Abstract
The continuous cropping obstacles in monoculture fields are a major production constraint for peanuts. Application of the endophytic fungus Phomopsis liquidambari has increased peanut yields, and nodulation and N2 fixation increases have been considered as important factors for P. liquidambari infection-improved peanut yield. However, the mechanisms involved in this process remain unknown. This work showed that compared with only Bradyrhizobium inoculation, co-inoculation with P. liquidambari significantly elevated endogenous H2O2 and NO levels in peanut roots. Pre-treatment of seedlings with specific scavengers of H2O2 (CAT) and NO (cPTIO) blocked P. liquidambari-induced nodulation and N2 fixation. CAT not only suppressed the P. liquidambari-induced nodulation and N2 fixation, but also suppressed the enhanced H2O2 and NO generation. Nevertheless, the cPTIO did not significantly inhibit the induced H2O2 biosynthesis, implying that H2O2 acted upstream of NO production. These results were confirmed by observations that exogenous H2O2 and sodium nitroprusside (SNP) reversed the inhibition of P. liquidambari-increased nodulation and N2 fixation by the specific scavengers. The transcriptional activities of the symbiosis-related genes SymRK and CCaMK of peanut–Bradyrhizobium interactions also increased significantly in response to P. liquidambari, H2O2 and SNP treatments. The pot experiment further confirmed that the P. liquidambari infection-enhanced H2O2 and NO signalling pathways were significantly related to the increase in peanut nodulation and N2 fixation. This is the first report that endophytic fungus P. liquidambari can increase peanut–Bradyrhizobium interactions via enhanced H2O2/NO-dependent signalling crosstalk, which is conducive to the alleviation of continuous cropping obstacles via an increase in nodulation and N2 fixation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The peanut (Arachis hypogeae L.) is an important oil and cash crop worldwide. China is a major producer of peanuts, with approximately 5 million hectares cultivated in each growing season, accounting for 30% of the total oilseed production [1, 2]. Due to increasing land scarcity and agro-industrialisation, peanuts are usually continuously monocropped on a large scale in the same field for many years without any crop rotation [1, 3]. However, there are various problems associated with such consecutive monoculture, including a continuous decline in yield and quality, which is described as ‘peanut continuous cropping obstaclesʼ [1–3]. Fan et al. [4] and Wang et al. [5] demonstrated that decreases in nodulation and N2 fixation were important causes of peanut replant obstacles in the long-term monoculture field. Our previous studies showed that the application of an endophytic fungus, Phomopsis liquidambari [6], can effectively alleviate peanut continuous cropping obstacles [1, 5]. Further analysis demonstrated that the increase in nodulation and N2 fixation may play an important role in P. liquidambari enhancement of peanut yield [7, 8]. However, the detailed mechanisms by which P. liquidambari improve nodulation and N2 fixation in peanut are still unclear.
A recent study by Zhang et al. [8] demonstrated that P. liquidambari co-inoculated with Bradyrhizobium significantly increased root flavonoid compound (peanut rhizobial nod gene inducer) secretion and lateral root (peanut rhizobial infection site) formation, which contributed to the induction of peanut-specific rhizobia enrichment and increased the efficiency of rhizobial infection. Further follow-up revealed that P. liquidambari infection indeed increased bradyrhizobial colonisation, the peanut nodulation and N2 fixation [8]. It is well known that during legume–rhizobium interactions, rhizobial infection is dominant and host-specific, and it can occur via two different modes of entry into the roots (root ‘hair curling invasionʼ and ‘cortex crack-entryʼ), followed by initiation of the nodule primordium and finally development of an effective nodule [9, 10]. During the early stages of the legume–rhizobium interaction, regardless of whether the initial rhizobial entry is through an infection thread or epidermal cracks, a subtle and complicated molecular dialogue between the two partners is required to coordinate this symbiotic process [9–12]. Therefore, in our previous study, although the inoculation of endophytic fungus P. liquidambari significantly increased the Bradyrhizobium infection efficiency and accelerated nodule initiation, the molecular regulations involved in enhancing peanut–Bradyrhizobium interaction in response to P. liquidambari infection remain unknown.
Many studies have demonstrated that the development of legume–rhizobium symbiosis involves the activities of a variety of endogenous signalling molecules in host plants [13, 14], which are important for controlling or mediating symbiotic responses. Among these signalling molecules, particularly hydrogen peroxide (H2O2) and nitric oxide (NO) have been widely accepted as the signalling response to biotic and abiotic stress processes, and there is currently compelling evidence that H2O2 and NO play important role in signalling processes during the establishment of legume–Rhizobium symbioses [15–20]. Previous studies have shown that high levels of H2O2 and NO can be detected during the early stages of the legume–rhizobium symbiotic interaction and those changes in the levels of H2O2 and NO or modifications of the H2O2 and NO generation-related genes significantly impact legume–rhizobium symbiosis, nodule initiation and development [15–20]. Therefore, during the process of legume–Rhizobium symbiosis, H2O2 and NO appear to play a key signalling role in the establishment and function of this symbiotic interaction.
Endophytic fungi, as a large and novel microbial resource, can be found in healthy plant tissues without causing any apparent symptoms of disease. Many studies have suggested that endophytic fungi can exert multifunctional ecological functions for promoting host growth [21]. For the horizontal transmission of endophytic fungi, specific or non-specific infection forming a mutualistic symbiosis with various hosts can be randomly established at the proper moment in natural environments. During this symbiotic process, endophytic fungal infection can trigger host defence reactions, in which H2O2 and NO are also important early events in the induction of plant systematic acquired resistance in response to this external infection stressor [22–24], finally establishing a subtle antagonistic balance between hosts and endophytic fungi via the complicated signalling molecule dialogue [22, 24, 25]. In addition, our previous studies have also provided evidence that endophytic fungal infection-induced H2O2 and NO biosynthesis promotes Atractylodes lancea volatile oil accumulation [22, 23, 26]. It is believed that some signalling molecules, such as H2O2 and NO, are shared during simultaneous rhizobial and specific endophytic fungal infection of their shared hosts. Therefore, in this study, we hypothesised that the H2O2 and NO biosynthesis induced by P. liquidambari infection are important for promoting symbiotic peanut–Bradyrhizobium interactions, nodulation and N2 fixation, which is responsible for alleviating continuous cropping obstacles associated with peanut.
The aims of this study were to investigate whether infection caused by the endophytic fungus P. liquidambari could induce an increase in endogenous H2O2 and NO during the early stage of the symbiotic peanut–Bradyrhizobium interaction and to further investigate the cross-communication between H2O2 and NO signalling to determine whether the enhancement of this signalling pathway in response to P. liquidambari infection promotes the peanut–Bradyrhizobium interaction, nodulation and N2 fixation.
Materials and Methods
Experimental Strains and Soil
The endophytic fungus P. liquidambari was previously isolated from the inner bark of the stem of Bischofia polycarpa [6]. It was labelled with green fluorescent protein (GFP) by an expression vector, pCT74 [8, 27], and incubated in PDB medium (200 g L−1 potato extract, 20 g L−1 glucose, pH 7.0) for 48 h at 28 °C on a shaker at 180 rpm. Fungal mycelia were collected by filtering, washed three times with sterile distilled water and re-suspended in sterile distilled water to serve as the fungal inoculum. Bradyrhizobium yuanmingense was obtained from China Agricultural University; it had been previously isolated from the surface-sterilized nodules of peanut, cultured in YMB medium (D-Mannitol, 10 g L−1; yeast extract, 3 g L−1; MgSO4, 0.1 g L−1; NaCl, 0.1 g L−1; K2HPO4, 0.25 g L−1; KH2PO4, 0.25 g L−1) in an orbital shaker at 180 rpm and 28 °C for 6 days. The bacterial cells were centrifuged at 4000×g for 10 min, re-suspended in sterile distilled water and then diluted to an OD600 of 0.8, corresponding to a concentration of 108 cells mL−1, to generate the Bradyrhizobium inoculum.
The soil was collected from an agricultural field at the Ecological Experimental Station of Red Soil, Chinese Academy of Sciences (Yingtan, Jiangxi Province, China, 28°13′ N, 116°55′ E). The field had continuously cropped peanuts for 5 years, and the selected soil was classified as Udic Ferrosol [FAO (1998) classification]. After removal of visible coarse plant material, the collected surface layer soil (0–20 cm) was passed through a sieve (2 mm) and stored for the bioassay. The basic soil physicochemical properties were as follows: organic matter 9.5 g kg−1, total N 0.6 g kg−1, total P 0.3 g kg−1, total K 5.7 g kg−1, available P 10.3 mg kg−1, available K 113.8 mg kg−1, and pH (1:2.5, w/v) 5.7.
Plant Material, Pre-germination and In Vitro Inoculation
The peanut cultivar Guanhua-5 is a common planting variety that is grown in the red soil regions of Jiangxi Province [1], China, and was used in this study. The seeds were soaked for 5 min in 70% ethanol, followed by five washes with sterile distilled water, and further surface disinfection with 1% (v/v) sodium hypochlorite (NaOCl) for 3 min followed by another wash with sterile distilled water. The surface-sterilized seeds were pre-germinated in the dark at 28 °C until the radicles reached 2–3 cm. Germinated seeds exposed to similar growth conditions were transplanted into pots (12 cm in diameter, 15 cm in height) containing sterile vermiculite; each pot contained one peanut seedling. All pots were cultivated in a growth chamber at 28 °C with 16 h of light and at 25 °C with 8 h of dark, with 70% relative humidity. After 3 days of cultivation, the peanut seedling was subjected to four treatments: (1) non-inoculated control; (2) seedling inoculated with only B. yuanmingense (leading to an inoculation level of 109 cells/seedling) (B treatment); (3) seedling inoculated with only P. liquidambari (9.4 mg mycelial dry weight/seedling) (P treatment); and (4) seedling co-inoculation with P. liquidambari and B. yuanmingense (P + B treatment). The non-inoculated seedlings received sterilized water. Hoagland’s nutrient solution was added every 5 days, and sterilized distilled water was added when needed. Three replicates were evaluated for each treatment, and three plants (from three pots) were pooled as one replicate for each sampling point. All seedlings were randomly distributed in the growth chamber, and the culture conditions were consistent with those described above.
Determination of Endophytic Fungal Infection in Peanut Roots
The root samples were collected after 3 and 7 days of inoculation and processed for microscopy [27]. The fungal structures were observed under a Zeiss Axio Imager A1 microscope (Zeiss, Jena, Germany). These images were then collected and analysed using a Sensicam QE cooled digital camera system (Cooke Corporation, Germany) with a MetaMorph/MetaFluor combination software package (Universal Imaging, West Chester, PA, USA). Meanwhile, the collected peanut roots were carefully washed with sterile distilled water, and the root total genomic DNA was further extracted from freeze-dried powdered material using an AxyPrep Multisource Genomic DNA Miniprep Kit (Axygen Bio-sciences, Union City, CA, USA) following the manufacturerʼs instruction. A fragment of the P. liquidambari-specific ITS locus was amplified with previously designed primers Bf1 (5′-CTGGCCCCCTCGGGGTCCCTGG-3′) and Br1 (5′-TTTCAGGGCCTGCCCTTTTACAGGC-3′) to further confirm endophytic fungal colonisation [28]. The PCR mixture and thermal cycling conditions were similar to those reported by Wang et al. [28]. In addition, the amounts of the endophytic fungus P. liquidambari in the infected roots were also estimated by quantitative PCR (qPCR) according to the method described by Yang et al. [27] and Wang et al. [28].
Chemicals and Treatments
Catalase (CAT) and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO) were used as specific scavengers of H2O2 and NO, respectively. H2O2 solution and sodium nitroprusside (SNP) were used as donors of H2O2 and NO, respectively. CAT, cPTIO, H2O2, and SNP were purchased from Sigma-Aldrich (St. Louis, MO, USA). All scavengers and exogenous donors were dissolved in sterile distilled water and filtered through 0.22-μm diameter sterile filters before use. The reagents and their dosages were chosen based on our preliminary study. Scavengers were sprayed on seedling leaves and roots 1 day before the application of exogenous donors or microbial inoculation. An equal volume of sterile distilled water was used as the control. Three replicates were assessed for each treatment.
Peanut Nodulation and N2 Fixation Parameter Analysis
The peanut seedlings were collected after 21 and 42 days of inoculation. The roots (21 days) were carefully cleaned with sterile distilled water, and the number of nodule initials (primordia) was counted using a staining procedure [8]. In addition, the active nodules (pink colour) were excised from the roots (42 days) with scalpels to measure the nodule number, individual nodule dry weight, individual nodule size and total nodule dry weight [29]. Peanut growth parameters such as shoot (leaf and stem) dry weight and root dry weight were also measured at 42 days of inoculation. The nodules, shoots and roots were dried in an oven at 80 °C to a constant weight before weighing. The harvested shoot samples were ground to a fine powder, and the N concentration was determined using the Kjeldahl method. The amount of fixed N2 was estimated by the N difference method, in which the N yield of the shoot of the uninoculated peanut was subtracted from that of the inoculated peanut, and the difference was assumed to be derived from N2 fixation.
Extraction and Measurement of H2O2 and NO
The production of H2O2 and NO was measured using the H2O2 and NO detection kit (Nanjing Jiancheng Bio-engineering Institute, China) according to the manufacturer’s instructions [22, 26]. Approximately 1 g of fresh root sample was ground in 5 mL of sterile distilled water to evaluate H2O2 and 5 mL of 40 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.2) to assess NO. The homogenate was centrifuged at 14,000×g for 10 min. The supernatant was used to measure H2O2 and NO, respectively.
RNA Extraction and qPCR
Total RNA was extracted from peanut roots using TRIzol reagent, according to the manufacturer’s recommendations (Sigma). The RNA samples were treated with RNase-free DNase (1 U μL−1) at 37 °C for 15 min and then at 65 °C for 10 min to remove genomic DNA contamination. The RNA integrity and concentration were measured by electrophoresis and NanoDrop spectrophotometry, respectively. First-strand cDNA was synthesized from 1 μg of total RNA (PrimeScript One Step RT Reagent Kit; Takara). qPCR was performed using the StepOne Real-time PCR system (Applied Biosystems) with SYBR Green I fluorescent dye (Takara, Dalian, China). Peanut Actin and Ubiquitin were used as internal controls as previously described [30, 31], with the gene-specific primers AhActin-Fw (5′-CTGGCATCATACCTTCTACAACG-3′) and AhActin-Rv (5′-GAATGGCAACATACATAGCAGGG-3′), and AhUbiquitin-Fw (5′-AAGCCGAAGAAGATCAAGCAC-3′) and AhUbiquitin-Rv (5′-GGTTAGCCATGAAGGTTCCA-3′), respectively. Moreover, the specific primer pairs used for quantitative measurement of the transcription of SymRK and CCaMK, respectively, were designed according to their partial mRNA sequences as follows: AhSymRK-Fw (5′-TGCTCTTACCCACTCTGA-3′) and AhSymRK-Rv (5′-ATCTGTAGTTGGACCCTC-3′) (GenBank Accession No. JQ780692.1); AhCCaMK-Fw (5′-TCTGTTGTCAGGAAAGGCATAA-3′) and AhCCaMK-Rv (5′-CCGCAATCTGGCGAATAA-3′) (GenBank Accession No. EU395429.1). The qPCR reaction mixture contained 10 μL of SYBR Premix Ex Taq (dNTP, Ex Taq polymerase, SYBR Green I, and Ex Taq reaction buffer; Takara), 0.4 μL of each primer (10 μM) and 0.4 μL ROX Reference Dye (50×), 20 ng cDNA template, and sterile distilled water up to a final volume of 20 μL. The qPCR protocol was as follows: 94 °C for 1 min, followed by 45 cycles of 94 °C for 15 s, 60 °C for 45 s and 72 °C for 30 s. The specificity of the amplification was verified by a melting curve analysis and the presence of single PCR products in the electrophoretic gel. The relative abundance of SymRK and CCaMK transcription was normalized to the levels of the two reference genes and calculated using the ΔΔCt method. Each sample was amplified in triplicate in each experiment.
Pot Experiment
The pot experiment was performed to further test the proposed hypothesis. One kilogramme of non-sterile soil was weighed and added to a plastic pot (15-cm diameter, 13-cm high). The germinated peanut seeds were transplanted into these pots, and each pot contained one seedling. After 5 days of cultivation, the peanut seedlings were inoculated with P. liquidambari (9.4 mg mycelial dry weight/seedling) for the P treatment. The specific scavengers CAT and cPTIO of H2O2 and NO were applied to evaluate the role of H2O2 and NO signalling in P. liquidambari-induced nodulation and N2 fixation, serving as the P + 5 mKat L−1 CAT and P + 2 mM cPTIO treatments, respectively. Exogenous H2O2 and NO supplementation were set up as P + 5 mKat L−1 CAT+ H2O2 and P + 2 mM cPTIO + SNP, respectively. Non-inoculated seedlings were used as controls. Experimental pots were randomly arranged in the growth chamber, and the culture conditions were consistent with the descriptions provided above. All treatments were performed in triplicate. After 5 days of inoculation, the partial plants were collected to detect endogenous H2O2, NO and the transcription levels of the SymRK and CCaMK genes. In addition, peanut nodulation and N2 fixation parameters were also analysed after 40 days of inoculation.
Statistical Analysis
All statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Mean values and standard deviations (SD) were calculated, and the final experimental data are presented as the mean (±SD) of three replicates of each treatment. When an analysis consisted of only a control and an experimental group, an independent t test was performed using SPSS 13.0 software, and when three or more groups were compared, one-way analysis of variance (ANOVA) was performed, followed by Tukey’s multiple-comparison test. Comparisons between different treatments were considered significant at p < 0.05.
Results
Infection and Quantification of P. liquidambari in Peanut Roots
In order to visualize the infection process of endophytic fungus P. liquidambari in peanut roots, the GFP-tagged P. liquidambari was used and observed by fluorescence microscopy after 3 and 7 days of inoculation. In the early stage of infection (3 days) (Fig. 1a), although autofluorescence was observed in the roots, a large number of P. liquidambari hyphae interweaved together and distributed on the root surface, and only small amount of fungal hyphae could penetrate root epidermis and infect into the epidermal cell layer. After 7 days of inoculation (Fig. 1b), the GFP-tagged P. liquidambari was easily recognisable by its shape and size, and fungal hyphae can undergo intracellular infection and branched growth in the epidermal cell layer. There was no significant difference in fungal infection between P. liquidambari only inoculated (P) and co-inoculated (P + B) peanut roots, and P. liquidambari was not observed in non-inoculated roots. Figure 1c shows that the P. liquidambari-specific ITS fragment could be amplified from P and P + B treatments. In addition, the concentration of P. liquidambari within peanut roots was expressed as the number of P. liquidambari-specific ITS copies per ng total (root + fungal) genomic DNA in a qPCR analysis. As shown in Fig. 1d, the colonisation levels in roots between P and P + B treatments were similar at the same sampling time, and colonisation concentration was 6.35 (P) and 6.47 (P + B) and 14.25 (P) and 14.17 (P + B) copies ng−1 gDNA at 3 and 7 days of inoculation, respectively.
The analysis of endophytic fungal infection and colonisation in peanut roots. Fluorescence microscopy determination of endophytic fungus P. liquidambari-infected peanut roots after 3 days (a, scale bar = 20 μm) and 7 days (b, scale bar = 10 μm) of inoculation. Agarose gel electrophoresis (c) and qPCR (d) analysis of the colonisation of peanut roots by endophytic fungus P. liquidambari after 3 and 7 days of inoculation. Values are the means of three independent experiments ± SD. Data in columns marked by different letters are significantly different between the different treatments at the same sampling time (p < 0.05)
P. liquidambari Improves Nodulation and N2 Fixation Parameters
As shown in Table 1, compared to Bradyrhizobium infection treatment alone (B), co-inoculation with P. liquidambari and Bradyrhizobium (P + B) significantly increased the peanut nodule primordium and nodule numbers by 51.55 and 39.1%, respectively. Further nodule trait analysis indicated that P + B also significantly improved the individual nodule weight, size and total nodule weight by 8.24, 7.21 and 50.98%, respectively. Moreover, no difference was observed in the N content of shoots between control peanuts and ones infected with only P. liquidambari, but Bradyrhizobium infection alone significantly increased shoot N by 34.68%. However, the highest shoot N content was observed in response to P + B treatment, with an increase of 95.65% compared with the control. N2 fixation analysis indicated that the co-inoculated peanuts fixed more nitrogen than the peanuts inoculated with only Bradyrhizobium, with an increase of 174.54%. In addition, compared with the control, the peanut shoot and root dry weight were enhanced by 31.03 and 56.52%, respectively, in the co-inoculation experiment. In contrast, no significant change was observed in the peanut biomass following inoculation with Bradyrhizobium alone, but infection with P. liquidambari alone significantly increased the root dry weight by 30.43%.
Involvement of the Increase in H2O2 in P. liquidambari-Induced Nodulation and N2 Fixation Enhancement
Figure 2a shows that compared with the control, the endogenous H2O2 content in peanut roots increased significantly over time after Bradyrhizobium inoculation (B). Further analysis revealed that P. liquidambari-infected peanuts (P) consistently had a higher H2O2 content than peanuts that received the B treatment. After 3 days of inoculation, a significant difference in H2O2 content was observed between P + B and the other three treatments, and the highest H2O2 levels occurred from the 5- to 7-day sampling time. This result demonstrated that P. liquidambari and Bradyrhizobium co-inoculation could further induce the generation of H2O2 in peanut roots. However, after 7 days from inoculation, the levels of H2O2 in the B, P and P + B treatments all gradually declined with longer sampling time.
Involvement of H2O2 in Bradyrhizobium-induced and P. liquidambari-enhanced nodulation and N2 fixation in peanut. a Bradyrhizobium- and P. liquidambari-induced H2O2 production in peanut. b Effects of CAT (H2O2 scavenger) on Bradyrhizobium-induced and P. liquidambari-enhanced H2O2, nodulation and N2 fixation. Values are the means of three independent experiments ± SD. The points on the line graph with different letters indicate significant differences between different treatments at the same sampling time. The data in columns marked by different letters are significantly different between the different treatments (p < 0.05)
As shown in Fig. 2b, the addition of H2O2 scavenger (CAT) suppressed not only Bradyrhizobium-induced H2O2 production but also Bradyrhizobium-elicited nodulation and N2 fixation. Nodulation and N2 fixation in peanuts treated with 2, 5 and 10 mKat L−1 CAT were 16.39, 27.87 and 34.43% (nodulation) and 20.99, 31.79 and 41.51% (N2 fixation) lower, respectively, than those in the groups treated with Bradyrhizobium alone. A similar phenomenon was also observed in the experimental groups derived from P + B treatment, and the application of 2, 5 and 10 mKat L−1 CAT also significantly reduced peanut nodulation and N2 fixation by 10.11, 31.46 and 40.45% (nodulation) and 12.62, 34.27 and 42.8% (N2 fixation) compared with the P + B treatment. Therefore, in this study, 5 mKat L−1 CAT was chosen for the follow-up experiments. The results indicated that the generation of H2O2 indeed played a non-negligible role in Bradyrhizobium-elicited and P. liquidambari-enhanced peanut nodulation and N2 fixation.
Involvement of the Increase in NO in P. liquidambari-Induced Nodulation and N2 Fixation Enhancement
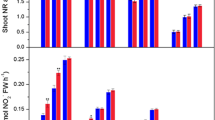
As shown in Fig. 3a, inoculation with Bradyrhizobium alone (B) significantly induced the biosynthesis of NO in peanut roots, and elevated NO was observed from 5 to 7 days. Similarly, P. liquidambari infection (P) also triggered the production of endogenous NO, while the content of NO in response to P was always relatively higher than that in peanuts that received only B treatment. The highest NO content was observed in co-inoculated peanuts (P + B), which showed significantly differences from the other three treatments after 5 days, suggesting that the co-inoculation could further improve the generation of NO. Additionally, after 7 days of incubation, the NO contents all gradually decreased in the B, P and P + B treatments.
Involvement of NO in Bradyrhizobium-induced and P. liquidambari-enhanced nodulation and N2 fixation in peanut. a Bradyrhizobium- and P. liquidambari-induced NO production in peanut. b Effects of cPTIO (NO scavenger) on Bradyrhizobium-induced and P. liquidambari-enhanced NO, nodulation and N2 fixation. Values are the means of three independent experiments ± SD. The points on the line graph with different letters indicate significant differences between different treatments at the same sampling time. Data in columns marked by different letters are significantly different between the different treatments (p < 0.05)
Figure 3b shows that NO scavenger (cPTIO) suppressed NO accumulation in peanut roots, and the nodulation and N2 fixation in the B + 2 mM cPTIO and B + 5 mM cPTIO treatments were both significantly decreased by 31.67 and 38.33% (nodulation) and 34.57 and 36.9% (N2 fixation), respectively, compared with the B treatment. A similar trend was observed in the P + B treatments supplemented with cPTIO; the application of 2 and 5 mM cPTIO significantly reduced nodulation by 30.77 and 35.16% and N2 fixation by 29.66 and 37.67% compared to the P + B treatment. Therefore, 2 mM cPTIO was used for the follow-up experiments. These data implied that the enhancement of peanut nodulation and N2 fixation induced by P. liquidambari infection likely depends on the improvement of endogenous H2O2 and NO biosynthesis in peanut roots.
Interaction Between H2O2 and NO Biosynthesis in P. liquidambari-Induced Nodulation and N2 Fixation Enhancement
The results obtained above indicated that H2O2 and NO biosynthesis are involved in Bradyrhizobium-induced or P. liquidambari-stimulated peanut nodulation and N2 fixation; however, their relationship and involvement in this process remained unclear. As shown in Fig. 4a, b, in the B and P + B treatments, Bradyrhizobium-induced or P. liquidambari-stimulated NO generation was strongly inhibited by the H2O2 scavenger CAT, whereas the NO scavenger (cPTIO) did not significantly affect Bradyrhizobium-induced or P. liquidambari-stimulated H2O2 production, indicating that the H2O2 and NO signalling pathways were closely linked and that H2O2 might act as an upstream signalling molecule during Bradyrhizobium-induced or P. liquidambari-stimulated NO biosynthesis. In addition, the application of exogenous H2O2 and SNP could reverse the inhibition of Bradyrhizobium-induced or P. liquidambari-stimulated peanut nodulation and N2 fixation by CAT and cPTIO, which further confirmed the results described above.
Interactions between H2O2 and NO signalling pathways for Bradyrhizobium-induced (a) and P. liquidambari-enhanced (b) nodulation and N2 fixation in peanut. Values are the means of three independent experiments ± SD. Data in columns marked by different letters are significantly different between the different treatments (p < 0.05)
P. liquidambari-Induced NO Dependence on H2O2 Production
The results shown in Fig. 5a indicated that in the group inoculated with Bradyrhizobium alone, both exogenous H2O2 and SNP significantly increased nodulation and N2 fixation. The increased nodulation and N2 fixation produced by H2O2 were completely inhibited by cPTIO, whereas the enhancement induced by SNP remained almost unaffected by CAT. Exogenous H2O2 could promote NO generation, but the application of SNP did not influence H2O2. However, compared with the P + B treatment (Fig. 5b), exogenous H2O2 addition enhanced the levels of endogenous H2O2 and NO but significantly decreased nodulation and N2 fixation, while SNP addition had no significant differential effect on nodulation and N2 fixation between the P + B and P + B + SNP groups. In addition, the application of cPTIO significantly inhibited nodulation and N2 fixation in the P + B + H2O2 group, whereas in the P + B + SNP group, CAT did not appear to have any effect. Further analysis also indicated that H2O2 stimulated NO generation, but no impact on H2O2 was observed in response to exogenous SNP for the P + B treatment. These findings further demonstrated that H2O2 may act upstream of NO production in P. liquidambari-induced nodulation and N2 fixation; however, excessive H2O2 accumulation could have unfavourable effects on peanut nodulation and N2 fixation.
Transcriptional Responses of the Symbiosis-Related Genes SymRK and CCaMK to Different Experimental Treatments
As shown in Fig. 6a, following Bradyrhizobium-only infection, exogenous H2O2 and SNP significantly up-regulated the transcription of SymRK and CCaMK, which were decreased following the application of CAT and cPTIO. H2O2 supplementation could alleviate this inhibition by CAT but did not affect cPTIO inhibition. However, SNP supplementation reversed the transcriptional suppression of SymRK and CCaMK produced by both CAT and cPTIO. For the P + B treatment (Fig. 6b), exogenous H2O2 down-regulated the transcription of SymRK and CCaMK, but no significant influence was observed for SNP addition. Both CAT and cPTIO decreased the expression levels of SymRK and CCaMK. Similarly, exogenous H2O2 reversed the suppression produced by CAT, but no obvious effect was observed following cPTIO addition. Additionally, exogenous SNP supplementation could significantly up-regulate the expression levels of SymRK and CCaMK in response to CAT and cPTIO inhibition. These findings were consistent with the observations described above. Additionally, P. liquidambari-only infection did not affect the transcription of SymRK and CCaMK.
The transcriptional activities of the symbiosis-related genes SymRK and CCaMK during Bradyrhizobium-induced (a) and P. liquidambari-enhanced (b) nodulation and N2 fixation in peanuts. Values are the means ± SD of three independent experiments. Data in columns marked by different letters are significantly different between the different treatments (p < 0.05)
P. liquidambari-Induced Nodulation and N2 Fixation Increase via Enhancement of the H2O2 and NO Signalling Pathway Under Pot Conditions
In the pot experiment, P. liquidambari inoculation significantly increased peanut nodule primordium formation, nodulation and N2 fixation, and exogenous H2O2 and SNP could effectively reverse the inhibition of nodulation and N2 fixation produced by CAT and cPTIO, respectively (Fig. 7a). Figure 7b shows that the levels of H2O2, NO and SymRK and CCaMK transcription were all significantly increased after P. liquidambari infection. Exogenous CAT slightly inhibited P. liquidambari-stimulated H2O2 and NO, while cPTIO reduced NO generation but did not affect H2O2 generation. Moreover, both CAT and cPTIO effectively down-regulated SymRK and CCaMK gene expression. However, exogenous H2O2 and SNP not only reversed the inhibition of H2O2 and NO production by CAT and cPTIO but also clearly up-regulated the transcription of SymRK and CCaMK. This further demonstrated that the P. liquidambari-induced H2O2 and NO signalling pathway improved the peanut–Bradyrhizobium interaction, ultimately resulting in increased peanut nodulation and N2 fixation.
Effects of CAT, cPTIO and exogenous H2O2 and SNP on P. liquidambari-induced nodulation and N2 fixation, H2O2 and NO, and SymRK and CCaMK transcription under pot conditions. Values are the means ± SD of three independent experiments. Data in columns marked by different letters are significantly different between the different treatments (p < 0.05)
Discussion
The use of beneficial microorganisms to increase leguminous plant nodulation and N2 fixation is a promising strategy and supplies an irreplaceable advantage over other conventional methods [8, 32–35]. Garg and Pandey [35] showed a positive correlation between arbuscular mycorrhizal fungal (AMF) colonisation and the increased legume–rhizobium symbiosis, nodulation and N2 fixation. Therefore, efficient infection or colonization is pivotal for the ability of exogenous microorganisms to exert their potential ecological functions [8, 27, 35, 36]. Compared with our previous study [8], this study further indicated that endophytic fungus P. liquidambari could successfully infect and colonise peanut roots in a relative short incubation time. P. liquidambari infection effectively increased individual nodule weight and size and total nodule weight, implying that the improvement of nodule characteristics should be responsible for increasing the peanut N2 fixation capacity. Although many studies have demonstrated that some bacteria, fungi and other rhizobial helper microbes could significantly increase plant nodulation and N2 fixation [32, 33, 35, 37–39], little information was available concerning the impact of endophytes on legume–rhizobium symbiosis interaction. Several studies have suggested the positive effects of endophytic actinomycetes Streptomyces sp. based on their ability to influence legume nodules by increasing the root-nodulating frequency [34, 40–42]. Subramanian et al. [43] demonstrated that plant endophytic bacteria Bacillus megaterium LNL6 isolated from the root nodules of Lesperdeza sp. and endophytic bacteria isolated from rice leaves significantly improved nodule function and N2 fixation in soybean co-inoculated with Bradyrhizobium japonicum MN110. In comparison to these studies, we first reported that an endophytic fungus has the potential to increase peanut-Bradyrhizobium interactions, and P. liquidambari appear to provide improved nodulation and N2 fixation activity than those previously reported microbes.
The signalling crosstalk between H2O2 and NO appears to be considered an essential factor that allows plants to adapt to complicated external conditions and respond to different biotic and abiotic stresses [16–19, 44]. H2O2 may act as a cofactor to promote endogenous NO synthesis [45, 46]. However, NO may also induce H2O2 production [47, 48]. Therefore, the forms of signalling crosstalk between H2O2 and NO are varied and mainly depend on the plants and the external stresses. Although an increasing number of studies have demonstrated that H2O2 and NO may have important functions in legume-rhizobium symbiosis, nodulation and N2 fixation [15–20], no studies have investigated the relationship between increased nodulation and the regulation of H2O2 and NO signalling crosstalk by co-inoculation with beneficial microorganisms.
To date, research investigating the important roles of H2O2 and NO during legume–Rhizobium symbiosis and nodulation have mainly focused on the pair-wise interactions between hosts and their corresponding symbiotic microorganisms [16, 19] while overlooking the interplay among multiple endosymbionts and their combined effects on host plants. Regarding N2 fixing interactions, several studies have also shown that co-inoculation with symbiotic microorganisms (AMF or endophytes) can effectively increase legume–Rhizobium symbiosis, nodulation and N2 fixation [33, 35, 37, 41–43, 49]. However, the detailed mechanisms underlying symbiosis interaction by multiple endosymbionts, and the involvement of H2O2 and NO crosstalk in this process had not been previously assessed. The present findings demonstrated that enhanced H2O2 and NO signalling are involved in P. liquidambari-induced peanut–Bradyrhizobium symbiosis, nodulation and N2 fixation. To our knowledge, this is the first report to demonstrate the enhanced signalling crosstalk between H2O2 and NO in endophytic fungal promotion of peanut–Bradyrhizobium interactions. However, we also observed that excessive H2O2 accumulation may induce oxidative damage in roots, resulting in unfavourable conditions for nodulation and N2 fixation.
In this study, compared with only Bradyrhizobium inoculation, P. liquidambari-infected peanuts consistently had a higher H2O2 and NO contents in roots, which suggested that P. liquidambari infection significantly improved the production of H2O2 and NO. Many studies showed that inoculation with AMF could significantly increase H2O2 and NO accumulations in the early stages of AMF-host interactions [16, 24, 50], which played an important role involved in symbiosis, growth promotion, defence reaction and secondary metabolite synthesis. Our previous studies indicated that endophytic fungus- and bacterium-triggered H2O2 and NO could directly increase total volatile oil accumulation and oxygenous sesquiterpenoid diversity in A. lancea [22, 51]. Therefore, we believe that the improvement of H2O2 and NO production in peanut roots may be due to the induction of systemic acquired resistance responded to endophytic fungus P. liquidambari infection; thus, the elevated H2O2 and NO can be used to enhance peanut–Bradyrhizobium interactions. In addition to responding P. liquidambari-infected stress, the stimulated H2O2 and NO might be considered as important signalling pathway to mediate peanut growth promotion, secondary metabolite production and even the other potential ecological functions produced from P. liquidambari. Therefore, the detailed action mechanisms for enhancing H2O2 and NO biosynthesis in peanut roots with P. liquidambari infection need further investigation.
Many studies have demonstrated that H2O2 and NO generation have important functions in regulating the expression of some symbiosis-related genes during the early stages of the legume–Rhizobium interaction, which are important for initiating the subsequent Rhizobium infection and formation of the nodule primordium [15, 16, 18, 52]. Regarding the symbiotic interaction between legumes and their cognate Rhizobium symbionts, Arthikala et al. [17] suggest that the symbiotic signals are transduced by the so-called Rhizobium symbiosis pathway, in which the symbiotic marker genes SymRK and CCaMK participate in the early signalling pathway that recognizes Rhizobium endosymbionts and further induces downstream signalling events to regulate the symbiotic process. The study reported by Sinharoy et al. [30] demonstrated that the early stages of the symbiotic peanut–Bradyrhizobium interaction are also regulated by the symbiotic marker genes SymRK and CCaMK. Therefore, the transcriptional activities of SymRK and CCaMK can directly reflect the interaction strength between peanut and Bradyrhizobium symbiosis. In this study, P. liquidambari co-inoculation significantly increased transcription of the SymRK and CCaMK genes, consistent with the enhanced peanut nodulation and N2 fixation. However, the enhanced SymRK and CCaMK transcription was completely abolished by specific H2O2 and NO scavengers, and exogenous supplementation of H2O2 and SNP could effectively reverse their transcriptions. Therefore, these results further confirm that the enhanced H2O2 and NO signalling crosstalk in response to P. liquidambari infection might be responsible for the increased symbiotic peanut–Bradyrhizobium interaction. In addition to SymRK and CCaMK genes, some other symbiosis-related genes, such as CASTOR, POLLUX, NUP85 and NUP133, which are also required and may be significantly regulated by H2O2 and NO during the early stages of the legume-Rhizobium interaction [15, 17, 18]. However, no information is available about the functions of these symbiosis-related genes or their homologous genes in the peanut–Bradyrhizobium symbiosis interaction. Therefore, more studies are needed to further investigate those genes involved in the early peanut–Bradyrhizobium symbiosis interaction and their possible relationships with enhanced H2O2 and NO signalling crosstalk.
Although endophytic fungi are broad-spectrum endosymbionts that are widely distributed in various hosts, very few studies have investigated improvements in legume nodulation and N2 fixation in response to endophytic fungal infection. As an environmentally adaptive endophytic fungus, P. liquidambari can efficiently improve soil biochemical and microbiological properties, which could provide a beneficial habitat for plant–microbe interactions [28, 53–56]. As a symbiotic fungus, P. liquidambari has been observed to significantly increase nitrogen uptake and accumulation in rice (Oryza sativa L.) by establishing a symbiotic system [27, 36, 56]. The discovery of the ability of P. liquidambari to increase peanut nodulation and N2 fixation may provide a new research field to elaborate novel ecological functions of endophytic fungi. The controlled pot experiment further confirmed that the increase in peanut nodulation and N2 fixation mainly resulted from the enhancement of H2O2 and NO signalling crosstalk induced by P. liquidambari (Fig. 8). Additionally, exogenous H2O2 and SNP addition effectively increased nodulation and N2 fixation. Therefore, we speculate that for practical agricultural production, direct application of H2O2 or SNP can improve symbiotic peanut–Bradyrhizobium interactions. However, this effect requires further investigation because of the presence of various factors in the natural agri-ecosystem. In contrast, based on the results of our previous studies and in this study, we believe that P. liquidambari can be directly used as an endosymbiotic elicitor to alleviate the long-term continuous cropping obstacles associated with the peanut monoculture field by facilitating the peanut–Bradyrhizobium interaction, nodulation and N2 fixation. However, additional research is needed to further understand the detailed molecular regulation of the peanut-Bradyrhizobium interaction in response to P. liquidambari-induced H2O2 and NO.
A model in which P. liquidambari infection enhances H2O2 and NO signalling crosstalk in peanut, resulting in an increase in peanut–Bradyrhizobium interactions, nodulation and N2 fixation. The symbol “+” represents a positive effect on peanut–Bradyrhizobium interactions in response to P. liquidambari infection in peanut roots
Conclusions
Consecutive monoculturing of the peanut has seriously affected its yield and quality. Different measures used for improving peanut production should be explored to ensure sustainable development of the peanut industry. In this work, we demonstrated that infection by the endophytic fungus P. liquidambari significantly increased H2O2 and NO generation compared with Bradyrhizobium inoculation alone. Specific H2O2 and NO scavengers and exogenous H2O2 and SNP supplementation experiments showed that P. liquidambari-induced increases in peanut nodulation and N2 fixation were attributed to enhanced signalling crosstalk between H2O2 and NO. The transcriptional levels of the symbiosis-related genes SymRK and CCaMK also increased significantly in response to P. liquidambari infection. The pot experiment further confirmed that the P. liquidambari infection-enhanced H2O2 and NO signalling pathway was responsible for the increased symbiotic peanut–Bradyrhizobium interaction, which was required for improving nodulation and N2 fixation. This is the first study to report that P. liquidambari acts as an endophytic elicitor to increase peanut–Bradyrhizobium interactions via enhanced H2O2/NO-dependent signalling crosstalk, alleviating the long-term continuous cropping obstacles associated with peanut by increasing nodulation and N2 fixation.
References
Li XG, Wang XX, Dai CC, Zhang TL, Xie XG, Ding CF, Wang HW (2014) Effects of intercropping with Atractylodes lancea and application of bio-organic fertiliser on soil invertebrates, disease control and peanut productivity in continuous peanut cropping field in subtropical China. Agroforest Syst 88:41–52
Li XG, Ding CF, Hua K, Zhang TL, Zhang YN, Zhao L, Yang YR, Liu JG, Wang XX (2014) Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 78:149–159
Ma HY, Yang B, Wang HW, Yang QY, Dai CC (2016) Application of Serratia marcescens RZ-21 significantly enhances peanut yield and remediates continuously cropped peanut soil. J Sci Food Agr 96:245–253
Fan TQ, Wang SB, Jiang SQ, Cheng B, Li Y, Chu CJ, Wang CB (2007) Effect of continuous cropping on photosynthesis and accumulation of dry matter in peanut. Journal of Peanut Science 36:35–37
Wang HW, Wang XX, Lü LX, Xiao Y, Dai CC (2012) Effects of applying endophytic fungi on the soil biological characteristics and enzyme activities under continuously cropped peanut. China J Appl Ecol 23:2693–2700
Chen Y, Peng Y, Dai CC, Ju Q (2011) Biodegradation of 4-hydroxybenzoic acid by Phomopsis liquidambari. Appl. Soil Ecol. 51:102–110
Zhou J, Kang L, Wang HW, Yang T, Dai CC (2014) Liquid laccase production by Phomopsis liquidambari B3 accelerated phenolic acids degradation in long-term cropping soil of peanut. Acta Agricult. Scand. Sect. B Soil Plant Sci. 64:683–693
Zhang W, Wang HW, Wang XX, Xie XG, Siddikee MA, Xu RS, Dai CC (2016) Enhanced nodulation of peanut when co-inoculated with fungal endophyte Phomopsis liquidambari and bradyrhizobium. Plant Physiol Bioch 98:1–11
Goormachtig S, Capoen W, Holsters M (2004) Rhizobium infection: lessons from the versatile nodulation behaviour of water-tolerant legumes. Trends Plant Sci. 9:518–522
Suzaki T, Kawaguchi M (2014) Root nodulation: a developmental program involving cell fate conversion triggered by symbiotic bacterial infection. Curr. Opin. Plant Biol. 21:16–22
Fabra A, Castro S, Taurian T, Angelini J, Ibañez F, Dardanelli M, Tonelli M, Bianucci E, Valetti L (2010) Interaction among Arachis hypogaea L. (peanut) and beneficial soil microorganisms: how much is it known? Crit. Rev. Microbiol. 36:179–194
Muñoz V, Ibáñez F, Tordable M, Megías M, Fabra A (2015) Role of reactive oxygen species generation and Nod factors during the early symbiotic interaction between bradyrhizobia and peanut, a legume infected by crack entry. J. Appl. Microbiol. 118:182–192
Mulder L, Hogg B, Bersoult A, Cullimore JV (2005) Integration of signalling pathways in the establishment of the legume-rhizobia symbiosis. Physiol Plantarum 123:207–218
Ferguson BJ, Mathesius U (2014) Phytohormone regulation of legume-rhizobia interactions. J. Chem. Ecol. 40:770–790
Andrio E, Marino D, Marmeys A, Dunoyer de Segonzac M, Damiani I, Genre A, Huguet S, Frendo P, Puppo A, Pauly N (2013) Hydrogen peroxide–regulated genes in the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytol 198:190–202
Puppo A, Pauly N, Boscari A, Mandon K, Brouquisse R (2013) Hydrogen peroxide and nitric oxide: key regulators of the legume-Rhizobium and mycorrhizal symbioses. Antioxid Redox Sign 18:2202–2219
Arthikala MK, Sánchez-López R, Nava N, Santana O, Cárdenas L, Quinto C (2014) RbohB, a Phaseolus vulgaris NADPH oxidase gene, enhances symbiosome number, bacteroid size, and nitrogen fixation in nodules and impairs mycorrhizal colonization. New Phytol 202:886–900
del Giudice J, Cam Y, Damiani I, Fung-Chat F, Meilhoc E, Bruand C, Brouquisse R, Puppo A, Boscari A (2011) Nitric oxide is required for an optimal establishment of the Medicago truncatula–Sinorhizobium meliloti, symbiosis. New Phytol 191:405–417
Meilhoc E, Boscari A, Bruand C, Puppo A, Brouquisse R (2011) Nitric oxide in legume–rhizobium symbiosis. Plant Sci. 181:573–581
Nagata M, Murakami E, Shimoda Y, Shimoda-Sasakura F, Kucho K, Suzuki A, Abe M, Higashi S, Uchiumi T (2008) Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol Plant Microbe In 21:1175–1183
Rodriguez RJ, White Jr JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330
Wang Y, Dai CC, Zhao YW, Peng Y (2011) Fungal endophyte-induced volatile oil accumulation in Atractylodes lancea plantlets is mediated by nitric oxide, salicylic acid and hydrogen peroxide. Process Biochem. 46:730–735
Ren CG, Dai CC (2013) Nitric oxide and brassinosteroids mediated fungal endophyte-induced volatile oil production through protein phosphorylation pathways in Atractylodes lancea plantlets. J. Integr. Plant Biol. 55:1136–1146
Espinosa F, Garrido I, Ortega A, Casimiro I, Álvarez-Tinaut MC (2014) Redox activities and ROS, NO and phenylpropanoids production by axenically cultured intact olive seedling roots after interaction with a mycorrhizal or a pathogenic fungus. PLoS One 9:e100132
Wang XM, Yang B, Wang HW, Yang T, Ren CG, Zheng HL, Dai CC (2015) Consequences of antagonistic interactions between endophytic fungus and bacterium on plant growth and defense responses in Atractylodes lancea. J Basic Microb 55:659–670
Yuan J, Sun K, Deng-Wang MY, Dai CC (2016) The mechanism of ethylene signaling induced by endophytic fungus Gilmaniella sp. AL12 mediating sesquiterpenoids biosynthesis in Atractylodes lancea. Front. Plant Sci. 7:361
Yang B, Wang XM, Ma HY, Jia Y, Li X, Dai CC (2014) Effects of the fungal endophyte Phomopsis liquidambari on nitrogen uptake and metabolism in rice. Plant Growth Regul. 73:165–179
Wang HW, Dai CC, Zhu H, Wang XX (2014) Survival of a novel endophytic fungus Phomopsis liquidambari B3 in the indole-contaminated soil detected by real-time PCR and its effects on the indigenous microbial community. Microbiol. Res. 169:881–887
Hwang S, Ray JD, Cregan PB, King CA, Davies MK, Purcell LC (2014) Genetics and mapping of quantitative traits for nodule number, weight, and size in soybean (Glycine max L.[Merr.]). Euphytica 195:419–434
Sinharoy S, Saha S, Chaudhury SR, Dasgupta M (2009) Transformed hairy roots of Arachis hypogea: a tool for studying root nodule symbiosis in a non-infection thread legume of the aeschynomeneae tribe. Mol Plant Microbe In 22:132–142
Morgante CV, Guimarães PM, Martins ACQ, Araújo ACG, Leal-Bertioli SC, Bertioli DJ, Brasileiro ACM (2011) Reference genes for quantitative reverse transcription-polymerase chain reaction expression studies in wild and cultivated peanut. BMC Res Notes 4:339
Egamberdieva D, Berg G, Lindström K, Räsänen LA (2010) Co-inoculation of Pseudomonas spp. with Rhizobium improves growth and symbiotic performance of fodder galega (Galega orientalis lam.). Eur. J. Soil Biol. 46:269–272
Sakamoto K, Ogiwara N, Kaji T (2013) Involvement of autoregulation in the interaction between rhizobial nodulation and AM fungal colonization in soybean roots. Biol Fert Soils 49:1141–1152
Le XH, Ballard RA, Franco CMM (2016) Effects of endophytic Streptomyces and mineral nitrogen on lucerne (Medicago sativa L.) growth and its symbiosis with rhizobia. Plant Soil 405:25–34
Garg N, Pandey R (2016) High effectiveness of exotic arbuscular mycorrhizal fungi is reflected in improved rhizobial symbiosis and trehalose turnover in Cajanus cajan, genotypes grown under salinity stress. Fungal Ecol. 21:57–67
Yang B, Ma HY, Wang XM, Jia Y, Hu J, Li X, Dia CC (2014) Improvement of nitrogen accumulation and metabolism in rice (Oryza sativa, l.) by the endophyte Phomopsis liquidambari. Plant Physiol Bioch 82:172–182
Tavasolee A, Aliasgharzad N, Salehi GR, Mardi M, Asgharzadeh A, Akbarivala S (2011) Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on fungal occupancy in chickpea root and nodule determined by real-time PCR. Curr. Microbiol. 63:107–114
Masciarelli O, Llanes A, Luna V (2013) A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol. Res. 169:609–615
Vicario JC, Primo ED, Dardanelli MS, Giordano W (2016) Promotion of peanut growth by co-inoculation with selected strains of Bradyrhizobium and Azospirillum. J. Plant Growth Regul. 35:413–419
Tokala RK, Strap JL, Jung CM, Crawford DL, Salove MH, Deobald LA, Bailey JF, Morra MJ (2002) Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl Environ Microb 68:2161–2171
Soe KM, Bhromsiri A, Karladee D, Yamakawa T (2012) Effects of endophytic actinomycetes and Bradyrhizobium japonicum strains on growth, nodulation, nitrogen fixation and seed weight of different soybean varieties. Soil Sci. Plant Nutr. 58:319–325
Le XH, Franco CMM, Ballard RA, Drew EA (2015) Isolation and characterisation of endophytic actinobacteria and their effect on the early growth and nodulation of lucerne (Medicago sativa L.). Plant Soil 405:13–24
Subramanian P, Kim K, Krishnamoorthy R, Sundaram S, Sa T (2015) Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobium japonicum MN110. Plant Growth Regul. 76:327–332
Niu LJ, Liao WB (2016) Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front. Plant Sci. 7:230
Lin AH, Wang YQ, Tang JY, Xue P, Li CL, Liu LC, Hu B, Yang FQ, Loake GJ, Chu CC (2012) Nitric oxide and protein S-Nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 158:451–464
Shi K, Li X, Zhang H, Zhang GQ, Liu YR, Zhou YH, Xia XJ, Chen ZX, Yu JQ (2015) Guard cell hydrogen peroxide and nitric oxide mediate elevated CO2-induced stomatal movement in tomato. New Phytol 208:342–353
Liao WB, Huang GB, Yu JH, Zhang ML, Shi XL (2011) Nitric oxide and hydrogen peroxide are involved in indole-3-butyric acid-induced adventitious root development in marigold. J. Hortic. Sci. Biotechnol. 86:159–165
Zhang F, Wang YP, Yang YL, Wu H, Wang D, Liu JQ (2007) Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ. 30:775–785
André S, Neyra M, Duponnois R (2003) Arbuscular mycorrhizal symbiosis changes the colonization pattern of Acacia tortilis spp. raddiana rhizosphere by two strains of rhizobia. Microb. Ecol. 45:137–144
Zhang RQ, Zhu HH, Zhao HQ, Yao Q (2013) Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J. Plant Physiol. 170:74–79
Zhou JY, Yuan J, Li X, Ning YF, Dai CC (2015) Endophytic bacterium-triggered reactive oxygen species directly increase oxygenous sesquiterpenoid content and diversity in Atractylodes lancea. Appl. Environ. Microbiol. 82:1577–1585
Sánchez C, Cabrera JJ, Gates AJ, Bedmar EJ, Richardson DJ, Delgado MJ (2011) Nitric oxide detoxification in the rhizobia-legume symbiosis. Biochem Soc T 39:184–188
Chen Y, Wang HW, Li L, Dai CC (2013) The potential application of the endophyte Phomopsis liquidambari to the ecological remediation of long-term cropping soil. Appl. Soil Ecol. 67:20–26
Chen Y, Ren CG, Yang B, Peng Y, Dai CC (2013) Priming effects of the endophytic fungus Phomopsis liquidambari on soil mineral n transformations. Microb. Ecol. 65:161–170
Xie XG, Dai CC (2015) Biodegradation of a model allelochemical cinnamic acid by a novel endophytic fungus Phomopsis liquidambari. Int Biodeter Biodeg 104:498–507
Yang B, Wang XM, Ma HY, Yang T, Jia Y, Zhou J, Dai CC (2015) Fungal endophyte Phomopsis liquidambari affects nitrogen transformation processes and related microorganisms in the rice rhizosphere. Front. Microbiol. 6:982
Acknowledgements
We would like to acknowledge the National Natural Science Foundation of China (NSFC No. 31370507), the Ph.D. Programs Foundation of Ministry of Education of China (No. 20133207110001), the Major Natural Science Research Programs of Jiangsu Higher Education Institutions (No. 13KJA180003), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions of China, and the Graduate Education Innovation Project of Jiangsu Province (KYZZ15_0215). We also express our great thanks to the anonymous reviewers and editorial staff for their time and attention.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Xie, XG., Fu, WQ., Zhang, FM. et al. The Endophytic Fungus Phomopsis liquidambari Increases Nodulation and N2 Fixation in Arachis hypogaea by Enhancing Hydrogen Peroxide and Nitric Oxide Signalling. Microb Ecol 74, 427–440 (2017). https://doi.org/10.1007/s00248-017-0944-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-0944-8