Abstract
Ammonia-oxidizing bacteria (AOB) and archaea (AOA) are the main nitrifiers which are well studied in natural environments, and AOA frequently outnumber AOB by orders especially in acidic conditions, making AOA the most promising ammonia oxidizers. The phylogeny of AOA revealed in related studies, however, often varied and hardly reach a consensus on functional phylotypes. The objective of this study was to compare ammonia-oxidizing communities by amoA gene and transcript based on both genomic DNA and RNA in extremely acidic forest soils (pH <4.5). Our results support the numerical and functional dominance of AOA over AOB in acidic soils as bacterial amoA gene and transcript were both under detection limits and archaeal amoA, in contrast, were abundant and responded to the fluctuations of environmental factors. Organic matter from tree residues was proposed as the main source of microbial available nitrogen, and the potential co-precipitation of dissolved organic matter (DOM) with soluble Al3+ species in acidic soil matrix may further restrict the amount of nitrogen sources required by AOB besides NH3/NH4 + equilibrium. Although AOA were better adapted to oligotrophic environments, they were susceptible to the toxicity of exchangeable Al3+. Phylotypes affiliated to Nitrososphaera, Nitrososphaera sister group, and Nitrosotalea were detected by amoA gene and transcript. Nitrosotalea devantaerra and Nitrososphaera sister group were the major AOA. Compared to the genomic DNA data, higher relative abundances of Nitrososphaera and Nitrososphaera sister group were recognized in amoA transcript inferred AOA communities, where Nitrosotalea relative abundance was found lower, implying the functional activities of Nitrososphaera sister group and Nitrososphaera were easily underestimated and Nitrosotalea did not attribute proportionally to nitrification in extremely acidic soils. Further comparison of the different AOA community compositions and relative abundance of each phylotypes revealed by amoA genes and transcripts make it possible to identify the functional AOA species and assess their ecological role in extremely acidic soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrification is considered as an essential and regulatory process in the whole nitrogen cycle, responsible to either nitrogen retention or loss in the ecosystem. As the first and rate-limiting step, microbial ammonia oxidation has been intensively investigated and previously thought to be mediated only by ammonia-oxidizing bacteria (AOB) over a century. Later, the first discovery of archaeal ammonia monooxygenase (amo) genes from soil [1] and marine [2] through metagenomic and culturing studies has unveiled the presence of archaeal ammonia oxidizers and clustered ammonia-oxidizing archaea (AOA) into two distinct clades, Group 1.1a and 1.1b, further enabling the design of PCR primers for environmental surveys of crenarchaeal amoA. Archaeal amoA genes have been retrieved from various environments such as marine waters [2] and sediment [3, 4], estuarine sediments [5,6,7], corals [8], sponges [9], soils [10], wastewater bioreactors [11], wetland [12, 13], oil reservoirs [14], and terrestrial hot springs [15]. Most importantly, archaeal amoA genes were found to be ubiquitous and show a substantial congruence in the phylogeny with ribosomal RNA genes, granting amoA gene an effective tool to resolve the community compositions of AOA and a functional gene for detection of ammonia oxidation and inference on the potential activities of AOA communities. Another group of nitrifiers, named complete ammonia oxidizers (comammox) which combined the two steps of nitrification, was recently reported and found in diverse environments, such as soils, freshwater, and wastewater treatment plants [16, 17]. There are only four drafted genomes and two enrichment cultures of comammox available up to date [16,17,18]. Moreover, comammox are deeply buried in the Nitrospira genus and hard to tell apart from the others according to 16S rRNA or nxrB. Primers targeting amoA gene have been designed with reasonably high coverage of the two clades of recognized comammox, but based on the limited information [19]. Therefore, more work is needed to probe and resolve the potentially ecologically important nitrifiers.
Molecular techniques of detecting amoA gene based on genomic DNA have confirmed the widespread occurrence of bacterial and archaeal groups, but it is difficult to link specific microbial groups to ecosystem function. Total genomic DNA can be extracted from cells in various states of activity, and the ones in starvation and dormant status are less likely to contribute significantly to the overall microbial functions. Culture-dependent methods, such as enrichment and isolation, can only reveal the physiology of a few representatives due to the extremely limited successes in culturing majority of the microorganisms and the physiological features detected in the culture medium may deviate from field studies leading to biased information on functional activities of ammonia oxidizers. Therefore, there is a strong need of detection methods for the active communities of microorganisms to better understand the functional groups. Detection of transcripts of the functional genes has provided opportunities for evaluation of active microbial communities inferred from such analysis. Felske et al. (1998) were the first to use reverse transcription (RT)-PCR to demonstrate that uncultured soil bacteria were active, making it feasible to future applications. Some concerns, however, persist, for example, it is not necessary to tell the function is operating if the transcript of the corresponding functional gene is detected. In the case of AOB, bacterial AMO is a multifunctional enzyme, capable of oxidizing methane, carbon monoxide, and a range of organic compounds. Nevertheless, the alternative substrates for archaeal AMO have been rarely reported, and the validity and reliability of amoA transcript in assessing ammonia-oxidizing function have been tested by many related studies [1, 20, 34].
Nitrification in acidic forest soils has received increasing interests of studies, as the substantial distribution of acidic soils globally and crucial ecological function of forests in carbon and nitrogen cycling [21]. AOA are recognized as the main driver of nitrification in acidic soils, and they are found much more abundant and actively respond to acidic pH conditions than AOB [22, 23]. The recovered AOA communities in acidic soils were dominated by two main groups, Nitrososphaera/Group 1.1b/soil AOA frequently found abundant in terrestrial environments and Nitrosotalea/Group 1.1a associated AOA noticeably with the acidophilic isolates, Nitrosotalea devanterra or Nitrosotalea Nd2 [24, 25]. A more resolved phylogeny of AOA revealed a new group diversified from Nitrososphaera/Group 1.1b/soil AOA, recently described as Nitrososphaera sister groups [26], and found the dominant AOA in some acidic soils [27], which points out the possibility of overestimation of Nitrososphaera group functional importance in acidic soils previously. Nitrosotalea/Group 1.1a associated AOA showed a high preference to acidic soils, while the isolates presented a relatively low efficiency of ammonia oxidation [24, 28, 29], which may need further verification of their functional roles of nitrification in acidic soils.
Comparison of AOA communities revealed by both amoA gene and transcript has been used to understand the overall microbial community compositions of ammonia oxidizers and the responsible functional groups to differentiate the functional phylotypes from the inhabited ones and deduce the influences of environmental stresses on their functional activities. To better understand re-forestry on ammonia oxidation, forest soils from two re-vegetated forests both planted with Chinese fir (Cunninghamia lanceolata) but in different re-forestry stages, 8 and 18 years, were examined in this study to advance information on microbial community responses to restoration and management to provide further basis on practice.
Materials and Methods
Site Description and Sample Collection
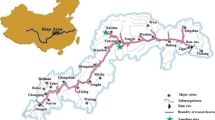
Forest soil samples were collected from two re-vegetated forests in the Nanling National Nature Reserve in Guangdong province, China (24° 37′–24° 57′ N, 112° 30′–113° 04′ E), on August 5, 2015. The young (Y) and matured (M) re-vegetated fir forests were located in Nanling Mountains and were re-planted with the same Chinese fir (Cunninghamia lanceolata), but at different restoration ages, 8 and 18 years, respectively.
For young or matured forest, both surface layer (A0 layer, 0–2 cm, with the removal of litter coverage; noted as YS and MS) and lower layer (B layer, 18–20 cm; noted as YL and ML) soil samples were collected from triplicated locations with randomized selection. Approximately 2 g of homogenized soil samples for RNA extraction were preserved in 5 mL of LifeGuard Soil Preservation Solution (MO BIO, Carlsbad, CA, USA) on site at the time of sampling. Around 2 kg of soil samples of each replicates were collected into plastic bags and kept in a cooler on site and transported back to laboratory for physicochemical analysis and DNA extraction.
Physicochemical Analysis
Physicochemical analysis of soil samples was proceeded following the guidelines of the Methods of Soil Analysis [30]. Briefly, pH vales were measured with a pH meter at soil to water ratio of 1:1 (Starter 3C, OHAUS, Pine Brook, NJ, USA). The standard sulfuric acid dichromate digestion method was used to detect soil organic carbon and the corresponding quantity of organic matter was calculated accordingly (organic matter to organic carbon, 1.724) [31]. Total nitrogen (N) was determined by Kjeldahl method. 2 M KCl extracted soil samples were analyzed for NH4 +-N and NO3 −–N by Nessler’s reagent colorimetry and ultra-violet spectrophotometry (UV–Vis spectrophotometer, 752 N type, Shanghai Jingke Co., China), respectively. Exchangeable Al was extracted from soil samples with 2 M KCl, and the concentration was determined by ICP-OES (Perkin Elmer Optima 8300, Waltham, MA, USA).
Nucleic Acid Extraction and Reverse Transcription
Total genomic DNA (gDNA) was extracted from replicates of each soil sample separately using PowerSoil DNA Isolation Kit (MO BIO, Carlsbad, CA, USA) according to the manual of the manufacturer, and DNA extracts were stored under −20 °C for polymerase chain reaction.
Total RNA was obtained from the preserved soil samples following the manual of RNA PowerSoil Total RNA Isolation Kit (MO BIO, Carlsbad, CA, USA) with proper modifications of optimizing bead beating duration, adjustment of elution buffer, and application of DNase I (Thermo Fisher Scientific, Basingstoke, UK) incubation to maximize the final yield and quality of RNA extracts. Quality and concentration of the acquired RNA were detected by NanoDrop Spectrophotometer ND-1000 and RNA gel electrophoresis. RNA was reversely transcripted into complementary DNA (cDNA) by random hexamers using PrimeScript First Strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan).
amoA Gene Amplification
Amplification of archaeal and bacterial amoA genes and transcripts was performed using the primer sets as shown in Table 1. The reaction mixture (50 μL) contained 2 μL of DNA template (20–40 ng per reaction), 1 μL of bovine serum albumin (BSA) (100 mg mL−1, Roche), 5 μL of 10× GoTaq Flexi buffer (Promega, Hong Kong), 1 μL of forward and 1 μL of reverse primers (20 μM), and 0.5 μL of GoTaq Flexi polymerase (5 U μL−1; Promega, Hong Kong). The PCR condition was set as 95 °C for 5 min followed by 30 cycles (archaeal amoA) or 35 cycles (bacterial amoA) of 95 °C for 45 s, 53 °C (archaeal amoA) or 55 °C (bacterial amoA) for 60 s, and 72 °C for 60 s. For samples with low abundances of bacterial amoA, nested PCR was carried out to further extend the detection capacity according to the previous studies by using primer set A189 and amoA-2R for the first round amplification and amoA-1F/2R for the second (Table 1) [32].
Quantitative Analysis by Real-Time PCR with Internal Control
Composite samples, mixture of DNA extracts from the triplicates, were used as templates to quantify archaeal amoA in iTaq Universal SYBR Green Supermix (Bio-Rad, Richmond, CA, USA) by real-time PCR (ABI StepOnePlus, ABI, Life Tech., Foster City, CA, USA). Primer sets used here were same with the ones in general PCR (Table 1). The internal control method was implemented as described previously to normalize the potentially inhibitory effects from extract matrix (Wu et al., non-published data). The known amount of plasmid DNA (pUC 19 plasmid DNA, Invitrogen, Carlsbad, CA, USA) was used as the internal control, mixed, and amplified under the identical PCR condition as archaeal amoA gene by the primer set M13 R/F to calibrate the differences of PCR efficiency among samples [32]. Melting curve analysis was performed to determine the melting points of the amplification products, in order to access the reaction specificity.
Clone Library Construction and Sequencing
Clone libraries were constructed using the amplified amoA genes, and the triplicates of each samples were analyzed independently. PCR products were obtained and purified from size-verified gel bands by illustra GFX PCR DNA and Gel Band Purification Kits (GE Healthcare Sciences, USB Corporation, UK). The purified DNA were ligate to PMD18 T-vector (pMD™ 18-T Vector Cloning Kit, Takara, Japan) and transformed into Escherichia coli DH5α competent cells (Takara, Japan), subjected to clone library construction. Thirty colonies from each clone library were randomly selected for sequencing after size verification of the PCR amplicons using M13 F/R primer set. Sequencing was performed with ABI 3730xl DNA analyzer (Applied Biosystems, Foster City, CA, USA).
Phylogenetic Analysis
The quality sequences after screening out the short or failed ones were fed to the RDP FunGene analyzing platform [33]. The nucleotide sequences were translated into amino acid sequence, checked, and corrected the potential frameshifts using FrameBot (RDP FunGene). The deduced amino acid sequences were aligned by HMMER3 aligner and clustered into OTUs by mcCLUST (RDP) using the complete-linkage clustering method with distance cutoff of 3%. The representative sequences from each OTU were selected and aligned with the published phylogenetic relatives retrieved from the GenBank database using Protein BLAST (National Center For Biotechnology Information) by the method of ClustalW in the MEGA 6 software [34, 35]. The aligned amino acid sequences were used to construct the phylogenetic trees using neighbor-joining method in MEGA 6 with 1000 bootstraps.
Statistical Analysis
Diversity indexes of each sampling site, Chao1 and Shannon, were calculated with cutoff of 3% by RDP FunGene analyzing platform [33]. Significance of differences within two groups and multiple groups was evaluated by Student’s t test and one-way ANOVA with Turkey post test (version 5.0, GraphPad Software), respectively. Data were expressed as mean ± standard deviation (SD) and considered significantly different at p < 0.05. The correlation between two variables was tested by Pearson product–moment correlation method and considered significant at p < 0.05 (SPSS, 16.0).
Amino Acid Sequence Accession Numbers
The representatives of amoA genes and transcript sequences reported in this study have been deposited in GenBank under accession numbers, KX619853–KX619934 and KX650406–KX650439, respectively.
Results
Physicochemical Characteristics of the Re-Vegetated Forests
Even though the two closely located re-vegetated forests were planted with the same trees, physicochemical characteristics of the soils showed significant differences among the different soil layers and forests in terms of organic matter, total N, NO3 −-N, NH4 +-N, and exchangeable Al3+ (Table 2).
Soil pH values were from 4.26 to 4.38, indicating extremely acidic condition. The concentrations of organic matter, total N, and NO3 −-N were significantly higher in surface layers than in the corresponding lower layers for both young and matured re-vegetated forests (p < 0.05). A more remarkable increase of organic matter contents in the surface and lower layer was detected in matured forest than the corresponding layers in young forest, possibly due to the relatively higher productivity and more decomposition in mature forest with denser canopies in the lower layers than young forest. The higher organic matter contents in surface soil layers for both young and matured forests further supported that decomposition of surface layer deposition was the main source of organic carbon detected in the lower layers (YS and YL, p < 0.05; MS and ML, p < 0.05). Similar to organic matter, total N and NO3 −-N levels were also higher in the surface soil layers than the lower layers (p < 0.05). Matured forest possessed higher quantities of total N and exchangeable Al3+, especially in the surface layer (YS and MS, p < 0.05).
The calculated C/N among all sampling sites were less than 20, implying high nutritional turnover rates in the two re-vegetated forests as the nitrogen nutrient can be effectively integrated into microbial biomass, facilitating nutrient cycling.
Seasonal and Spatial Variation of amoA Gene and Transcript Abundances
Bacterial amoA gene and transcripts were under the detection limit in both young and matured forests using either gDNA or cDNA even after additional efforts, nested PCR. Unlike AOB, AOA were abundant at all sampling sites, with gene copy number ranging from 1.90 ± 0.07 × 108 (MS) to 5.20 ± 0.25 × 108 (ML) and transcript copies from 2.40 ± 0.21 × 106 (MS) to 8.30 ± 0.13 × 106 (YS) (Fig. 1).
For the young forest, both amoA gene and transcript abundances were higher in the surface layer than lower one. Matured forest, on the contrary, were detected with higher abundance of amoA genes and transcripts in the lower layer (p < 0.05). Moreover, the abundances of amoA transcripts were in agreement with the gene abundances, except for ML where copies number of amoA transcript dropped significantly.
Phylogeny Reconstruction of AOA Based on amoA Genes and Transcripts
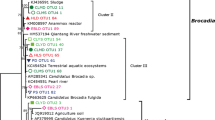
All verified archaeal amoA gene and transcript sequences of this study were clustered into 34 OTUs with 3% cutoff, and the representatives were grouped into seven clusters with robust phylogenetic support based on the relative similarity to phylogenetic reference sequences, covering three out of five main lineages currently recognized in AOA phylogeny (Fig. 2).
Sequences identified as Nitrososphaera/Group 1.1b/soil-like AOA were grouped into Cluster 1 (95–96% similar to Nitrososphaera viennensis or Nitrososphaera gargensis), Cluster 2 (91–95% similar to N. gargensis or N. JG1), and Cluster 3 (94–95% similar to N. gargensis). Sequences grouped into Cluster 1 and Cluster 3 were only detected by amoA transcripts and Cluster 2 included both gene and transcript sequences.
Cluster 4, containing sequences detected among all sampling sites by both amoA gene and transcripts, fell into a phylogenetic group named as Nitrososphaera sister group which was first proposed in 2011 [26], as sequences showed the identical indexes with all the reference sequences (N. viennensis, N. JG1, Nitrososphaera evergladensis and N. gargensis) in Nitrososphaera/Group 1.1b/soil AOA. Similar to the two subclusters suggested by Pester et al. (2012), Cluster 5 was also affiliated to the Nitrososphaera sister group and grouped with the recently cultured sister group isolate, Candidatus Nitrosocosmicus franklandus [36]. Sequences in Cluster 4 can be retrieved from both amoA genes and transcripts and prevalently found among all sampling sites, and Cluster 5 sequences were only detected by amoA transcripts.
The remaining sequences were identified as Nitrosotalea devanterra or Nitrosotalea Nd2-like AOA, belonging to Nitrosotalea/Group 1.1a associated AOA and divided into two clusters, Cluster 6 and Cluster 7. In Cluster 6, sequences were less similar to Nitrosotalea devanterra or Nitrosotalea Nd2 (89–93%) and only detected in the lower soil layers of matured and young re-vegetated forests. Cluster 7 sequences showed relatively higher identities to Nitrosotalea devanterra or Nitrosotalea Nd2 (96–98%) and distributed in all soil samples. Cluster 6 may be a new cluster as the sequences presented the similarity indexes much smaller than the one shared between the two isolates within Nitrosotalea (98%, Table S1.).
Seasonal and Spatial Variations of AOA and AOB Diversity and Community Structure
The sufficient sampling efforts were justified by the rarefaction curves shown in Fig. S1 for both amoA gene and transcript inferred AOA communities. AOA communities revealed by amoA gene and transcript showed no obvious spatial variation by soil layers and forest types, but higher diversity (OTU number and Shannon) and richness (Chao1) were noticed in AOA communities from amoA transcripts (Table 3).
All verified amino acidic sequences deduced from amoA gDNA and cDNA were pooled to make comparisons among microbial community structures through principal coordinates analysis (PCoA) based on weighted UniFrac distances (Fig. 3). AOA communities revealed by cDNA was deviated from those by gDNA as they were located at the opposite sides of the axis with the highest explanation percentage, P1 (60.0%), except for YL (Fig. 3a). cDNA-sinferred AOA communities tended to group together, separating from the gDNA inferred ones, possibly resulting from the highly diversified cDNA–AOA communities possessing specific OTUs only shared among themselves (Fig. 3b). Even though gDNA and cDNA revealed different AOA communities, the relative similarities between the sampling sites were detected, as both gDNA and cDNA inferred structures showed that YS was more similar with ML, but not MS (Fig. 3c). Different from the gDNA–AOA communities, distinct community structures between YS and YL was resolved by cDNA–AOA communities (Fig. 3d). The inconsistency in community structures by gDNA–AOA and cDNA–AOA communities may provide more insights into the identification of the functionally active AOA differing from the niche-inhabiting species.
Seasonal and Spatial Variation of AOA and AOB Community Compositions
All verified amoA amino acidic sequences deduced from gDNA and cDNA were clustered into 34 OTUs (3%) and displayed in the heatmaps to show the microbial compositions of all soil samples in this study (Fig. 4a). The identified OTUs further referred back to the phylogenetic structures and grouped into species. The proportions of the detected species in each soil sample are shown in Fig. 4b.
OTU1 to OTU6 belonged to Nitrosotalea/Group 1.1a associated-like AOA, and OTU7 to OTU20 were Nitrososphaera sister group-like AOA. The rest OTUs were classified into Nitrososphaera/Group 1.1b/soil AOA (Fig. 4a). Although Nitrososphaera/Group 1.1b/soil AOA were counted for a minor proportion of the overall communities compared to the other two lineages, they were found most diversified and divided into 14 OTUs, among which 11 OTUs (OTU21–23, OTU27–29, and OTU30–34) were only detected by cDNA. For the two dominant AOA, Nitrososphaera sister group and Nitrosotalea, 6 (OTU7–9, OTU14, OTU19–20) out of 14 OTUs and 1 (OTU3) out of 6 OTUs, respectively, were able to be detected only in cDNA revealed AOA communities. There were only two OTUs, OTU5 (Nitrosotalea) and OTU16 (Nitrososphaera sister group), that showed to be gDNA inferred AOA communities specific. All the three identified AOA lineages can be detected by both gDNA and cDNA. The most abundant OTUs, OTU1 and OTU2 (Nitrosotalea), and OTU12 and OTU18 (Nitrososphaera sister group), were retrieved from both AOA communities among all soil samples. cDNA revealed more diverse AOA communities with more unique OTUs and divergent frequencies of the common OTUs from ones detected by gDNA.
Nitrosotalea devantaerra and Nitrososphaera sister group were the major AOA identified among all sampling sites. AOA belonging to Nitrososphaera sister group were more abundant in cDNA revealed communities (except for MS), while Nitrosotalea devantaerra were more often found to dominate gDNA inferred AOA communities (except for MS).
Discussion
AOA Were Selected over AOB Due to the Limited Substrates in Acidic Soils
AOB were not detected successfully in both young and matured forest soils with extremely acidic pH (pH <4.5) by either gDNA or cDNA, indicating that archaeal ammonia oxidation is the dominant process for the first step of nitrification, which is consistent with other related studies on this topic [20, 37, 38]. Ammonia (NH3) rather than ammonium (NH4 +) is the substrate for autotrophic ammonia oxidation, and its availability drops exponentially with decreasing pH due to the high pKa of the NH4 + (NH3 + H+ → NH4 +; pKa = 9.25), resulting in a serious substrate shortage for ammonia oxidizers in acidic soils. AOA with a relative higher affinity for ammonia by 200-fold compared to AOB, granting them a competitive advantage inhabiting acidic niches to carry out the biochemical process [39].
The concentration of organic matter showed a strong positive correlation with nitrogen, especially total N and NH4 + (p < 0.01, Table 4), suggesting that microbial available nitrogen or ammonia from mineralization of organic matter can be a main source of substrate for autotrophic ammonia oxidizers in acidic soils. This was also observed in soils that high mineralization rate was frequently coupled with high net nitrification rate [40, 41]. As a result, the a slow releasing and low concentration of ammonia from organic matter mineralization select the growth of AOA than AOB and the latter are usually below the detection limits [41]. Without the pronounced competition with the bacterial counterparts, AOA communities in the two re-vegetated forests with extremely acidic soils would be ideal subjects to further investigate the functional role of archaeal ammonia oxidizers in nitrification.
Potential Impacts of the Physicochemical Factors on Overall and Functional AOA Abundances
In order to provide a comprehensive picture of AOA community composition and functional activities in extremely acidic soils, both the amoA gene retrieved from genomic DNA and the corresponding transcript from RNA were analyzed and compared. The abundance of amoA transcripts was detected to be approximately 100 times lower than those of genomic amoA gene, which has been also observed in other studies and suggested to be due to the low recovery rate of messenger RNA (mRNA) and/or suppressed nitrification function of a large proportion of AOA detected. As mentioned in the “Results,” gDNA– and cDNA–AOA communities do not agree on a single AOA lineage as the dominant one even at the same site. We therefore speculate that the different copies between amoA gene and transcript were not merely resulted from the easy degradation of mRNA and would further provide information of their differentiated function and activity.
The relatively high concentration of NH4 + for AOA growth found at sites and insignificant correlation between nitrogen level and microbial abundance implies a surplus of NH3 to support a fraction of AOA communities inferred by genomic amoA gene. Therefore, nitrogen may not be the limiting factor of AOA leading to the drastic spatial variation by soil layers and forest types. Among the physicochemical parameters, exchangeable Al3+ was negatively correlated with both amoA gene and transcript abundance (amoA gene, p < 0.01; amoA transcript, p < 0.05), indicating exchangeable Al3+ may be a potent factor affecting both overall AOA community abundances and also the functional groups. Excessive amount of Al3+ in acidic soils may be toxic to ammonia oxidizers as observed a significant drop of AOA abundance at MS where exchangeable Al3+ concentration was the highest [42]. Therefore, Al speciation and disassociation in acidic soils should be crucial to the survival and functioning of ammonia oxidizers.
There was a strong positive correlation between organic matter and exchangeable Al3+, implying organic matter interact with Al3+ in acid soils and conversely contribute to the regulation of soil-exchangeable Al. Organic matter in acidic forest soils can be immobilized and stabilized by polyvalent Al cation species through the precipitation of dissolved organic matter (DOM) [43]. As a result, DOM precipitated with soluble Al species would divert partial organic matter away from mineralization and the precipitates were found less susceptible to microbial decay, which may further limit NH3 generation to support the growth of demanding AOB [44]. Besides the direct toxicity to microbial cells, Al species also exert an impact on the available nitrogen sources of ammonia oxidizers in acidic soils.
A more Complex Archaeal Ammonia Oxidizer Community Revealed by the Cross-Comparison Between amoA Genes and Transcripts Data
It is interesting to notice that cDNA recovered much more diverse AOA communities than gDNA did, indicated by more cDNA specific OTUs, higher diversity indexes (Shannon and Chao1), and distinct community structures. The unique OTUs are not arbitrary as they were well aligned with either cultured or uncultured reference sequences. Therefore, it may signify higher sensitivity and diversification of transcripts than genes in response to the environmental stresses or influences and would better resolve ammonia oxidizer communities in addition to function indexation.
Related research has shown the importance of soil pH affecting the distribution of terrestrial AOA and proposed the common ancestor of Thaumarchaeota was originated from neutral pH, followed by further independent diversification into lower pH lineages, Nitrosotalea and Nitrosopumilus, and higher pH lineage, Nitrososphaera [45]. Indeed, Nitrosotalea were retrieved from both AOA genes and transcripts, encompassing the sequences similar to Nitrosotalea devanterra or Nitrosotalea Nd2, cultured obligate acidophilic AOA [24, 25]. Their relative abundances shown in the gDNA– and cDNA–AOA communities, however, differed from each other, as Nitrosotalea-like AOA were the most abundant in gDNA–AOA (except for MS) but not in cDNA–AOA, which may imply that although Nitrosotalea can adapt to acidic environments and dominate gDNA–AOA communities, they did not contribute proportionally to the overall nitrification. Some culture-based studies have also revealed their relatively lower specific cell activities, more than 10 times inefficient in nitrite generation than other AOA isolates [24, 28, 29]. In contrast, Nitrososphaera may be underestimated their functional role of nitrification in acidic soils, as substantial amounts of AOA similar to N. viennensis, N. gargensis, and N. JG1 were recovered by cDNA. Pester et al. (2012) also reported the relative dominance of Nitrososphaera in four acidic soils with pH from 4.4 and 5.1. Therefore, it may indicate Nitrososphaera cluster possesses some acidophilic phylotypes that are not widely recognized but capable to actively contribute to nitrification in acidic soils, which would further broaden our understanding of niche specification of AOA.
The third identified AOA lineage, Nitrososphaera sister group, was found most abundant especially in cDNA–AOA communities. Nitrososphaera sister group comprising two subclusters was first proposed by Pester et al. (2012) and later reported about their active nitrification in acidic red soils [27]. Recently, the first isolate as the representative of Nitrososphaera sister group, named as Candidatus Nitrosocosmicus franklandus, was cultured from arable soils with pH 7.5 and grouped with sequences in Cluster 5 (Fig. 2), and they were counted for a minor proportion of cDNA–AOA communities [36]. Candidatus Nitrosocosmicus franklandus were suggested to be capable of ureolysis and aggregate formation due to discovery of elicited proliferation by urea addition and cell-associated extracellular polymeric materials. These competitive advantages may promise them with their possible growth and active nitrification in acidic soils. Cluster 4 was comprised of sequences with the same identity indexes to all the reference sequences in Nitrososphaera/Group 1.1b/soil AOA, forming a new mirrored structure to Nitrososphaera and recognized as a subcluster and the major Nitrososphaera sister group. As Cluster 4 did not show the comparable relative branching order with Cluster 5 in the reconstructed phylogenetic tree, it may be easily misclassified into Nitrososphaera lineage and underestimated their potentially crucial role of nitrification in acidic soils as indicated in this study. The distinct phylogenetic distances of Cluster 4 and Cluster 5 from Nitrososphaera/Group 1.1b/soil AOA may imply a gradual speciation process that more specialized phylotypes adapted to the niches diversified from the ancestors.
In the correlation analysis, the relative abundance of Nitrososphaera, unlike the other two AOA lineages, revealed by cDNA rather than gDNA is positively correlated with the detected physicochemical parameters, especially exchangeable Al3+, which may again support the possibility of newly evolved acidophilic Nitrososphaera phylotypes in extremely acidic soils where Al toxicity is pronounced. For the two dominant AOA, only gDNA inferred relative abundances of Nitrososphaera sister group and Nitrosotalea demonstrated positive and negative correlation with organic matter, nitrogen-related factors, and exchangeable Al3+, respectively (Table 4). This may be a hint of species redundancy that the environments would prefer the growth of species or ecotypes that can adapt and eliminate the ones that are susceptible to the environmental stresses, which contribute to overall diversity and stability of the microbial communities, and the functional groups, however, are counted for only a part and form functional niches and contribute significantly to the functional activities [46]. The positive correlation of the organic matter, nitrogen-related factors, and exchangeable Al3+ with the relative abundance of Nitrososphaera sister group, in contrast to the negative correlation with Nitrosotalea, may propose that Nitrososphaera sister group is relatively more nitrogen demanding and/or Al tolerant. The physiology study of Candidatus Nitrosocosmicus franklandus, indeed, suggest a lower cell yield (7.6 × 103 cells μM−1 NH3) and larger cell size (smaller specific surface area, 1.1 diam) [36].
Conclusion
AOA are ammonia oxidizers not only numerically but also functionally dominated over the bacterial counterpart in extremely acidic soils. Nitrososphaera, Nitrososphaera sister group, and Nitrosotalea were three lineages detected, and Nitrososphaera sister group and Nitrosotalea were the two dominated AOA at all sites. The variations in their relative abundances by gDNA– and cDNA–AOA communities and responses to environmental influences may shed light on their contrasting functional roles in nitrification and species redundancy within each lineage. The nitrification activities of Nitrososphaera sister group and Nitrososphaera were highlighted here as they may be underestimated previously in extremely acidic soils. Physicochemical parameters of organic matter and exchangeable Al3+ showed significant impacts on both AOA abundance and community compositions.
References
Treusch AH, Leininger S, Kletzin A, Schuster SC, Klenk HP, Schleper C (2005) Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ Microbiol 7(12):1985–1995. https://doi.org/10.1111/j.1462-2920.2005.00906.x
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu D, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers YH, Smith HO (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304(5667):66–74. https://doi.org/10.1126/science.1093857
Cao H, Hong Y, Li M, Gu JD (2011) Phylogenetic diversity and ecological pattern of ammonia-oxidizing archaea in the surface sediments of the western Pacific. Microb Ecol 62(4):813–823. https://doi.org/10.1007/s00248-011-9901-0
Cao H, Hong Y, Li M, Gu J-D (2012) Lower abundance of ammonia-oxidizing archaea than ammonia-oxidizing bacteria detected in the subsurface sediments of the Northern South China Sea. Geomicrobiol J 29(4):332–339
Beman JM, Francis CA (2006) Diversity of ammonia-oxidizing archaea and bacteria in the sediments of a hypernutrified subtropical estuary: Bahia del Tobari, Mexico. Appl Environ Microbiol 72(12):7767–7777. https://doi.org/10.1128/AEM.00946-06
Cao H, Hong Y, Li M, Gu JD (2011) Diversity and abundance of ammonia-oxidizing prokaryotes in sediments from the coastal Pearl River estuary to the South China Sea. Antonie Van Leeuwenhoek 100(4):545–556. https://doi.org/10.1007/s10482-011-9610-1
Cao H, Li M, Hong Y, Gu JD (2011) Diversity and abundance of ammonia-oxidizing archaea and bacteria in polluted mangrove sediment. Syst Appl Microbiol 34(7):513–523. https://doi.org/10.1016/j.syapm.2010.11.023
Beman JM, Roberts KJ, Wegley L, Rohwer F, Francis CA (2007) Distribution and diversity of archaeal ammonia monooxygenase genes associated with corals. Appl Environ Microbiol 73(17):5642–5647. https://doi.org/10.1128/AEM.00461-07
Steger D, Ettinger-Epstein P, Whalan S, Hentschel U, de Nys R, Wagner M, Taylor MW (2008) Diversity and mode of transmission of ammonia-oxidizing archaea in marine sponges. Environ Microbiol 10(4):1087–1094. https://doi.org/10.1111/j.1462-2920.2007.01515.x
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442(7104):806–809. https://doi.org/10.1038/nature04983
Park HD, Wells GF, Bae H, Criddle CS, Francis CA (2006) Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Appl Environ Microbiol 72(8):5643–5647. https://doi.org/10.1128/AEM.00402-06
Li M, Cao HL, Hong YG, Gu JD (2011) Spatial distribution and abundances of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in mangrove sediments. Appl Microbiol Biot 89(4):1243–1254. https://doi.org/10.1007/s00253-010-2929-0
Wang YF, Gu JD (2013) Higher diversity of ammonia/ammonium-oxidizing prokaryotes in constructed freshwater wetland than natural coastal marine wetland. Appl Microbiol Biot 97(15):7015–7033. https://doi.org/10.1007/s00253-012-4430-4
Li H, Mu BZ, Jiang Y, Gu JD (2011) Production processes affected prokaryotic amoA gene abundance and distribution in high-temperature petroleum reservoirs. Geomicrobiol J 28(8):692–704. https://doi.org/10.1080/01490451.2010.514026
Weidler GW, Dornmayr-Pfaffenhuemer M, Gerbl FW, Heinen W, Stan-Lotter H (2007) Communities of archaea and bacteria in a subsurface radioactive thermal spring in the Austrian Central Alps, and evidence of ammonia-oxidizing .Crenarchaeota Appl Environ Microbiol 73(1):259–270. https://doi.org/10.1128/AEM.01570-06
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, von Bergen M, Rattei T, Bendinger B, Nielsen PH, Wagner M (2015) Complete nitrification by Nitrospira bacteria. Nature 528(7583):504–509. https://doi.org/10.1038/nature16461
van Kessel MA, Speth DR, Albertsen M, Nielsen PH, Op den Camp HJ, Kartal B, Jetten MS, Lucker S (2015) Complete nitrification by a single microorganism. Nature 528(7583):555–559. https://doi.org/10.1038/nature16459
Palomo A, Jane Fowler S, Gulay A, Rasmussen S, Sicheritz-Ponten T, Smets BF (2016) Metagenomic analysis of rapid gravity sand filter microbial communities suggests novel physiology of Nitrospira spp. Isme J 10(11):2569–2581. https://doi.org/10.1038/ismej.2016.63
Pjevac P, Schauberger C, Poghosyan L, Herbold CW, van Kessel MA, Daebeler A, Steinberger M, Jetten MS, Lücker S, Wagner M (2016) amoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. bioRxiv:096891. https://doi.org/10.1101/096891
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10(11):2966–2978. https://doi.org/10.1111/j.1462-2920.2008.01701.x
Vonuexkull HR, Mutert E (1995) Global extent, development and economic-impact of acid soils. Plant Soil 171(1):1–15
You J, Das A, Dolan EM, Hu ZQ (2009) Ammonia-oxidizing archaea involved in nitrogen removal. Water Res 43(7):1801–1809. https://doi.org/10.1016/j.watres.2009.01.016
Zhang LM, Hu HW, Shen JP, He JZ (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. Isme J 6(5):1032–1045. https://doi.org/10.1038/ismej.2011.168
Lehtovirta-Morley LE, Ge CR, Ross J, Yao HY, Nicol GW, Prosser JI (2014) Characterisation of terrestrial acidophilic archaeal ammonia oxidisers and their inhibition and stimulation by organic compounds FEMS. Microbiol Ecol 89(3):542–552. https://doi.org/10.1111/1574-6941.12353
Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW (2011) Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. P Natl Acad Sci USA 108(38):15892–15897. https://doi.org/10.1073/pnas.1107196108
Pester MRT, Flechl S, Gröngröft A, Richter A, Overmann J, Reinhold-Hurek B, Loy A, Wagner M (2012) amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ Microbiol 14(2):525–539. https://doi.org/10.1111/j.1462-2920.2011.02666.x
Wu Y, Conrad R (2014) Ammonia oxidation-dependent growth of group I.1b Thaumarchaeota in acidic red soil microcosms FEMS. Microbiol Ecol 89(1):127–134. https://doi.org/10.1111/1574-6941.12340
Jung MYPS, Min D, Kim JS, Rijpstra WI, Damsté JS, Kim GJ, Madsen EL, Rhee SK (2011) Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl Environ Microb 77(24):8635–8647. https://doi.org/10.1128/Aem.05787-11
Konneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437(7058):543–546. https://doi.org/10.1038/nature03911
Swift R, Sparks D (1996) Methods of soil analysis: part 3, chemical methods. Soil Science Society of America. Book Series 5:1018–1020
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. American Society of Agronomy, Madison, Wisconsin, USA, pp. 539–579
Horz HP, Barbrook A, Field CB, Bohannan BJM (2004) Ammonia-oxidizing bacteria respond to multifactorial global change. P Natl Acad Sci USA 101(42):15136–15141. https://doi.org/10.1073/pnas.0406616101
Fish JA, Chai BL, Wang Q, Sun YN, Brown CT, Tiedje JM, Cole JR (2013) FunGene: the functional gene pipeline and repository. Front Microbiol 4:291. https://doi.org/10.3389/fmicb.2013.00291
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Lehtovirta-Morley LE, Ross J, Hink L, Weber EB, Gubry-Rangin C, Thion C, Prosser JI, Nicol GW (2016) Isolation of ‘Candidatus Nitrosocosmicus franklandus’, a novel ureolytic soil archaeal ammonia oxidiser with tolerance to high ammonia concentration FEMS. Microbiol Ecol 92(5):fiw057. https://doi.org/10.1093/femsec/fiw057
He J, Shen J, Zhang L, Zhu Y, Zheng Y, Xu M, Di HJ (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9(9):2364–2374. https://doi.org/10.1111/j.1462-2920.2007.01358.x
Yao H, Gao Y, Nicol GW, Campbell CD, Prosser JI, Zhang L, Han W, Singh BK (2011) Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl Environ Microb 77(13):4618–4625. https://doi.org/10.1128/Aem.00136-11
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying archaea and bacteria. Nature 461(7266):976–U234. https://doi.org/10.1038/nature08465
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75(2):139–157. https://doi.org/10.1890/04-0988
Stopnisek N, Gubry-Rangin C, Hofferle S, Nicol GW, Mandic-Mulec I, Prosser JI (2010) Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl Environ Microbiol 76(22):7626–7634. https://doi.org/10.1128/AEM.00595-10
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11(10):1111–1120. https://doi.org/10.1111/j.1461-0248.2008.01230.x
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org Geochem 31(7–8):697–710. https://doi.org/10.1016/S0146-6380(00)00049-8
Scheel T, Dorfler C, Kalbitz K (2007) Precipitation of dissolved organic matter by aluminum stabilizes carbon in acidic forest soils. Soil Sci Soc Am J 71(1):64–74. https://doi.org/10.2136/sssaj2006.0111
Gubry-Rangin C, Kratsch C, Williams TA, McHardy AC, Embley TM, Prosser JI, Macqueen DJ (2015) Coupling of diversification and pH adaptation during the evolution of terrestrial Thaumarchaeota. P Natl Acad Sci USA 112(30):9370–9375. https://doi.org/10.1073/pnas.1419329112
Yin B, Crowley D, Sparovek G, De Melo WJ, Borneman J (2000) Bacterial functional redundancy along a soil reclamation gradient. Appl Environ Microb 66(10):4361–4365. https://doi.org/10.1128/Aem.66.10.4361-4365.2000
Acknowledgements
This research was financially supported by a PhD studentship from the University of Hong Kong (RW), a research grant from the National Natural Science Foundation of China [31470562] (Y-FW), and a Shenzhen Scientific Grant (JCYJ20140722112616608 to WSL) from the Shenzhen Science and Technology Innovation Committee of P.R. China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 31470562 to YFW), a Shenzhen Scientific Grant (JCYJ20140722112616608 to WSL) from the Shenzhen Science and Technology Innovation Committee, and a Hong Kong PhD Fellowship (RW).
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic Supplementary Material
ESM 1
(PDF 75 kb)
Rights and permissions
About this article
Cite this article
Wu, RN., Meng, H., Wang, YF. et al. A More Comprehensive Community of Ammonia-Oxidizing Archaea (AOA) Revealed by Genomic DNA and RNA Analyses of amoA Gene in Subtropical Acidic Forest Soils. Microb Ecol 74, 910–922 (2017). https://doi.org/10.1007/s00248-017-1045-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-1045-4