesis (Kirkland et al. 2005). The production of oosporein, a major secondary metabolite in B. bassiana, has also proved pH-dependent (Luo et al. 2014). Despite the special importance, the Pal pathway required for pH regulation has been unexplored in filamentous insect pathogens. Therefore, this study seeks to characterize the B. bassiana Pal pathway comprising PacC, PalA, PalB, PalC, PalF, PalH, and PalI orthologous to those in A. nidulans.
Materials and methods
Microbial strains and media
The wild-type strain B. bassiana ARSEF 2860 (designated WT) and its mutants were grown at optimal 25 °C in Sabouraud dextrose agar (SDAY, 4 % glucose, 1 % peptone, and 1.5 % agar plus 1 % yeast extract) buffered with 50 mM Na2HPO4-citric acid to pH 3 or 7 and with 50 mM Tris-HCl to pH 9 for phenotypic experiments or not buffered for standard cultures. Their stress responses were assayed in 1/4 SDAY (1/4 of each SDAY nutrient) buffered as above. Escherichia coli TOP10 and E. coli DH5α from Invitrogen (Shanghai, China) were cultivated in Luria-Bertani media at 37 °C for plasmid propagation. Agrobacterium tumefaciens AGL-1 cultivated in YEB (Fang et al. 2004) was used as a T-DNA donor for fungal transformation.
Cloning and analysis of pacC and pal genes
All PacC and Pal sequences of A. nidulans, S. cerevisiae, and Candida albicans in the NCBI database were used as queries to search through the B. bassiana genome (Xiao et al. 2012) at http://blast.ncbi.nlm.nih.gov/blast.cgi. The coding sequences of located PacC and Pal orthologues were amplified from the WT via PCR with designed primers (Table S1 in the Supplementary Material), followed by sequencing at Invitrogen. The protein sequences derived from the verified genes were individually analyzed online to reveal their domains, followed by phylogenetic analysis with MEGA5 software (Tamura et al. 2011).
Constructing single-gene deletion and complementary mutants
The 5′ and 3′ fragments of pacC and each pal gene were cloned from the WT via PCR with paired primers (Table S1 in the Supplementary Material) under the action of LaTaq DNA polymerase (TaKaRa, Dalian, China) and inserted into the proper enzymes sites (listed in Table S1 in the Supplementary Material) of the backbone plasmid p0380-bar (Xie et al. 2012), yielding p0380-5′x-bar-3′x (x: pacC or each pal gene). Further, a full-length nucleotide sequence of each target gene with flanking regions was amplified from the WT and ligated into p0380-sur-gateway under the action of Gateway® BP Clonase™ II Enzyme Mix (Invitrogen) to exchange for the gateway fragment, resulting in p0380-sur-x. Each target gene was deleted by the recombination of p0380-5′x-bar-3′x in the WT and rescued by ectopic integration of p0380-sur-x into its deletion mutant by means of Agrobacterium-mediated transformation (Fang et al. 2004). Putative mutant colonies on selective plates were screened in terms of the bar resistance to phosphinothricin (200 μg/ml for deletion mutants) or the sur resistance to chlorimuron ethyl (10 μg/ml for complementary mutants). Correct recombination events in the mutants were identified via PCR and Southern blotting with paired primers and amplified probe (Fig. S1 in the Supplementary Material). Probe preparation, membrane hybridization, and visualization were carried out using DIG High Primer DNA Labeling and Detection Starter Kit II (Roche, Mannheim, Germany). Positive deletion mutants were evaluated together with the parental WT and complementary mutants (control strains) in triplicate experiments.
Assessing culture acidification rates, organic acid levels, and accumulated ammonia levels
For each of the fungal strains, three aliquots of 50 ml 105 conidia/ml Czapek broth (CZB, 3 % sucrose, 0.3 % NaNO3, 0.1 % K2HPO4, 0.05 % KCl, 0.05 % MgSO4, and 0.001 % FeSO4; pH 7.3) in flasks were shaken at 150 rpm for 7 days at 25 °C. During the period, the pH level of each liquid culture was measured daily using an electronic pH detector. When pH levels largely differed between deletion mutants and control strains on day 5, a 10-ml sample taken from each flask was filtered through filter papers. Hyphal cells of 0.2 g from each sample were ground in liquid nitrogen and suspended in 10 ml ultrapure water, followed by centrifugation at 11,000×g to remove hyphal debris. Possible proteins in the supernatant were removed by adding an equal volume of chloroform/isopropanol (25:1). Organic acids in the filtered supernatant and those in the deproteinized solution were quantified as extracellular concentrations (μg/ml) and intracellular contents (mg/g), respectively, via ion-exchange chromatography on a Dionex ICS-2000 (Dionex Corporation, Sunnuvale, CA, USA), as described elsewhere (Wang et al. 2014). The examined acids were lactic, oxalic, citric, and pyruvic acids. A solution of each acid (10 μg/ml) was used as the standard for each of the quantified acids in the samples.
Assaying cellular responses to osmotic agents
Three aliquots of 1 μl 106 conidia/ml suspension of each strain were spotted onto the plates of 1/4 SDAY (pH adjusted to 3, 7, or 9) supplemented with NaCl (0.8 M), KCl (0.8 M), LiCl (10 mM), and sorbitol (1.5 M), respectively. After 7 days of incubation at 25 °C in a light/dark cycle of 12:12 h, diameters of all colonies were cross-measured as indices of growth rates in response to each of the osmotic agents.
Transcriptional profiling of target genes
To quantify transcript levels of pacC and pal genes in the WT, 100 μl aliquots of 107 conidia/ml suspension were evenly spread on cellophane-overlaid SDAY plates, followed by 3 days of incubation at 25 °C to collect cultures for RNA extraction. Hyphal cells from the 5-day-old CZB cultures of the WT and deletion mutants were also collected for RNA extraction to assess gene transcripts of several enzymes involved in the metabolism of different organic acids. Total RNAs were separately extracted from the collected cultures under the action of an RNAiso™ Plus Reagent (TaKaRa) and reversed into complementary DNAs (cDNAs) under the action of a PrimerScript® RT reagent kit (TaKaRa). Tenfold dilution of each cDNA was used as a template to assess transcript levels of the target genes in each strain via quantitative real-time PCR (qRT-PCR) with paired primers (Table S1 or S2 in the Supplementary Material). The γ-actin transcript of B. bassiana was used as an internal standard. Three cDNA samples were analyzed under the action of a SYBR® Premix Ex Taq™ (TaKaRa). Relative transcript levels of each gene were calculated as ratio of its transcripts over the γ-actin transcripts in the WT or of the transcripts in a deletion mutant over that in the WT using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Analysis of intracellular vacuolation
Conidia from the SDAY culture of each strain were suspended in Sabouraud dextrose broth (SDB = agar-free SDAY, adjusted pH = 9) alone (control) or supplemented with 0.8 M NaCl (treatment). All the SDB suspensions were standardized to 106 conidia/ml and shaken at 25 °C for 3 days. Hyphal cells collected from the cultures were repeatedly washed with 50 mM phosphate buffer saline (PBS), followed by a series of treatments required for transmission electronic microscopy (TEM) of their ultrathin sections, as described previously (Wang et al. 2014).
Assays for phenotypes associated with biological control potential
A germination medium (2 % sucrose and 0.5 % peptone) at pH = 3, 7, or 9 was used to quantify the trend of conidial germination of each strain over a period of 24-h incubation at 25 °C, and median germination time (GT50) was estimated by modeling analysis of the trend (Xie et al. 2013). Colony growth rates at the three pH levels were evaluated by measuring the diameters of 7-day-old colonies initiated by spotting 1 μl of conidial suspension per SDAY plate. To quantify conidiation capacity, SDAY cultures at the three pH levels were initiated by spreading 100 μl of conidial suspension per plate. After 7 days of incubation at 25 °C and 12:12 h, three colony discs (5-mm diameter) were bored from each plate culture using a cork borer. Conidia on each disc were washed off into 1 ml of 0.02 % Tween-80 via vortex. The conidial concentration in the suspension was determined using a hemocytometer and converted to the number of conidia per square centimeter culture. In addition, morphologic features of the conidia produced at different pH levels were examined under a microscope. Conidial size and density of fungal strains showing morphological changes at pH 9 were quantified as the readings of forward scatter (FSc) and side scatter (SSc) detectors from the flow cytometry of three samples of 2 × 104 conidia per strain, as described previously (Qiu et al. 2014).
Conidia from 7-day-old standard SDAY cultures were assayed for the virulence of each strain to Galleria mellonella larvae (∼300 mg per capita) infected through cuticular and cuticle-bypassing routes, respectively. For cuticular infection, batches of ∼35 larvae were immersed in 30 ml aliquots of 107 conidia/ml suspension (treatment) or 0.02 % Tween-80 (control) for 10 s, followed by transferring the larvae onto towel paper for the removal of excessive water. The cuticle-bypassing infection was achieved by injecting 5 μl of 105 conidia/ml suspension (treatment) or 0.02 % Tween-80 (control) into the hemocoel of each larva in each batch. After inoculation, each batch of the treated larvae was maintained in a large Petri dish for 7 days at 25 °C and monitored daily for mortality records. Median lethal time (LT50 in day) of each strain against the larvae was estimated by probit analysis of time-mortality trend. The bioassay of each type was repeated three times. Conidial thermotolerance and UV-B resistance were assayed by exposing three samples of conidia to a wet-heat stress at 45 °C for 0–90 min and UV-B irradiation (weighted wavelength 312 nm) at the doses of 0 –0.5 J/cm2 as described previously (Xie et al. 2012, 2013). The median lethal time LT50 (min) for conidial thermotolerance and the medium lethal dose LD50 (J/cm2) for conidial UV-B resistance were estimated by modeling analysis of conidial survival trends over the gradient intensities of the two stresses, respectively.
Data analysis
All phenotypic observations, measurements, and fitted parameters from the experiments of three replicates were subjected to one-factor (strain) analysis of variance (ANOVA), followed by Tukey’s honestly significant difference (HSD) test to distinguish the means of each phenotype between each deletion mutant and its two control strains.
Results
Features of PacC and Pal orthologues in B. bassiana
PacC, PalA, PalB, PalC, PalF, PalH, and PalI orthologous to those in A. nidulans, S. cerevisiae, and C. albicans were located in the genome database of B. bassiana (Xiao et al. 2012) through the online search. As illustrated in Fig. S2a in the Supplementary Material, the located PacC (590 aa) harbors two conserved C2H2-type zinc-finger overlapping domains (residues 98–123 and 112–134). All the located Pal orthologues structurally coincide well with those described in A. nidulans (Peñalva et al. 2008, 2014). PalH (858 aa) and PalI (701 aa) contain multiple transmembrane domains, and PalF (905 aa) is a typical arrestin-related protein. PalA (865 aa) has a BRO1 domain and a YPXL/I motif, hinting to a likelihood that it interacts with ESCRT components and binds to the signaling protease cleavage site of PacC. The identified PalB (833 aa) possesses microtubule interacting and transport (MIT), calpain (CysPc), and calpain III domains. PalC (495 aa) has a domain typical for the BRO1_Alix_Like superfamily. In sequence alignment analysis, all the orthologues identified in B. bassiana are relatively closer to those in Cordyceps militaris, Fusarium oxysporum, and Metarhizium robertsii (previously classified to M. anisopliae sensu lato) than Aspergilli and the yeasts (Fig. S2b–h in the Supplementary Material).
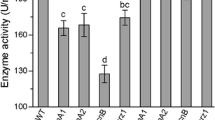
Transcript levels of pacC and pal genes in the 3-day-old WT culture grown in SDAY at 25 °C varied with pH level (Fig. 1). These genes were transcribed more abundantly at pH 9, but less abundantly at pH 3, than at pH 7. Exceptionally, palF and palH were slightly upregulated under the acidic condition. Thus, the pacC and all pal genes in B. bassiana were expressed in a pH-dependent manner.
Deletions of pacC and pal genes slowed down culture acidification
During 7 days of culturing the suspension of 1 × 106 conidia/ml CZB (initial pH = 7.3) at 25 °C, all deletion mutants except ΔpalI showed similar culture pH levels (Fig. 2a) on days 4 (7.10–7.15) and 5 (6.27–6.81), and these levels were significantly higher than those of the control strains (day 4, 6.40–6.91; day 5, 4.47–4.93). The culture was acidified more rapidly in ΔpalI (pH = 6.95 on day 4 and 5.51 on day 5) than in other deletion mutants but significantly slower than in the WT (Tukey’s HSD, P = 0.0113 on day 4 and 0.0244 on day 5). These pH changes disappeared on day 6 (4.34–4.47, F 10,22 = 1.92, P = 0.066) and day 7 (data not shown). The significant delay of the culture acidification by either pacC or any pal deletion indicated positive roles of all the target genes in the fungal pH regulation.
The Pal pathway regulates the culture acidification of B. bassiana. a pH levels in the cultures of deletion mutants (white bars) and control strains (WT and complemented strains; gray bars) grown at 25 °C in CZB (pH 7.3) for 4, 5, and 6 days, respectively. b Contents (mg/g dry biomass) of lactic acid (LA), oxalic acid (OA), and citric acid (CA) in the hyphal cells from 5-day-old CZB cultures. c LA, OA, and CA concentrations (μg/ml) in the supernatants of the 5-day-old CZB cultures. Asterisked bars differ significantly from those unmarked (Tukey’s HSD, P < 0.05). d Relative transcript levels of five acid-related genes in seven deletion mutants versus WT. Error bars: SD from three replicates (a) or samples (b–d)
Intracellular contents (Fig. 2b) and extracellular concentrations (Fig. 2c) of three organic acids were quantified from the 5-day-old CZB cultures. Lactic acid content decreased in ΔpalC and ΔpalH versus the WT but increased in other deletion mutants except ΔpalF. The contents of oxalic and citric acids were decreased and increased, respectively, in six deletion mutants but unchanged in ΔpalI. Extracellular concentrations of lactic and oxalic acids dropped in the six mutants excluding ΔpalI, which showed minor or little change. In contrast, the citric acid concentration was elevated by 2.2- to 5.5-fold in all the ΔpacC and Δpal mutants compared to the WT. These changes were restored by targeted gene complementation. However, pyruvic acid was undetectable in the samples of all the tested strains.
Several genes involved in the metabolism of organic acids (Magnuson and Lasure 2004) were examined for their transcript levels in the cDNA samples derived from the 5-day-old CZB cultures. The gene transcript of a lactate dehydrogenase (Ldh1) required for lactic acid synthesis was reduced by 90–98 % in six deletion mutants and 50 % in ΔpalI compared with the WT (Fig. 2d). Another Ldh (Ldh2) transcript was decreased by 92 % in ΔpalB and 33–54 % in four deletion mutants but unaffected in ΔpalC and ΔpalH. The expression of an oxaloacetate acetylhydrolase (Oac) essential for oxalic acid synthesis was suppressed by 87–97 % in all the seven mutants. However, these mutants exhibited much less or little transcript change in citrate synthase (Cst) or pyruvate carboxylase (Pcb).
Deletions of pacC and pal genes increased pH-dependent osmosensitivity and fragmented intracellular vacuoles
During 7 days of cultivation at 25 °C, six deletion mutants excluding ΔpalI showed an osmotolerance decreased with increasing pH level (Fig. 3). NaCl and KCl added to the medium reduced their colony growth by 19–35 and 18–20 % at pH 3, 39–46 and 28–31 % at pH 7, and 60–62 and 83–100 % at pH 9, respectively. Co-cultivation with LiCl reduced their growth by 27–31 % at pH 7 and 55–58 % at pH 9 despite little effect at pH 3. Similarly, their sensitivity to sorbitol increased by 30–40 % at pH 7 and 57–68 % at pH 9 but changed slightly or insignificantly at pH 3. Exceptionally, ΔpalI showed null response to all the osmotic agents under the tested conditions (Tukey’s HSD, P > 0.05) except its response to NaCl at pH 3. All the control strains were equally responsive to each osmotic agent at each pH level. Apparently, pacC and all pal genes except palI regulated positively the fungal osmoresponse in a pH-dependent manner.
The Pal pathway regulates B. bassiana osmosensitivity in a pH-dependent manner. a–l Diameters of 7-day-old fungal colonies grown at 25 °C on the plates of 1/4 SDAY supplemented with 0.8 M NaCl (a–c), 0.8 M KCl (d–f), 10 mM LiCl (g–i), or 1.5 M sorbitol (j–l) and adjusted to the pH levels of 3 (left column), 7 (middle column), and 9 (right column), respectively. White bars: deletion mutants. Gray bars: WT and complementary strains. Asterisked bars differ significantly from those unmarked (Tukey’s HSD, P < 0.05). Error bars: SD from three replicates
To gain insight into the mutant hypersensitivity to osmotic stress under the alkaline condition, hyphal cells co-cultivated with or without NaCl at pH 9 were subjected to TEM analysis. Large vacuoles appeared in the NaCl-free cells of the WT and each mutant (Fig. 4a). Co-cultivation of the WT with NaCl did not affect either vacuolar size or morphology (Fig. 4b). In contrast, vacuoles became fragmented in the NaCl-stressed cells of all the deletion mutants except ΔpalI showing little change in vacuolation.
The alkaline pH-dependent regulation of cellular osmosensitivity by the Pal pathway is linked to intracellular vacuolation. a, b TEM images of hyphal cells (scale bars 0.1 μm) from the 3-day-old SDB cultures co-cultivated with or without 0.8 M NaCl at pH 9. Arrows indicate vacuolar changes of most mutant cells in the absence (a) and presence (b) of NaCl under the same alkaline condition
Contributions of pacC and pal genes to biological control potential
All the deletion mutants grew as well as their control strains in SDAY at pH 3 or 7 (data not shown). In contrast, colony growth at pH 9 was suppressed by 48–53 % for all the mutants except ΔpalI (Fig. 5a). The suppression was independent of conidial germination rates, which were similar for all the tested strains at each of the three pH levels (Fig. S3 in the Supplementary Material). Conidial yields from the 7-day-old SDAY cultures grown at pH 3 (Fig. 5b) and 7 (Fig. 5c) increased significantly (Tukey’s HSD, P < 0.05) in the mutants except ΔpalA and ΔpalC showing an insignificant change at pH 7. The yield at pH 9 decreased in five mutants but increased in ΔpalH and was unchanged in ΔpalI (Fig. 5d). Microscopic examination revealed remarkable changes in conidial size and morphology for most of the deletion mutants grown at pH 9, but such changes were inconspicuous at pH 3 and 7 (Figs. S4–S6 in the Supplementary Material). The FSc and SSc readings from the flow cytometry of 2 × 104 conidia produced at pH 9 indicated greater increases in the conidial size (Fig. 5e) and density (Fig. 5f) of six mutants (19–34 and 35–61 %, respectively) than of ΔpalI (12 and 14 %). These increases were significant compared with the readings from the control strains.
Deletions of pacC and pal genes resulted in phenotypic changes associated with the biological control potential of B. bassiana. a Diameters of fungal colonies grown for 7 days at 25 °C after each plate were spotted with 1 μl of conidial suspension. b–d Conidial yields measured from the 7-day-old SDAY cultures at three pH levels, respectively. Each plate culture was initiated by spreading 100 μl of conidial suspension. e, f Conidial size and density indicated by the respective FSc and SSc readings from the flow cytometry of 2 × 104 conidia produced at pH 9. g, h LT50s from time-mortality trends of G. mellonella larvae inoculated for normal cuticular penetration (immersion) and cuticle-bypassing infection (intrahemocoel injection), respectively. White bars: deletion mutants. Gray bars: WT and complementary strains. Asterisked bars differ significantly from those unmarked (Tukey’s HSD, P < 0.05). Error bars: SD from three replicates (a–d, g, and h) or samples (e, f)
Conidial virulence, thermotolerance, and UV-B resistance determinant to biological control potential were quantified as LT50 and LD50. In standardized bioassays, all the mutants took significantly longer time to kill 50 % of G. mellonella larvae than their control strains (Tukey’s HSD, P < 0.05) irrespective of topical application (immersion) of 107 conidia/ml suspension for cuticular infection (Fig. 5g) or hemocoel injection of 500 conidia per larva for cuticle-bypassing infection (Fig. 5h). However, neither conidial thermotolerance nor UV-B resistance differed significantly between each deletion mutant and the WT based on their LT50 (min) and LD50 (J/cm2) estimates (Fig. S7 in the Supplementary Material).
Discussion
In B. bassiana, pacC and all pal genes were transcribed most abundantly under the alkaline condition. This suggests a link of the pal genes to the fungal PacC activation like their orthologues in A. nidulans (Diez et al. 2002; Fernandez-Martinez et al. 2003). The positive roles of pacC and six pal genes in the fungal pH regulation are unveiled by delayed culture acidification and altered acid accumulation after any of the genes was deleted. Our mutants except ΔpalI displayed similar rates of culture acidification, a phenomenon not reported previously, although intra/extracellular levels of three organic acids were more or less different one another. As one of major acids secreted by many fungi, oxalic acid is often related to metal detoxification and pathogenesis (Gadd 1999; Fomina et al. 2005). In this study, similar reductions of intra/extracellular oxalic acid levels concurred with drastic oac depression in six of the mutants, indicating that the deletions of pacC and five pal genes hindered oxalic acid synthesis that requires the Oac activity (Magnuson and Lasure 2004). Tanscriptional suppression of ldh2 essential for lactic acid synthesis differed largely among the six mutants despite their ldh1 transcripts depressed consistently by >90 %. Perhaps for this reason, the six mutants showed greater variability in intra/extracellular lactic acid levels. These mutants showed higher intra/extracellular citric acid levels than the control strains, but the changes of their cst transcripts were ignorable, suggesting other enzymes likely involved in citric acid synthesis and secretion. This speculation also suits to ΔpalI, whose citric acid was maximal at extracellular level but unchanged at intracellular level. Taken together, pacC and pal genes could regulate differential gene expression required for synthesis and secretion of organic acids. Thus, transcriptional depression of some genes, such as oac and ldh1/2, in each deletion mutant could alter accumulation levels of different acids responsible for the delay of culture acidification.
Moreover, pacC and five pal genes excluding palI have proved essential for cellular response to osmotic salts/agents, and their osmoregulative roles are all pH-dependent in B. bassiana. The deletion mutants of these genes were considerably consistent in response to each of three osmotic metal salts and one osmotic carbohydrate tested in this study. Their osmosensitivity was maximal at pH 9, intermediate at pH 7, and minimal or unchanged at pH 3. Previously, a M. robertsii ΔpacC mutant showed higher sensitivity to NaCl, KCl, and LiCl, but null response to sorbitol, only at an unknown pH level (Huang et al. 2014). We consider that the pH-dependent sensitivities of our deletion mutants to different metal salts are the results of their responding to osmotic stress rather than metal toxicity because their responses to sorbitol were also pH-dependent. The high osmosensitivity shown by the mutants is largely attributable to fragmented vacuoles in hyphal cells under the alkaline condition. This is also supported by the exceptional ΔpalI mutant, which showed null response to all the osmotic metal salts and carbohydrate but unchanged vacuolation under the alkaline condition. Our observations in B. bassiana are in agreement with a report from Ustilago maydis, in which six deletion mutants except ΔpalI were highly sensitive to osmotic agents under alkaline conditions (Cervantes-Chávez et al. 2010).
Furthermore, the Pal pathway regulates growth and conidiation of B. bassiana in a pH-dependent manner. Growth defects exhibited by the abovementioned six mutants at pH 9 disappeared at pH 3 or 7, coinciding with the defective growth of the same mutants in U. maydis under an alkaline condition (Cervantes-Chávez et al. 2010). For all or most of our deletion mutants, conidiation was differentially facilitated at pH 3 or 7 but slightly suppressed at pH 9, accompanied with increased conidial size and density under the alkaline condition. This is the first report on the pH-dependent regulation of conidiation, conidial size, and conidial density by fungal pal genes. Notably, our ΔpacC conidiation levels at different pH levels are different from those of M. robertsii ΔpacC, in which conidiation was partially suppressed at pH 4 and completely inhibited at pH 10 (Huang et al. 2014).
Finally, pacC and six pal genes are all mild virulence regulators in B. bassiana. Our ΔpacC mutant showed a mild virulence defect as did the M. robertsii ΔpacC (Huang et al. 2014) but very different from a Cryptococcus neoformans pacC disruptant, in which host death was accelerated because in the latter, pacC was associated with cell wall remodeling and evasion of the host immune responses (O’Meara et al. 2013, 2014). Overall, the Pal pathway contributed significantly to the virulence of B. bassiana but little to conidial thermotolerance and UV-B resistance, which are also influential on the fungal potential against arthropod pests.
References
Arst HN Jr, Peñalva MA (2003) pH regulation in Aspergillus and parallels with higher eukaryotic regulatory systems. Trends Genet 19:224–231
Arst HN Jr, Bignell E, Tilburn J (1994) Two new genes involved in signaling ambient pH in Aspergillus nidulans. Mol Gen Genet 245:787–790
Calcagno-Pizarelli AM, Negrete-Urtasun S, Denison SH, Rudnicka JD, Bussink HJ, Múnera-Huertas T, Stanton L, Hervás-Aguilar A, Espeso EA, Tilburn J, Arst HN Jr, Peñalva MA (2007) Establishment of the ambient pH signaling complex in Aspergillus nidulans: PalI assists plasma membrane localization of PalH. Eukaryot Cell 6:2365–2375
Cervantes-Chávez JA, Oetiz-Castellanos L, Tejeda-Sartorius M, Gold S, Ruiz-Herrera J (2010) Functional analysis of the pH responsive pathway Pal/Rim in the phytopathogenic basidiomycete Ustilago maydis. Fungal Genet Biol 47:446–457
Chen Y, Zhu J, Ying SH, Feng MG (2014) Three mitogen-activated protein kinases required for cell wall integrity contribute greatly to biocontrol potential of a fungal entomopathogen. PLoS ONE 9, e87948
Cornet M, Gaillardin C (2014) pH signaling in human fungal pathogens: a new target for antifungal strategies. Eukaryot Cell 13:342–352
Cupertino FB, Freitas FZ, de Paula RM, Bertolini MC (2012) Ambient pH controls glycogen levels by regulating glycogen synthase gene expression in Neurospora crassa: new insight into pH signaling pathway. PLoS ONE 7, e44258
Daval S, Lebreton L, Gracianne C, Guillerm-Erckelboudt AY, Boutin M, Marchi M, Gazengel K, Sarniguet A (2013) Strain-specific variation in a soilborne phytopathogenic fungus for the expression of genes involved in pH signal transduction pathway, pathogenesis and saprophytic survival in response to environmental pH changes. Fungal Genet Biol 61:80–89
Díez E, Alvaro J, Espeso EA, Rainbow L, Suárez T, Tilburn J, Arst HN Jr, Peñalva MA (2002) Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J 21:1350–1359
Espeso EA, Tilburn J, Sanchez-Pulido L, Brown CV, Valencia A (1997) Specific DNA recognition by the Aspergillus nidulans three zinc finger transcription factor PacC. J Mol Biol 274:466–480
Fang WG, Zhang YJ, Yang XY, Zheng XL, Duan H, Li Y, Pei Y (2004) Agrobacterium tumefaciens-mediated transformation of Beauveria bassiana using an herbicide resistance gene as a selection marker. J Invertebr Pathol 85:18–24
Fernández-Martínez J, Brown CV, Diez E, Tilburn J, Arst HN Jr, Peñalva MA, Espesso EA (2003) Overlap of nuclear localization signal and specific DNA binding residues within the zinc finger domain of PacC. J Mol Biol 334:667–684
Fomina M, Hillier S, Charnock JM, Melville K, Alexander IJ, Gadd GM (2005) Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl Environ Microbiol 71:371–381
Fonseca-García C, León-Ramirez CG, Ruiz-Herrera J (2012) The regulation of different metabolic pathways through the Pal/Rim pathway in Ustilago maydis. FEMS Yeast Res 12:547–556
Gadd GM (1999) Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. Adv Microbiol Physiol 41:47–92
Galindo A, Calcagno-Pizarelli AM, Arst HN Jr, Peñalva MA (2012) An ordered pathway for the assembly of ESCRT-containing fungal ambient pH signaling complexes at the plasma membrane. J Cell Sci 125:1784–1795
Herranz S, Rodríguez JM, Bussink HJ, Sanchez-Ferrero JC, Arst HN Jr, Peñalva MA, Vincent O (2005) Arrestin-related proteins mediate pH signaling in fungi. Proc Natl Acad Sci U S A 102:12141–12146
Hervás-Aguilar A, Galindo A, Peñalva MA (2010) Receptor-independent ambient pH signaling by ubiquitin attachment to fungal arrestin-like PalF. J Biol Chem 285:18095–18102
Huang W, Shang YF, Chen PL, Gao Q, Wang CS (2014) MrPacC regulates sporulation, insect cuticle penetration and immune evasion in Metarhizium robertsii. Environ Microbiol 17:994–1008
Kirkland BH, Eisa A, Keyhani NO (2005) Acid as a fungal acaracidal virulence factor. J Med Entomol 42:346–351
Lamb TM, Mítchell AP (2003) The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol Cell Biol 23:677–686
Lewis MW, Robalino IV, Keyhani NO (2009) Uptake of the fluorescent probe FM4-64 by hyphae and haemolymph-derived in vivo hyphal bodies of the entomopathogenic fungus Beauveria bassiana. Microbiol-SGM 155:3110–3120
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lucena-Agell D, Galindo A, Arst HN Jr, Peñalva MA (2015) Aspergillus nidulans ambient pH signaling does not require endocytosis. Eukaryot Cell 14:545–553
Luo ZB, Li Y, Mousa J, Bruner S, Zhang Y, Pei Y, Keyhani NO (2014) Bbmsn2 acts as a pH-dependent negative regulator of secondary metabolite production in entomopathogenic fungus Beauveria bassiana. Environ Microbiol 17:1189–1202
Magnuson JK, Lasure LL (2004) Organic acid production by filamentous fungi. In: Tkacz JS, Lange L (eds) Advantages in fungal biotechnology for industry, agriculture, and medicine. Kluwer Academic/Plenum Publishers, Dordrecht, pp 307–340
Merhej J, Richard-Forget F, Barreau C (2011) The pH regulatory factor Pac1 regulates Tri gene expression and trichothecene production in Fusarium graminearum. Fungal Genet Biol 48:275–284
Mingot JM, Tilburn J, Diez E, Bignell E, Orejas M, Widdick DA, Sarkar S, Brown CV, Caddick MX, Espeso EA, Arst HN Jr, Peñalva MA (1999) Specificity determinants of proteolytic processing of Aspergillus PacC transcription factor are remote from the processing site, and processing occurs in yeast if pH signaling is bypassed. Mol Cell Biol 19:1390–1400
Mingot JM, Espeso EA, Díez E, Peñalva MA (2001) Ambient pH signaling regulates nuclear localization of the Aspergillus nidulans PacC transcription factor. Mol Cell Biol 21:1688–1699
Negrete-Urtasun S, Reiter W, Díez E, Dension SH, Tilburn J, Espeso EA, Peñalva MA, Arst HN Jr (1999) Ambient pH signal transduction in Aspergillus: completion of gene characterization. Mol Microbiol 33:994–1003
Nobile CJ, Solis N, Myers CL, Fray AJ, Deneault JS, Nantel A, Mitichell AP, Filler SG (2008) Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell Microbiol 10:2180–2196
O’Meara TR, Holmer SM, Selvig K, Dietrich F, Alspaugh JA (2013) Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. mBio 4:e00522–12
O’Meara TR, Xu WJ, Selvig KM, O’Meara MJ, Mitchell AP, Alspaugh JA (2014) The Cryptococcus neoformans Rim101 transcription factor directly regulates genes required for adaption to the host. Mol Cell Biol 34:673–684
Peñalva MA, Arst HN Jr (2002) Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol Mol Biol Rev 66:426–446
Peñalva MA, Arst HN Jr (2004) Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu Rev Microbiol 58:425–451
Peñalva MA, Tilburn J, Bignell E, Arst HN Jr (2008) Ambient pH gene regulation in fungi: making connections. Trends Microbiol 16:291–300
Peñalva MA, Lucena-Agell D, Arst HN Jr (2014) Liaison alcaline: Pals entice non-endosomal ESCRTs to the plasma membrane for pH signaling. Curr Opin Microbiol 22:49–59
Prusky D, Yacoby N (2003) Pathogenic fungi: leading or led by ambient pH? Mol Plant Pathol 4:509–516
Qiu L, Wang JJ, Chu ZJ, Ying SH, Feng MG (2014) Phytochrome controls conidiation in response to red/far-red light and daylight length and regulates multistress tolerance in Beauveria bassiana. Environ Microbiol 16:2316–2318
Selvig K, Alspaugh JA (2011) pH response pathways in fungi: adapting to host-derived and environmental signals. Mycobiology 39:245–256
St Leger RJ, Joshi L, Roberts DW (1997) Adaptation of proteases and carbohydrates of saprophytic, phytopathogenic and entomopathogenic fungi to the requirements of their ecological niches. Microbiol-SGM 143:1983–1992
St Leger RJ, Joshi L, Roberts D (1998) Ambient pH is a major determinant in the expression of cuticle-degrading enzymes and hydrophobin by Metarhizium anisopliae. Appl Environ Microbiol 64:709–713
St Leger RJ, Nelson JO, Screen SE (1999) The entomopathogenic fungus Metarhizium anisopliae alters ambient pH, allowing extracellular protease production and activity. Microbiol-SGM 145:2691–2699
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tilburn J, Sarkar S, Widdick DA, Espeso EA, Orejas M, Mungroo J, Peñalva MA, Arst HN Jr (1995) The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J 14:779–790
Trushina N, Levin M, Mukherjee PK, Horwitz BA (2013) PacC and pH dependent transcriptome of the mycotrophic fungus Trichoderma virens. BMC Genomics 14:138
Vincent O, Rainbow L, Tilburn J, Arst HN Jr, Peñalva MA (2003) YPXL/I is a protein interaction motif recognized by Aspergillus PalA and its human homologue AIP1/Alix. Mol Cell Biol 23:1647–1655
Wang CS, Feng MG (2014) Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol Control 68:129–135
Wang XX, He PH, Feng MG, Ying SH (2014) BbSNF1 contributes to cell differential, extracellular acidification, and virulence in Beauveria bassiana, a filamentous entomopathogenic fungus. Appl Microbiol Biotechnol 98:8657–8673
Xiao GH, Ying SH, Zheng P, Wang ZL, Xie XQ, St Leger RJ, Zhao GP, Wang CS, Feng MG (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep-UK 2:483
Xie XQ, Li F, Ying SH, Feng MG (2012) Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS ONE 7, e30298
Xie XQ, Guan Y, Ying SH, Feng MG (2013) Differentiated functions of Ras1 and Ras2 proteins in regulating the germination, growth, conidiation, multi-stress tolerance and virulence of Beauveria bassiana. Environ Microbiol 15:447–462
Zou CG, Tu HH, Liu XY, Tao N, Zhang KQ (2010) PacC in the nematophagous fungus Clonostachys rosea controls virulence to nematodes. Environ Microbiol 12:1868–1877
Acknowledgments
We thank Jun-Ying Li (Analysis Center of Agrobiology and Environmental Sciences, Zhejiang University) for technical assistance with TEM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Numbers 31270537, 3157110241, and 31321063).
Conflict of interest
All the authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 3667 kb)
Rights and permissions
About this article
Cite this article
Zhu, J., Ying, SH. & Feng, MG. The Pal pathway required for ambient pH adaptation regulates growth, conidiation, and osmotolerance of Beauveria bassiana in a pH-dependent manner. Appl Microbiol Biotechnol 100, 4423–4433 (2016). https://doi.org/10.1007/s00253-016-7282-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7282-5