Abstract
Protein phosphatase type 1 (PP1) plays an important role in cellular metabolism and development in yeast. In PP1 enzyme complex, Glc8 protein is a global regulatory subunit and regulates many physiological processes. However, its biological roles are unexplored in filamentous fungi. In this study, we characterized a yeast ortholog of Glc8 in Beauveria bassiana, a filamentous entomopathogenic fungus. Gene disruption of BbGlc8 had no significant effect on vegetative growth, but resulted in a significant reduction in conidiation (51%) and blastospore yield (55%) in the mutant. The ΔBbGlc8 mutant displayed an enhanced sensitivity to oxidative stress and a weakened virulence as indicated by cuticle infection and intrahemocoel injection assays. Transcriptomic analysis indicated that the genes regulated by BbGlc8 during conidiation were primarily associated with metabolism, cell rescue and cell wall formation. Notably, as a down-regulated gene in ΔBbGlc8 mutant, BbOsmC2 (a member of OsmC protein family) contributes to fungal resistance to salt stress, spore differentiation and virulence. Thus, BbOsmC2 functions as a down-stream target of BbGlc8 during spore differentiation, but not in stress response. Our findings indicate that BbGlc8 contributes to the biocontrol potential of B. bassiana by mediating comprehensive genetic pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein phosphatase type 1 (PP1), belonging to the PPP family of protein phosphatase, regulates a plethora of physiological processes in yeast cells (e.g., budding yeast and fission yeast), including ion homeostasis, glycogen metabolism, transcription, sporulation and so on (Williams-Hart et al. 2002; Cannon 2010; Martín and Lopez‑Aviles 2018). In yeast, PP1 enzyme functions as a heterotrimeric complex containing a catalytic subunit (Glc7) and its distinctive substrate specificities are dependent on variable regulatory subunits (e.g., Glc8, Pan1 and Pex31) (Cannon 2010). In fungi, Glc8 gene was initially characterized in a glycogen-deficient mutant of budding yeast, and encodes a homologue of PP1 inhibitor-2 (I-2) in mammalian cells (Cannon et al. 1994). Inhibitor I-2 regulates the PP1 activity via changing the conformation of the catalytic subunit (Tan et al. 2003). As a global regulator, Glc8 protein functions in vivo as a major activator of Glc7 protein phosphatase activity, but does not change the Glc7 protein level in cells (Cannon 2010). Under certain physiological conditions, the excessive Glc8 protein also acts as an inhibitor of Glc7 acitivity (Tung et al. 1995). In Saccharomyces cerevisiae, Glc8 is a heat-stable protein and specific for PP1 enzyme (Li et al. 2007). As a global regulator, Glc8 is involved in many physiological processes in yeast. For example, Glc8 regulates the metabolism of carbohydrates by sensing the changes of glucose content. Glc8 expression is repressed by extracellular glucose, and Glc7 protein is completely activated in the absence of glucose (Nigavekar et al. 2002). Glc8 is involved in regulation of glycogen synthase, and its loss reduces glycogen accumulation in cells (Cannon et al. 1994). Additionally, Glc8 controls the chromosome segregation in yeast cell cycle (Tung et al. 1995). To date, the Glc8 roles in yeasts have been well-studied, but its roles in filamentous fungi are not completely revealed.
Filamentous fungi are characterized with extensive hyphal networks and have the comprehensive impacts on ecosystems (Klein and Paschke 2004). Beauveria bassiana, a filamentous fungus, is a ubiquitous insect pathogen with broad host spectrum and has been widely used for the biocontrol of insect pests (De la Cruz Quiroz et al. 2015). In nature, fungal conidia attach to the body surface of host and invade into host by breaching the cuticle (Lewis et al. 2009; Wanchoo et al. 2009). After reaching the hemocoel, invasive hyphae undergo morphological transition to generate yeast-like hyphal bodies (in vivo blastospore) which utilize various nutrients in hemolymph (Lewis et al. 2009). After killing the host, hyphae penetrate through the cuticle again and grow on the host cadaver followed by generation of numerous conidia (He et al. 2015, 2016). In B. bassiana, several virulence-related pathways have been characterized to be associated with autophagy (e.g., BbATG1), cell cycle (e.g., BbCdc14), metabolism (e.g., BbSNF1), cytoskeleton (e.g., BbGEL1) and so on (Ying et al. 2016; Wang et al. 2013, 2014; He et al. 2016), but none was associated with the protein dephosphorylation pathway regulated by PP1.
In this report, we used B. bassiana as a representative of filamentous fungus to explore the roles of Glc8 in fungal development and pathogenicity. The results indicate that B. bassiana Glc8 (BbGlc8) significantly contributes to fungal sporulation (including conidial and blastospore development) and virulence. Comparative transcriptomics between the wild-type and ΔBbGlc8 mutant strains indicated that BbGlc8 gene mediated sets of genes during conidial development. Among the differentially repressed genes, BbOsmC2 (a member of osmotically inducible protein C family) was verified to be involved in spore development and virulence. This study suggests that Glc8 gene functions as an important role in fungal asexual development associated with lifecycle of B. bassiana.
Materials and methods
Microbial strains and cultivation
Microbial strains were cultivated as previously described (Ying et al. 2014). The wild type of B. bassiana ARSEF2860 (Bb2860) (US Plant, Soil and Nutrition Laboratory, Tower Road, Ithaca, NY, USA) is the first isolate whose genome has been sequenced, and it is used as model strain of B. bassiana (Xiao et al. 2012). Fungal strain was maintained on Sabouraud dextrose agar (SDAY: 4% glucose, 1% peptone and 1.5% agar plus 1% yeast extract). Escherichia coli DH5α (Invitrogen, Carlsbad, CA, USA) was cultured in Luria–Bertani (LB) medium with required antibiotics for plasmid proliferation. Agrobacterium tumefaciens AGL-1 (a bacterium) was proliferated in YEB broth (w/v: 0.5% sucrose, 1% peptone, 0.1% yeast extract and 0.05% MgSO4) and added as a donor strain in fungal transformation. Czapek-Dox agar (CZA) (3% glucose, 0.3% NaNO3, 0.1% K2HPO4, 0.05% KCl, 0.05% MgSO4 and 0.001% FeSO4 plus 1.5% agar) was used to screen the transformants.
Phylogenetic analysis of the B. bassiana Glc8 protein
The sequence of yeast Glc8 protein (GenBank No.: AAA53673) was used as a query to search the potential homologs in the Bb2860 genome (Xiao et al. 2012), and then the B. bassiana homolog (Locus tag: BBA_06202) was identified and named as BbGlc8. Then, the cDNA sequence of BbGlc8 protein was mapped onto the genome sequence of B. bassiana, and its whole open reading frame (ORF) was obtained.
The Glc8 orthologs in fungi were obtained from the NCBI protein database using the online BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/). The protein sequences of all orthologs were aligned with ClustalW, and their phylogenetic relationships were constructed with MEGA version 5 (Tamura et al. 2011).
Construction of BbGlc8 gene disruption and complemented strains
The BbGlc8 gene disruption vector was constructed as previously described (Gao et al. 2018b), and all the required primers are listed in Supporting Information Table S1. In brief, upstream (1.04 kb) and downstream (1.35 kb) flanking sequences of BbGlc8 ORF were prepared by PCR reaction with the primer pair PG1/PG2 and PG3/PG4, respectively. The resultant PCR fragments were ligated into the EcoRI/BamHI and XbaI/HpaI sites of p0380-bar (Xie et al. 2012), respectively, using ClonExpress II One Step Cloning Kit (Vazyme Biotech, Nanjing, China). The resulting vector was named p0380-bar-Glc8 and used for gene disruption. To complement the gene disruption mutant, the BbGlc8 ORF plus 2.13 kb of upstream and 0.48 kb of downstream sequences was amplified with primers PG7/PG8. The resultant DNA fragment was cloned into the vector p0380-sur-gateway as previously described (Xie et al. 2012), generating the plasmid p0380-sur-Glc8 with sulfonylurea resistance marker. The disruption and complementation plasmids were introduced into the wild-type and gene disruption mutant strains, respectively, using Agrobacterium-mediated transformation procedure (Fang et al. 2004). Putative disruption mutants were screened on Czapek-Dox agar (CZA) supplemented with 200 µg/ml phosphinothricin, and the complemented strains were screened on CZA plates with 15 µg/ml chlorimuron ethyl. To confirm the correct recombination events, PCR reaction was performed in candidate transformants with primer PG5 and PG6 (Supplementary Table S1). Recombination events were further confirmed by Southern blot analysis, using DIG High Prime DNA Labeling and Detection Starter Kit II (Roche, Penzberg, Germany). The probes were labeled with digoxin, using DNA fragment (416 bp) amplified with primers PG9/GP10 (Supporting Information Table S1) as template. The target fragments were hybridized and visualized according to the manufacturer’s instruction.
Phenotypic analyses of fungal strains
Phenotypic assays, including conidial germination, radial growth, spore production, stress response and virulence, were performed as previously described (Ying et al. 2016). All assays were repeated three times.
Radial growth on plates
Aliquots of 1 µl conidial suspension (1 × 106 conidia/ml) were spotted on SDAY or CZA modified with different carbon and nitrogen sources. In carbon-modified CZA, carbon sources included (final concentration, 3%) glucose, sucrose, trehalose, glycerol, mannitol, fructose and maltose. In nitrogen-modified CZA, nitrogen sources (final concentration) included urea (0.3%), NH4Cl (0.3%), N-acetylglucosamine (0.5%), chitin (0.3%), proline (0.5%) and gelatin (0.3%). After 7 days of cultivation at 25 °C, the colony diameters were measured.
Responses to chemical stress
Chemical stress was initiated by adding different chemical reagents (final concentration) into CZA plates, including hypertonic reagents (0.5 M NaCl and 1M sorbitol), oxidation reagents (2 mM hydrogen peroxide and 0.02 mM menadione) and cell wall stressor (3 µg/ml Congo red). Aliquots of 1 µl suspensions (1 × 106 conidia/ml) were placed on plates and cultured at 25 °C for a week. Colony diameters were examined, using CZA plates without stress chemicals as a control.
Spore development and production
To assess conidial yield, aliquots of 100 µl of conidia suspension (107 conidia/ml) were smeared evenly on SDAY agar plates and incubated for 7 days at 25 °C. Mycelial disks (Ø 5 mm) were rinsed with 0.02% Tween 80 solution by vigorous vortex, and the conidial concentration was used to calculate conidial yield on mycelia as the number of conidia/cm2.
For blastospore production, conidia were inoculated into SDB broth at a final concentration of 106 conidia/ml. After an incubation of 3 days at 25 °C with constant shaking (150 rpm), spore concentration was measured and calculated as the number of cells per ml.
Conidial virulence
The conidial virulence was determined using Galleria mellonella larvae as bioassay insects via two infection routes. The 7-day old conidia were grown on SDAY plates. In cuticle infection bioassay, the larvae were submerged in the conidial suspension (1 × 107 conidia/ml) for 10 s. In intrahemocoel injection bioassay, 5 µl of conidial suspension (1 × 105 conidia/ml) was injected into the host hemocoel. Tween 80 solution (0.02%) was used as a blank control. The treated insects (30–35 larvae per treatment) were reared at 25 °C for 7 days. The median lethal time (LT50) was estimated by Probit analysis from the trend of mortality recorded daily.
Unraveling the BbGlc8-mediated transcriptome
To probe the effects of BbGlc8 on global gene expression during conidial development, a comparative transcriptomic analysis was performed between the wild-type and ΔBbGlc8 mutant strains as previously described (Ying et al. 2014).
The wild-type and ΔBbGlc8 mutant strains were cultured on SDAY plates for 2 days, and total RNA was extracted from mycelia of indicated strains. RNA samples were constructed into two libraries which were sequenced on Illumina Hiseq X Ten platform at Vazyme Biotech Co., Ltd (Nanjing, China). Sequence data have been deposited in NCBI’s Gene Expression Ominibus under the accession No. GSE116102. Each library was replicated two times in the independent experiments.
All clean reads from each library were mapped onto the genome sequence of Bb2860 (Xiao et al. 2012), using the HISAT program (Kim et al. 2015). All mapped genes were quantified in terms of the expected number of fragments per kilobase of transcript sequence per millions base pairs sequenced (FPKM) with Cufflinks software (Trapnell et al. 2010). The differentially expressed genes (DEGs) between two libraries were screened with the Cuffdiff method (Trapnell et al. 2013). Genes with a q value of < 0.05 (5% false discovery rate) and the absolute value of log square ratio > 1 were considered significant. To probe the potential function of DEGs, an enrichment analysis was performed with Functional Catalog (FunCat) method (Ruepp et al. 2004), using online portal (http://mips.helmholtz-muenchen.de/funcatDB/) at the threshold of P < 0.01.
Validation of the downstream target of BbOsmC2
The significantly repressed genes in ΔBbGlc8 mutant were deemed as the BbGlc8-mediated downstream targets. To probe the potential targets involved in conidiation, a member of the OsmC (osmotically inducible protein C) family (locus tag: BBA_08760; named as BbOsmC2) was selected as a representative.
The roles of BbOsmC2 in conidial and blastospore development were elucidated via gene disruption and complementation, using the same strategy applied to functional analysis of BbGlc8. All required primers are also listed in Supporting Information Table S2. All phenotypic assays for disruption mutant of BbOsmC2 were performed with the same methods described in the section “Phenotypic analyses of fungal strains”.
Data analysis
Tukey’s honest significance test (Tukey’s HSD) was used to evaluate the significant difference in the indicated phenotype among the wild-type, disruption mutant and complemented strains.
Results
Bioinformatic features of BbGlc8 protein and construction of its gene disruption and complementation strains
On the basis of BLAST search, a single highly related B. bassiana gene, BBA_06202 (Identity: 30%; E-value: 3e-5) was identified and designated as BbGlc8. The ORF sequence of BbGlc8 gene was 923 bp long with one intron in genomic sequence, and coded for a 281-amino acid protein. The deduced BbGlc8 protein contained an IPP-2 domain which could be seen in Glc8 homologues in other fungi (Supporting Information Table S3). Phlyogenetic analysis indicated that the homologues of filamentous fungi and yeasts were sorted into two independent branches (Supporting Information Fig. S1). B. bassiana BbGlc8 was sorted into a cluster of entomopathogenic fungi, and is closely related to that of Cordyceps militaris than to homologues in two Metarhizium species.
To determine the potential roles of BbGlc8, its gene disruption mutant was constructed by replacing its partial ORF with the phosphinothricin resistance gene (bar), using a homologous recombination strategy (Supporting Information Fig. S2). The gene disruption mutant was complemented via ectopic integration of the full-length BbGlc8 ORF plus the upstream promoter sequence, using the sulfonylurea resistance gene (sur) as the second selection marker. The correct gene disruption mutant and complementation strains were screened by PCR reaction and further confirmed by Southern blotting.
Disruption of the BbGlc8 affected fungal oxidation tolerance, spore production and virulence
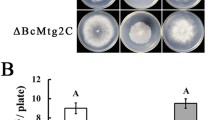
After 7 days of growth on various plates, the ΔBbGlc8 mutant did not show significant growth defects when compared with the wild-type and complemented strains (Fig. S3). On SDAY plates, disruption of BbGlc8 had no significant effect on the vegetative growth; however, fungal development was significantly impaired in the gene disruption mutant strain (Fig. 1a). On the culture surface, the wild-type and complemented strains produced 7.37 ± 0.37 and 6.67 ± 0.91 × 108 conidia/cm2 [mean ± standard deviation (SD)], respectively. However, the disruption strain only yielded 3.58 ± 0.39 × 108 conidia/cm2 at 7 days post incubation (dpi). In the submerged culture, the gene disruption strain also displayed a significantly decreased blastospore yield (5.47 ± 0.38 × 107 spores/ml (mean ± SD), whereas the wild-type and complemented strains produced 12.30 ± 0.61 and 10.50 ± 0.44 × 107 spores/ml (Fig. 1b), respectively. Additionally, gene loss enhanced fungal sensitivity to oxidation resistance (Fig. 1c). Mortality was measured over an 8 day period, and all strains tested were able to kill all test insects within 8 days post inoculation (Fig. 1d, f). As for fungal virulence, the loss of BbGlc8 resulted in a slight but statistically significant delay in LT50s from two kinds of bioassays [20% in topical test (Fig. 1e) and 14% in injection test (Fig. 1g)], indicating that fungal virulence had been slightly weakened.
Effects of the BbGlc8 loss on phenotypic aspects in B. bassiana. a Conidial yield. Fungal strains were grown on SDAY for conidiation up to 7 days, and the yield is calculated as the number of conidia per square centimeter. b Blastospore production. Fungal stains were inoculated into SDB broth (SDAY without agar) for 3 days, and the yield is presented as the cell number per milliliter of culture broth. c Fungal tolerance to chemical stress. Conidia were inoculated on Czapek-Dox agar plates supplemented with either NaCl (0.5 M), sorbitol (1.0 M), H2O2 (2 mM), menadione (0.02 mM) or Congo red (3 µg/ml). Fungal virulence was evaluated with two bioassay methods. Mortalities in topical (d) and intrahaemoceol injection (f) assays were recorded within 8 days post infection. The mean lethal time (LT50) for topical (e) and intrahaemoceol injection (g) assays was estimated by Probit analysis. Asterisks (*) on columns indicate a significant difference between the gene disruption mutant and the wild-type or complemented strain (Tukey’s HSD: P < 0.05). Error bars: standard deviation for three replicates
Ablation of BbGlc8 significantly affected global transcriptome
To identify the potential BbGlc8-mediated gene targets during conidial development, the global expression profiles were compared between the wild-type and ΔBbGlc8 mutant strains. Ablation of BbGlc8 resulted in altered expression of 1185 genes, with 414 up-regulated (~ 4.0% of the genome) and 771 down-regulated (~ 7.4% of the genome) genes in the mutant compared with the wild type (Table S4). In addition, twenty-six genes were only transcribed in the wild-type strain, and four genes were only transcribed in ΔBbGlc8 mutant.
Enrichment analysis indicated that the down-regulated DEGs were over-presented in functional catalogs of metabolism, cell transport, cellular defense and so on (Fig. 2 and Table S5). Overall, repressed genes were involved in metabolism, including a large number of genes associated with amino acid and carbonhydrate metabolism (e.g., glycoside hydrolase, glucanosyltransferase, and alcohol dehydrogenase); cell rescue, defense and virulence (e.g., heat shock protein (Hsp) 30, laccase 2 and OsmC protein); cellular transport (e.g., major facilitator superfamily transporter and monocarboxylate permease). A set of genes related to cell wall were also found to be repressed in ∆BbGlc8 mutant strain, including cell surface protein (BBA_09174), hydrophobin (BBA_00530), cell wall glucanosyltransferase Mwg1 (BBA_08214) and so on. In addition, a mitogen-activated protein kinase (sty1) (BBA_09043) was also down-regulated in gene disruption mutant. Up-regulated genes in the BbGlc8 mutant were only enriched in three functional catalogs, including metabolism, cellular transport as well as cell rescue defense and virulence (Fig. 2 and Table S6). These genes were mainly involved in metabolism of prosthetic groups (e.g., pyruvate decarboxylase: BBA_08386), allantoate transport (e.g., allantoate permease: BBA_03541) and cell defense (e.g., drug resistance protein: BBA_06344).
Functional Catalog (FunCat) analysis of the BbGLC8-mediated trancriptome. Differentially expressed genes (DEGs) were determined by comparing the transcriptomes of the wild-type and ΔBbGLC8 mutant strains. FunCat analysis was used to sort all DEGs, and these DEGs were over-presented in six functional categories
Functional analyses of BbOsmC2 gene
The unidentified OsmC-like protein (locus tag: BBA_08760) belongs to the OsmC superfamily and is named as BbOsmC2. The BbOsmC2 ORF was 525 bp long without intron in the genomic sequence and coded a protein with 174 amino acid residues.
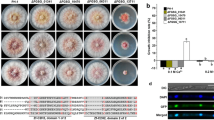
The physiological roles of BbOsmC2 gene in B. bassiana were revealed by gene disruption and complementation. PCR reaction and Southern blot indicated that the gene disruption mutant and complementation strains were successfully constructed (Fig. S4).
Disruption of BbOsmC2 did not significantly affect vegetative growth on various media (Fig. S5). However, BbOsmC2 significantly contributed to conidial and blastospore production. Conidial yield was significantly decreased in gene disruption mutant, which produced 2.19 ± 0.08 × 108 conidia/cm2 (mean ± SD), whereas the wild-type and the complementation strains yielded 7.37 ± 0.37 and 5.70 ± 0.43 × 108 conidia/cm2, respectively (Fig. 3a). The blastospore yield was shown as the spore number per milliliter of culture media. The loss of BbOsmC2 resulted in a significant reduction in blastospore yield, with a yield of 3.10 ± 0.85 × 107 spores/ml (mean ± SD). However, the wild-type and complemented strains generated 12.30 ± 0.61 and 10.73 ± 0.67 × 107 blastospores/ml, respectively, which did not significantly differ with each other (Fig. 3b). In addition, ablation of BbOsmC2 caused a reduced resistance to hyperosmotic stress (Fig. 3c). During 8 day bioassay, all strains could kill all inoculated insects (Fig. 3d, f). In cuticle infection bioassay (Fig. 3e), the LT50 values for the wild-type and ΔBbOsmC2 strains were 4.57 ± 0.25 days (mean ± SD) and 5.20 ± 0.11 days, respectively, indicating that a increase of 14.0% in time to kill 50% of insect hosts for ΔBbOsmC2 mutant when compared with the wild-type strain. In intrahaemocoel-infection bioassay (Fig. 3g), the LT50 values ΔBbOsmC2 strains was only elongated by approximately 7.0% as compared with the wild-type strain.
Phenotypic assays of the wild-type, ΔBbOsmC2 mutant and complemented strains. A series of phenotypic assays were performed to evaluate the gene disruption on fungal physiologies, including conidial production (a), blastospore yield (b), stress response (c), as well as virulence via cuticle penetration (d, e) and intrahaemoceol infection (f, g). All experiments were conducted as same as those used in phenotypic evaluation of ∆BbGlc8 mutant (as described in Fig. 1). Asterisks (*) on columns indicate a significant difference between the ΔBbOsmC2 mutant and the wild-type or complementation strain (Tukey’s HSD: P < 0.05). Error bars: standard deviation
Discussion
As presented above, Glc8 gene was proven to be involved in spore (conidium and blastospore) differentiation, oxidation resistance and virulence of the filamentous entomopathogenic fungus B. bassiana. In yeasts, Glc8 protein contributes to metabolism and chromosome segregation (Tung et al. 1995; Cannon 2010). Thus, Glc8 protein functions as an important regulator in single-cell yeast and filamentous fungi. The philological roles for Glc8 gene in the lifecycle of B. bassiana is discussed below.
The virulence of entomopathogenic fungi is crucial for their efficacies as biocontrol agents (St. Leger et al. 2011). The ability to perform dimorphic change between hyphae and yeast-like cells is an important determinant for the virulence of fungal pathogens (Gauthier 2015). In the host hemocoel, B. bassiana generates hyphal bodies (in vivo blastospores) via dimorphic transition (Lewis et al. 2009). The BbGlc8 gene contributes to 55% of blastospore yield in the wild-type strain. In B. bassiana, genes involved in dimorphic transition are associated with autophagy (e.g., BbATG5) (Zhang et al. 2013), cell cycle (e.g., BbCdc14) (Wang et al. 2013), energy sensing (e.g., BbSNF1) (Wang et al. 2014) and cytoskeleton (e.g., BbGEL1) (He et al. 2016). The current study adds new understanding to fungal dimorphic transition. Additionally, in the host hemocoel, the fungal growth will be inhibited by the oxidative stress caused by the insect’s immune reaction (Bergin et al. 2005). Fungal resistance to oxidation is another determinant for B. bassiana virulence (Ortiz-Urquiza and Keyhani 2015; Chu et al. 2018). The BbGlc8 gene is involved in fungal tolerance to oxidative stress caused by menadione. Thus, the weakened virulence of disruption mutants could be due, in part, to their defects in blastospore development and resistance to oxidative stress. Glc8 has been linked to glycogen accumulation and chromosome segregation in yeast (Cannon et al. 1994; Tung et al. 1995). Our data indicate that Glc8 links the fungal development and stress tolerance to the virulence of entomopathogenic fungi.
Asexual condiation promotes fungal disperse in nature (Gao et al. 2018a). At the last stage of infection cycle of B. bassiana, mycelia spread saprophytically on cadavers and generate plentiful conidia via asexual development (He et al. 2015, 2016). BbGlc8 contributes to conidiation, and acts as a regulator for asexual development in B. bassiana. This is the first report about the Glc8 roles in the asexual development of filamentous fungi, and no information is available for its mediated pathways. Thus, a comparative transcriptomic analysis was conducted to probe the potential targets of BbGlc8 during conidiation. BbGlc8 has a comprehensive influence on the global gene expression during fungal conidiation. First, BbGlc8 mediates the transcription of genes involved in amino acid and carbohydrate metabolism. B. bassiana SNF1 kinase contributes to conidiation by regulating the amino acid metabolism (He et al. 2015). These results suggest that amino acid metabolism during conidiation is regulated by multiple pathways. Second, transcriptomic analysis indicated that BbGlc8 is required for the expression of genes involved in cell rescue. For instance, several heat shock protein (Hsp) genes are repressed in BbGlc8 mutant strain, including Hsp30, Hsp70 and Hsp90 genes. Previous study indicated that an Hsp40 gene contributes to conidial development in B. bassiana (Wang et al. 2016), but is not found downstream of BbGlc8. In Trichoderma atroviride (a filamentous fungus), the pathways associated with metabolism and cell rescue are involved in fungal conidiation (Sanchez-Arregui et al. 2012). This suggests that the cellular metabolism is finely regulated during conidiation in filamentous fungi. Third, BbGlc8 regulates the expression of cell wall protein (e.g., hydrophobin). In B. bassiana, hydrophobins form a rodlet layer on conidial surface (Zhang et al. 2011). Although the hydrophobin roles in conidiation have not been examined in B. bassiana, a hydrophobin-like protein (BbHyd3) contributes to conidiation (He et al. 2016). In addition, the requirement of hydrophobin for conidiation has been established in other filamentous fungi (e.g., M. brunneum and Ma. grisea) (Kim et al. 2005; Sevim et al. 2012). Finally, BbGlc8 controls the expression of genes in signal transduction. For example, the mitogen-activated protein kinase (MAPK) cascade is an important signal transduction pathway (Liu et al. 2017). BbGlc8 is required for the expression of Mpk3 in MAPK pathway. This result suggests that BbGlc8 has an interaction with signaling transduction pathways, although the detailed mechanisms need to be investigated. In terms of physiological terms, BbGlc8 contributes to conidiation by mainly mediating cellular metabolism, stress defense, signal transduction and cell wall formation.
To explore more “cell rescue”-related genes involved in conidiation, we identified a member of OsmC family (BbOsmC2) as a contributor to conidiation in B. bassiana. OsmC gene was initially characterized in bacteria and induced by hyperosmosis caused by salt shock (Atichartpongkul et al. 2001; Shin et al. 2004), but it also plays a significant role in cellular defense against oxidative stress (Lesniak et al. 2003). In B. bassiana, BbOsmC2 contributes to fungal resistance to salt stress (an expected role), spore differentiation (including conidium and blastospore) and virulence, but not to fungal tolerance to oxidative stress. Dimorphic transition is crucial for fungal pathogenicity (Gauthier 2015). BbOsmC2 is involved in fungal virulence, which might be due to its significant role in blastospore production. These findings suggest that the OsmC proteins play divergent roles in prokaryotic and eukaryotic organisms. This is the first report about the biological roles of OsmC gene in fungi, including filamentous fungi and yeasts, although the members of OsmC family are prevalent in fungi (Meireles et al. 2017). As mentioned above, BbGlc8 significantly contributes to conidiation and blastospore production in B. bassiana. These results indicate that BbOsmC2 functions as a down-stream gene of BbGlc8 during spore development, but they are not in same pathway during fungal tolerance to environmental stresses. This finding suggests that BbOsmC2 is controlled by different upstream regulators as cell needs, although it entails much work to elucidate the detailed mechanisms.
Taken together, BbGlc8 plays an important role in oxidation tolerance, spore differentiation and virulence in B. bassiana. Transciptomic analysis unraveled that BbGlc8 has the comprehensive effects on genes involved in various cellular processes during fungal conidation. More importantly, we characterized BbOsmC2, a member of OsmC family, as a down-stream target of BbGlc8 during conidial formation. The present study links the PP1 pathway to fungal differentiation, and develops an initial framework to elucidate the BbGlc8-mediated pathways in conidiation. Future work, identifying more downstream genes and actual PP1 phosphatase regulated by Glc8 protein in B. bassiana, may enhance our understanding of the physiological processes in the infection cycle of entomopathogenic fungi.
References
Atichartpongkul S, Loprasert S, Vattanaviboon P, Whangsuk W, Helmann JD, Mongkolsuk S (2001) Bacterial Ohr and OsmC paralogues define two protein families with distinct functions and patterns of expression. Microbiology-SGM 147:1775–1782
Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K (2005) Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun 73:4161–4170
Cannon JF (2010) Function of protein phosphatase-1, Glc7, in Saccharomyces cerevisiae. Adv Appl Microbiol 73:27–59
Cannon JF, Pringle JR, Fiechter A, Kha M (1994) Characterization of glycogen-deficient glc mutants of Saccharomyces cerevisiae. Genetics 136:485–503
Chu XL, Dong WX, Ding JL, Feng MG, Ying SH (2018) Interactome analysis of transcriptional coactivator multiprotein bridging factor 1 unveils a yeast AP–1–like transcription factor involved in oxidation tolerance of mycopathogen Beauveria bassiana. Curr Genet 64:275–284
De la Cruz Quiroz R, Roussos S, Hernández D, Rodríguez R, Castillo F, Aguilar CN (2015) Challenges and opportunities of the biopesticides production by solid-state fermentation: filamentous fungi as a model. Crit Rev Biotechnol 35:326–333
Fang W, Zhang Y, Yang X, Zheng X, Duan H, Li Y, Pei Y (2004) Agrobacterium tumefaciens-mediated transformation of Beauveria bassiana using an herbicide resistance gene as a selection marker. J Invertebr Pathol 85:18–24
Gao X, Zhang J, Song C, Yuan K, Wang J, Jin Q, Xu JR (2018a) Phosphorylation by Prp4 kinase releases the self-inhibition of FgPrp31 in Fusarium graminearum. Curr Genet. https://doi.org/10.1007/s00294-018-0838-4
Gao Y, Feng MG, Ying SH (2018b) Oxaloacetate hydrolase gene links the cytoplasmic route of oxalate formation to differentiation and virulence of entomopathogenic fungus Beauveria bassiana. J Asia-Pac Entomol 21:211–216
Gauthier GM (2015) Dimorphism in fungal pathogens of mammals, plants, and insects. PLoS Pathog 11:e1004608
He PH, Wang XX, Chu XL, Feng MG, Ying SH (2015) RNA sequencing analysis identifies the metabolic and developmental genes regulated by BbSNF1 during conidiation of the entomopathogenic fungus Beauveria bassiana. Curr Genet 61:143–152
He PH, Dong WX, Chu XL, Feng MG, Ying SH (2016) The cellular proteome is affected by a gelsolin (BbGEL1) during morphological transitions in aerobic surface versus liquid growth in the entomopathogenic fungus Beauveria bassiana. Environ Microbiol 18:4153–4169
Kim S, Ahn IP, Rho HS, Lee YH (2005) MHP1, a Magnaporthe grisea hydrophobin gene, is required for fungal development and plant colonization. Mol Microbiol 57:1224–1237
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360
Klein DA, Paschke MW (2014) Filamentous fungi: the indeterminate lifestyle and microbial ecology. Microb Ecol 47:224–235
Lesniak J, Barton WA, Nikolov DB (2003) Structural and functional features of the Escherichia coli hydroperoxide resistance protein OsmC. Protein Sci 12:2838–2843
Lewis MW, Robalino IV, Keyhani NO (2009) Uptake of the fluorescent probe FM4-64 by hyphae and haemolymph-derived in vivo hyphal bodies of the entomopathogenic fungus Beauveria bassiana. Microbiology-SGM 155:3110–3120
Li M, Satinover DL, Brautigan DL (2007) Phosphorylation and functions of inhibitor-2 family of proteins. Biochemistry 46:2380–2389
Liu J, Sun HH, Ying SH, Feng MG (2017) The Hog1-like MAPK Mpk3 collaborates with Hog1 in response to heat shock and functions in sustaining the biological control potential of a fungal insect pathogen. Appl Microbiol Biotechnol 101:6941–6949
Martín R, Lopez–Aviles S (2018) Express yourself: how PP2A–B55Pab1 helps TORC1 talk to TORC2. Curr Genet 64:43–51
Meireles DA, Domingos RM, Gaiarsa JW, Ragnoni EG, Bannitz-Fernandes R, da Silva Neto JF, de Souza RF, Netto LES (2017) Functional and evolutionary characterization of Ohr proteins in eukaryotes reveals many active homologs among pathogenic fungi. Redox Biol 12:600–609
Nigavekar SS, Tan YSH, Cannon JF (2002) Glc8 is a glucose-repressible activator of Glc7 protein phosphatase-1. Arch Biochem Biophys 404:71–79
Ortiz-Urquiza A, Keyhani NO (2015) Stress response signaling and virulence: insights from entomopathogenic fungi. Curr Genet 61:239–249
Ruepp A, Zollner A, Maier D, Albermann K, Hani J, Mokrejs M, Tetko I, Güldener U, Mannhaupt G, Münsterkötter M, Mewes HW (2004) The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Res 32:5539–5545
Sanchez-Arregui A, Perez-Martinez AS, Herrera-Estrella A (2012) Proteomic analysis of Trichoderma atroviride reveals independent roles for transcription factors BLR-1 and BLR-2 in light and darkness. Eukaryot Cell 11:30–41
Sevim A, Donzelli BGG, Wu D, Demirbag Z, Gibson DM, Turgeon BG (2012) Hydrophobin genes of the entomopathogenic fungus, Metarhizium brunneum, are differentially expressed and corresponding mutants are decreased in virulence. Curr Genet 58:79–92
Shin DH, Choi I-G, Busso D, Jancarik J, Yokota H, Kim R, Kim SH (2004) Structure of OsmC from Escherichia coli: a salt-shock-induced protein. Acta Crystallogr D D60:903–911
St. Leger RJ, Wang C, Fang W (2011) New perspectives on insect pathogens. Fungal Biol Rev 25:84–88
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan YSH, Morcos PA, Cannon JF (2003) Pho85 phosphorylates the Glc7 protein phosphatase regulator Glc8 in vivo. J Biol Chem 278:147–153
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515
Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L (2013) Differential analysis of gene regulation at transcript resolution with RNA-sEq. Nat Biotechnol 31:46–53
Tung HY, Wang W, Chan CS (1995) Regulation of chromosome segregation by Glc8p, a structural homolog of mammalian inhibitor 2 that functions as both an activator and an inhibitor of yeast protein phosphatase 1. Mol Cell Biol 15:6064–6074
Wanchoo A, Lewis MW, Keyhani NO (2009) Lectin mapping reveals stage-specific display of surface carbohydrates in in vitro and haemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiology-SGM 155:3121–3133
Wang J, Liu J, Hu Y, Ying SH, Feng MG (2013) Cytokinesis-required Cdc14 is a signaling hub of asexual development and multi-stress tolerance in Beauveria bassiana. Sci Rep 3:3086
Wang XX, He PH, Feng MG, Ying SH (2014) BbSNF1 contributes to cell differentiation, extracellular acidification, and virulence in Beauveria bassiana, a filamentous entomopathogenic fungus. Appl Microbiol Biotechnol 98:8657–8673
Wang J, Ying SH, Hu Y, Feng MG (2016) Mas5, a homologue of bacterial DnaJ, is indispensable for the host infection and environmental adaptation of a filamentous fungal insect pathogen. Environ Microbiol 18:1037–1047
Williams-Hart T, Wu X, Tatchell K (2002) Protein phosphatase type 1 regulates ion homeostasis in Saccharomyces cerevisiae. Genetics 160:1423–1437
Xiao G, Ying SH, Zheng P, Wang ZL, Zhang S, Xie XQ, Shang Y, Zheng H, Zhou Y, St. Leger RJ, Zhao GP, Wang C, Feng MG (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep 2:483
Xie XQ, Li F, Ying SH, Feng MG (2012) Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS One 7:e30298
Ying SH, Ji XP, Wang XX, Feng MG, Keyhani NO (2014) The transcriptional co-activator multiprotein bridging factor 1 from the fungal insect pathogen, Beauveria bassiana, mediates regulation of hyphal morphogenesis, stress tolerance and virulence. Environ Microbiol 16:1879–1897
Ying SH, Liu J, Chu XL, Xie XQ, Feng MG (2016) The autophagy-related genes BbATG1 and BbATG8 have different functions in differentiation, stress resistance and virulence of mycopathogen Beauveria bassiana. Sci Rep 6:26376
Zhang SZ, Xia YX, Kim B, Keyhani NO (2011) Two hydrophobins are involved in fungal spore coat rodlet layer assembly and each play distinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveria bassiana. Mol Microbiol 80:811–826
Zhang L, Wang J, Xie XQ, Keyhani NO, Feng MG, Ying SH (2013) The autophagy gene BbATG5, involved in the formation of the autophagosome, contributes to cell differentiation and growth but is dispensable for pathogenesis in the entomopathogenic fungus Beauveria bassiana. Microbiology-SGM 159:243–252
Acknowledgements
This study was jointly supported by National Key R & D Program of China (2017YFD0200400) and National Natural Science Foundation of China (31670144).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Kupiec.
GEO reviewer link: Go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116102. Enter token wfwfeeeyjvwblur into the box.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, YJ., Ding, JL., Feng, MG. et al. Glc8, a regulator of protein phosphatase type 1, mediates oxidation tolerance, asexual development and virulence in Beauveria bassiana, a filamentous entomopathogenic fungus. Curr Genet 65, 283–291 (2019). https://doi.org/10.1007/s00294-018-0876-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-018-0876-y