Abstract
Phosphorus (P) is an important limiting element for the growth and yield of peanut (Arachis hypogeae L.). Mutualistic fungus Phomopsis liquidambaris (B3) effectively increases host P accumulation, but the underlying mechanism is poorly understood, and this study aimed to illustrate how and the mechanism by which B3 increase P accumulation of peanut under P limitation from the perspective of phytohormone crosstalk. In this study, greenhouse pot experiments were performed to investigate the effects of B3 colonization on the phytohormone auxin (IAA), gibberellins (GAs), and cytokinins (CKs) signaling pathways of peanut under different P levels (trace P, low P, and normal P). The results revealed that B3 is capable of significantly increasing the concentrations of IAA, GAs, and CKs in peanut under low P conditions. Complementation and functional inhibition experiments demonstrated that IAA and CKs may be upstream signaling molecules of GAs and function in B3-induced expression of the AhPTH1;3 and AhPTH1;4 genes, leading to increased levels of P in peanut and consequently promoting growth. Furthermore, the improved signal transduction of phytohormones should also be important for root system architecture (RSA) establishment, which is further helpful in enhancing P absorption in peanut. Our study suggests that the endophytic fungus B3-regulated crosstalk of phytohormone signaling pathways is an important factor for increasing P accumulation in peanuts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus (P) is an essential macronutrient for plant development and reproduction and is a major component of biogeochemistry (López-Arredondo et al., 2014). However, despite the abundance of P in both organic and inorganic forms in the soil, it is mostly unavailable for plant uptake due to its high reactivity with metal cations in the soil (Rawat et al., 2020). Thus, approximately 70% of global cultivated land suffers from P deficiency, which has become a limiting factor of primary productivity in agricultural ecosystems (López-Arredondo et al., 2014; Wen et al., 2022). Thus, inorganic P fertilizers are intensively used at high concentrations to support crop production. However, excessive application of P fertilizer ensures high crop yields, but environmental pollution problems also arise (Oldroyd and Leyser 2020; Pathak and Fagodiya 2022). At present, novel solutions are needed to increase the P uptake efficiency of crops, which reduces phosphate fertilizer inputs while securing high crop yields and contributes to sustainable agricultural development.
Plants have evolved intricate mechanisms to survive and flourish in low P stress conditions; these responses include modification of their root system architecture (RSA), increased expression of high affinity P transporter genes, and the induction of phytohormones (Oldroyd and Leyser 2020). Some growth phytohormones, including auxin (IAA), cytokinins (CKs), gibberellins (GAs), ethylene (ETH), and strigolactone (SL), participate in root tip perception of PO4− starvation signaling and drive the modification of RSA under low P conditions (Svistoonoff et al., 2007; Revalska and Iantcheva 2018). Many studies have suggested that biosynthesis, polar transport, and sensitivity to IAA signals play important roles in delaying taproot growth and inducing lateral root formation under low P conditions (Liu et al., 2013; Péret et al., 2011). Most often, the CKs signaling pathway is thought to be related to the induction of root development caused by P starvation responses (PSRs). Recent studies have shown that the formation of cluster-root primordia relies on moderate IAA concentration gradients, which are regulated by the CKs signaling pathway in P limitation (Puga et al., 2017; Wang et al., 2015). Additionally, Jiang et al. (2007) showed that GAs may play an important role of control root hair elongation when plant in a state of PSRs and suggested that GA-DELLA functions alongside IAA signals to coordinate root system development. Thus, multiple phytohormone interactions are usually involved during the response to P starvation and promotion of P uptake in plants, and the mechanisms of integration are being revealed.
Beyond physiological adaptations, plants have also developed associations with beneficial microorganisms, such as arbuscular mycorrhizal (AM) fungi and endophytes, to alleviate P limitation in most natural soils (Rodriguez et al., 2009; Zhang et al., 2020a; Paul and Rakshit 2021). It is worth noting the high potential of the endophytic community for plant growth promotion and improvement of plant P-stress resistance (Sessitsch et al., 2012). Several direct or indirect mechanisms by which root-endophytic fungi may influence plant development have been proposed, such as expansion of the interface for P capture, solubilization of sparingly soluble minerals, and higher expression of phosphate transporters (Rodriguez et al., 2009; Li et al., 2015; Oldroyd and Leyser 2020). Additionally, increasing evidence has revealed that those endophytic fungi enhance the growth of hosts by producing different phytohormones or manipulating phytohormone production (Mehmood et al., 2019; Zhang et al., 2019). Recently, a transcriptome study showed that dark septate endophyte (DSE) S16 increases the levels of IAA biosynthesis pathway-regulated genes to promote the growth and nutrient uptake of sweet cherry (Wu et al., 2021). Additionally, Rozpądek et al. (2018) reported that Mucor sp. altered ethylene metabolism in the host, leading to root hair elongation and growth promotion. However, the exact role of phytohormones in plant-fungus interactions remains elusive, particularly under P limitation.
Peanut (Arachis hypogeae L.) is a widespread oil and cash crop plant of great agricultural and economic significance cultivated in over 100 countries (Xie et al. 2019a). The stable production of peanuts has important significance for lifting local farmers’ income and developing social trade (Zhao et al., 2021a, b). For peanut, the optimum P supply ranges from 0.8 to 1.1 mM (Shi et al., 2020). However, in the hilly regions of southern China, due to limited arable land resources and the promotion of intensive farming methods, peanuts are successively planted on the same land for more than 20 years (Li et al. 2014). Continuous cropping leads to the acidic nature of red soils, which is commonly associated with P deficiency and a decline in peanut production (Guo et al. 2010; Li et al. 2014). Our previous studies reported that the endophytic fungus Phomopsis liquidambaris (B3) could establish a stable symbiosis with peanut, wheat, and rice (Tang et al. 2019; Xie et al. 2019a; Zhang et al. 2020a, b; Zhu et al. 2022). Several studies have suggested the positive effects of B3, which significantly promote nutrient uptake based on their ability to regulate the phytohormone IAA, CKs, and ETH signaling pathways. A further study showed that IAA signaling activated by B3 colonization enhanced the symbiosis between peanut and bradyrhizobial, thereby promoting nutrient accumulation in peanut (Li et al., 2018; Zhang et al., 2018). More interestingly, a consecutive 2-year field plot experiment observed that the promotion of peanut growth induced by B3 was commonly accompanied by an increase in the P uptake of peanut through the whole growth stage (Xie et al., 2019b). However, it is unclear whether the mechanism of B3-mediated increase in P accumulation is related to the phytohormone signaling pathway cascade of peanut.
Therefore, the aims of the present work were (1) to investigate whether B3 induce an increase in peanut P uptake by regulating phytohormone IAA, CK, and GA levels and (2) to further investigate the cross-communication between these phytohormone signaling pathways to determine whether enhancement of this signaling pathway promotes the expression of high affinity P transporter (e.g., AhPHT1;3 and AhPHT1;4) genes and the establishment of root morphological structure, thereby promoting P accumulation and contributing to peanut growth. The results of this study would explain the P uptake-promoting effects of the endophytic fungus B3 from the perspective of phytohormone signaling transduction and provide a basis for the practical application of B3 for increasing the P acquisition of peanuts in continuous cropping soils.
2 Materials and Methods
2.1 Experimental Strain, Soil, and Peanut Cultivar
The experimental strain endophytic fungus Ph. liquidambaris B3 was isolated from the inner bark of Bischofia polycarpa (Chen et al., 2011). The green fluorescent protein-tagged B3 was demonstrated to successfully colonize peanut root tissue (Zhang et al., 2016). B3 was first activated in potato dextrose broth (PDB, 200 g L−1 potato extract and 20 g L−1 glucose, pH 7.0) at 25 °C and shaken at 180 rpm for 48 h. Overall, 3.55 g (0.43 g dry weight) of fungal mycelia was collected, washed three times with sterile deionized water (SDW), and then diluted in 200 mL SDW as the inoculum for germinating grains.
Experimental red soil (5–20 cm) was deliberately collected from the Ecological Experimental Station of Red Soil, Chinese Academy of Science, Yingtan, Jiangxi Province of China (116° 55′ E, 28° 12′ N), where peanuts had been continuously cropped for 5 years and resulted in a low-P soil environment. The soil is classified as Udic Ferrosol (FAO 1998 classification), and the main physicochemical properties are as follows: organic matter, 8.51 g kg−1; total N, 0.55 g kg−1; total P, 0.28 g kg−1; total K, 5.64 g kg−1; available P, 7.84 mg kg−1; available K, 110.80 mg kg−1; and pH (1:2.5, w/v), 4.90 (Xie et al., 2019b). Ganhua-5, a common peanut cultivar grown in the hilly red soil regions of southern China, was used in this study.
2.2 Field Pot Experimental Design
The field pot experiment was performed at the botanical gardens of Nanjing Normal University, Jiangsu Province, China (118° 55′ E, 32° 6′ N) on April 4, 2021. The peanut seeds were first sterilized with 75% (v/v) ethanol for 5 min, followed by disinfestation in 3% (v/v) sodium hypochlorite for 2 min and rinsing thoroughly in SDW. Surface disinfected peanut seeds were germinated in cultivation boxes (length 33 cm, width 20 cm, height 10 cm) containing sterilized vermiculite in the dark at 28 °C until the radicle reached 2–3 cm. Then, germinated peanut seedlings were planted in pots (height 28 cm, diameter 23 cm) that contained approximately 9 kg of red soil. Each pot contained three peanut seedlings. The experimental design included two treatments: (1) non-inoculated treatment (CK) and (2) seedlings infected with B3 (B3). In the B3 infection treatment, 10 mL of the B3 inoculum containing 21.25 mg of mycelia (dry weight) was added to each planting hole (Sun et al., 2021). The non-inoculated group received 10 mL of SDW. Six replicates were prepared for each treatment. All plants were regularly watered during the growing season. Plant samples were collected at the maturation stage (120 days after planting) and used to evaluate peanut agronomic indices, including root length; plant height; tiller number; and the biomasses of shoots, roots, and pods.
2.3 Greenhouse Experiment and Hydroponic Experimental Design
A greenhouse experiment was performed in an illumination incubator (16/8 h day/night 25 °C, 60% relative humidity). The germinated seeds mentioned above were transplanted into plastic pots (11.80 cm in diameter, 15.20 cm in height, three seedlings per pot) with 300 g of sterilized vermiculite. Plants were arranged in a 3 × 2 factorial design, in which the main effects were the level of supplied P and B3 infection. The P treatments consisted of three gradients: (1) trace P (P0.1), applying Hoagland nutrient solution with 0.1 mM KH2PO4; (2) low P (P0.5), with 0.5 mM KH2PO4; and (3) normal P (P1.5), with 1.5 mM KH2PO4. Twelve biological replicates were performed for each treatment. Twenty milliliters of Hoagland nutrient solution was applied twice a week, and watering was performed with SDW when necessary. The peanut plants were harvested after 7, 14, 21, and 28 days of cultivation. Peanut roots were carefully washed with SDW to remove vermiculite. The roots were used to detect IAA, GA, and CK levels and analyze the RSA. In addition, the root and shoot samples were used to analyze the biomass (dry weight) and total P concentration after oven-drying at 60 °C for 72 h.
To explore the effects of B3 application on uptake rates of P by peanut, a hydroponic experiment was performed in an illumination incubator as previously mentioned. Peanut seeds were sterilized and germinated as mentioned previously and then placed in planting baskets and cultivated hydroponically in a hydroponic box containing 1 L of Hoagland nutrient solution with 0.5 mM KH2PO4. The peanut seedlings were grown in a growth cabinet for 21 days, and the P concentration of Hoagland nutrient solution was detected at 0, 3, 7, 14, and 21 days of cultivation.
2.4 Chemicals and Treatments
2,3,5-Triiodobenzoic acid (TIBA, 1 μM, 10 μM, 100 μM), paclobutrazol (PBZ, 10 μM, 50 μM, 100 μM), and lovastatin (1 μM, 10 μM, 100 μM) were used as specific inhibitors of IAA, GAs, and CKs, respectively (Peng et al., 2013; He et al., 2020). Indole-3-acetic acid (IAA, 10 μM), gibberellins acid 3 (GA3, 300 μM), and 6-benzylaminopurine (6-BA, 100 μM) were used as exogenous donors of IAA, GAs, and CKs, respectively. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Prior to use, all of the above solutions of exogenous donors and specific inhibitors were dissolved in 0.2% dimethyl sulfoxide (DMSO) and filtered through 0.22 µm diameter microporous membranes (Tullagreen, Carrigtwohill, Co. Cork, IRL). The chemicals and their dosages used here were selected based on the previous studies. According to the method of Zhou et al. (2016), specific inhibitors were sprayed on seedling leaves until dripping wet and 1 day before the application of exogenous donors or B3 inoculation. After 24 h, the signaling molecule solution (200 μL) was sprayed on plantlets and was allowed to flow from leaf surfaces to the roots. The reagents and their dosages used here were chosen based on our preliminary study which showed that these compounds could efficiently suppress P accumulation induced by B3 inoculation but did not cause compromised growth in peanut. For controls, the same volumes of vehicle solvent were added to plantlets.
2.5 Total P Concentration and Root Structure Analysis
Dried root and shoot samples were ground separately to a fine powder, and 0.1 g of powdered tissue samples was digested using an acid mixture following the procedure described by Pang et al. (2018) to further analyze the total P content in various tissues by the Mo-Sb anti-spectrophotometer method (Murphy and Riley 1962). Peanut samples after 3, 7, 14, and 21 days of cultivation were collected to detect RSA. Samples were separated into roots and shoots and washed carefully with SDW. Then, roots were spread out in a transparent plastic tray with a 3-mm-deep layer of water, and root images were obtained using a root scanner (Shanghai Microtek Technology Co., Ltd., China) following the manufacturer’s instructions. The images were analyzed using a plant root phenotypic analysis system (Zhejiang Top Cloud-agri Technology Co., Ltd., China) to obtain total root length, lateral root number, root surface, root volume, and mean root diameter.
2.6 Measurement of IAA, GAs, and CKs
IAA in plants was extracted and measured according to the method of Li et al. (2018). Briefly, 2 g of roots was ground in liquid nitrogen and extracted in 10 mL methanol with 50 mg butylated hydroxytoluene (BHT) and 50 mg polyvinyl pyrrolidone (PVP) overnight. The homogenate was centrifuged at 10,000 rpm for 15 min. The IAA content in the supernatant was determined by a Plant IAA Assay Kit (Shanghai Ruifan Biotechnology Co., Ltd., China). CKs were extracted from plants according to Li et al. (2018), 2 g peanut plant tissue was ground in liquid nitrogen with 40 ppm sodium diethyldithiocarbamate and 2% (w/w) PVP, and then 16 mL ice-cold methanol was used for extraction at 4 °C overnight. Next, the homogenate was centrifuged at 4000 × g for 15 min, and the supernatant was evaporated. Ethyl acetate was added to the residue. Finally, the mixture was evaporated to dryness and dissolved in 300 µL 95% ethylalcohol. CKs detection was conducted using a Plant Cytokinin Enzyme-Linked Immunosorbent Assay Kit (Shanghai Ruifan Biological Technology Co., Ltd., China) following the manufacturer’s instructions. GAs were extracted and measured using the following method (Zhou et al., 2016). One gram of plant root tissue was ground in liquid nitrogen with 40 ppm sodium diethyldithiocarbamate and 2% (w/w) PVP and extracted in 10 mL ice-cold phosphate-buffered saline. After then, the extract was adjusted to pH 7.4. The homogenate was centrifuged at 4000 × g for 20 min, and GAs were measured with a Plant Gibberellin Enzyme-Linked Immunosorbent Assay Kit (Shanghai Ruifan Biological Technology Co., Ltd., China). All samples were washed with 0.5 mM NaOH to remove non-specific interferences (Jones et al. 1987). [2-14C] IAA, [2H] CK, and [2H] GA as internal standards to calibrate loss during the regular sample-clean up stage (Tan et al., 2003; Sundberg et al., 1994; Emery et al., 1998). In this study, phytohormone standards were used to detect recovery rate of ELASA (Fang et al., 2013).
2.7 RNA Extraction and qPCR
The total RNA of the peanut root was extracted with a Spin Column Total RNA Purification Kit (Shanghai Sangon Biotech, Co., Ltd., China) according to the manufacturer’s instructions. Next, genomic DNA (gDNA) was removed, and first-strand cDNA was synthesized with an Evo M-MLV RT Mix kit (Hunan Accurate Biology Co., Ltd., China). Real-time qPCR was performed using Applied Biosystems Step One Real-Time PCR Systems (Carlsbad, CA, USA). All reactions were performed using a SYBR® Green Pro Taq HS qPCR Kit (No Rox) (Hunan Accurate Biology Co., Ltd., China) according to the manufacturer’s instructions. The selected study genes and specific primers for qRT-PCR are listed in Table 1. The reactive step of qPCR was consistent with the report of Zhang et al. (2020b). The 2−ΔΔCt method was used to calculate the relative expression of the target genes (Livak et al., 2001). The experiment was performed with six biological replicates.
2.8 Statistical Analysis
All of the experimental data in the study were analyzed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA). The data obtained were represented by the means of at least six biological replicates and their standard deviations (SD). An independent t test was employed if an analysis consisted of only a control and an experimental group (CK and B3 treatments). One-way ANOVA was used followed by Tukey’s multiple-comparison test when more than two datasets were compared, with a p < 0.05 threshold considered significant. Graphs and images were assembled with GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
3 Result
3.1 Effects of B3 Colonization on Peanut Growth in Low-P Soil
Under the low-P soil, the results in Fig. 1 demonstrate that B3 colonization had significant effects on peanut agronomic parameters. The root length (Fig. 1a), plant height (Fig. 1b), and tiller number (Fig. 1c) significantly increased following B3 treatment by 23.7%, 20.2%, and 47.5%, respectively, compared to those of CK treatment. Figure 1d-f shows that the shoot and root fresh weight (FW) of peanut in B3 treatment were also significantly increased by 47.7% and 94.4%, respectively, when compared to CK treatment. In addition, the parameters of pod yield were compared to evaluate the effects of the endophyte B3 on peanut production and observed that there were higher numbers of pods per plant (27.3%), FW per pod (20.8%), and yield (45.9%) upon B3 treatment than CK treatment. Further studies demonstrated that B3 colonization significantly improved the RSA under low P conditions. Table 2 shows that the number of lateral roots of peanut significantly increased in the B3 colonization group when compared with the control, and the length, diameter, volume, and superficial area of roots in the B3 treatment were also increased.
Effects of Ph. liquidambaris colonization on agronomic characters of peanut. Root length (a), plant height (b), tiller number (c), shoot FW (d), root FW (e), pod number (f), FW per pod (g), and yield (h). Values are means for twelve biological replicates with ± SD (standard deviation). CK, Ph. liquidambaris-uninfected plants; B3, Ph. liquidambaris-infected plants. Asterisk represents a significant difference between CK and B3 treatments (*, P ≤ 0.05; **, P ≤ 0.01, ***, P ≤ 0.001). FW, fresh weight
3.2 Effects of B3 Colonization on Peanut Biomass and P Concentration Under Different P Applications
Under P deficiency conditions (including trace P and low P), compared to CK treatments, B3 colonization significantly increased the shoot and root biomass (Fig. 2a-f) at 14, 21, and 28 days after infection (DAI). Correspondingly, the total P contents in shoots and roots under B3 treatments were significantly higher than those under CK treatment at the sampled stages (Fig. 2g-1). Interestingly, even at normal P levels, the colonization of B3 also effectively increased peanut biomass and total P content when compared to CK, indicating the potential function produced by B3. Additionally, hydroponic experiments further demonstrated that the colonization of endophytes significantly accelerated the rate of P clearance in Hoagland nutrition solution (Fig. S1).
Effects of Ph. liquidambaris colonization on peanut biomass (a–f) and P concentration (g–l) under different P levels. Values are means for twelve biological replicates with ± SD. Asterisk represents a significant difference between CK and B3 treatments (*, P ≤ 0.05; **, P ≤ 0.01). P0.1: trace P; P0.5: low P; P1.5: normal P. CK, Ph. liquidambaris-uninfected plants; B3, Ph. liquidambaris-infected plants. DW, dry weight; DAI, days after infection
3.3 Involvement of IAA, GAs, and CKs in B3-Induced P Accumulation
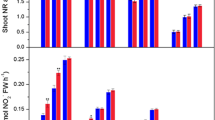
Figure 3a, c, e demonstrate that the contents of IAA, GAs, and CKs in B3-colonized peanuts were significantly increased and peaked at 21, 7, and 21 DAI, respectively. Further analysis found that the addition of exogenous TIBA, PBZ, and lovastatin suppressed B3-induced IAA, GAs, and CKs production and also significantly reduced the corresponding P accumulation in peanuts (Fig. 3b, d, f). The applications of 10 μM TIBA, 50 μM PBZ, and 10 μM lovastatin could significantly reduce the accumulation of total P in peanuts and did not cause compromised growth in peanut (Figs. 3 and S2). Thus, these concentrations were chosen for the follow-up experiments. Therefore, these results indicated that IAA, GAs, and CKs may play essential roles in B3-induced P accumulation in peanut plantlets.
Involvement of auxin (a), gibberellin (c), and cytokinin (e) in Ph. liquidambaris-induced P accumulation in peanuts. Effects of addition of TIBA (b), paclobutrazol (d), and lovastatin (f) on Ph. liquidambaris-induced P accumulation in peanut. Values are means for six biological replicates with ± SD. Asterisk represents a significant difference between CK and B3 treatments (*, P ≤ 0.05; **, P ≤ 0.01), and different lowercase letters indicate significant differences at P ≤ 0.05. CK, Ph. liquidambaris-uninfected plants; B3, Ph. liquidambaris-infected plants; TIBA, 2,3,5-triiodobenzoic acid; IAA, indole-3-acetic acid; GA, gibberellin; CTK, cytokinin. FW, fresh weight
3.4 Interaction Among IAA, GAs, and CKs in the Signaling Pathways of B3-Induced P Accumulation
The results of Fig. 4 show that the generations of IAA, GAs, and CKs induced by B3 inoculation were strongly inhibited by TIBA, PBZ, and lovastatin, respectively (Fig. 4b, d, f). Figure 4b d demonstrate that the generation of GAs induced by B3 inoculation was completely inhibited by TIBA and lovastatin, while PBZ was unable to completely inhibit IAA and CKs generation, indicating that IAA and CKs may act as upstream signals of GAs in the regulation of peanut P uptake. Further analysis found that the exogenous application of IAA and CKs could restore the adverse effects of TIBA, lovastatin, and PBZ on B3-induced P accumulation (Fig. 4a c). It was also observed that the addition of IAA and CKs promoted the generation of GAs, while exogenous GAs addition had no effect on the generation of IAA or CKs (Fig. 4b d). The results presented in Fig. 4e and f demonstrate that TIBA had no significant impact on B3-induced CKs content, and IAA content was not affected by lovastatin. On this basis, the application of exogenous IAA did not significantly affect the generation of CKs, and the IAA content was also not affected by the addition of CKs in B3-inoculated group treated with TIBA and lovastatin, respectively (Fig. 4f). Therefore, these results demonstrated that IAA and CKs may act as upstream signaling molecules in B3-induced P accumulation via GAs.
Interaction relationships among auxin, gibberellin, and cytokinin in the signaling pathways of Ph. liquidambaris-induced P accumulation. A total of 10 μM TIBA, 10 μM IAA, 300 μM GA, 50 μM paclobutrazol, 10 μM lovastatin, and 100 μM CTK were applied. Values are means for six biological replicates with ± SD. Different lowercase letters indicate significant differences at P ≤ 0.05. CK, Ph. liquidambaris-uninfected plants; B3, Ph. liquidambaris-infected plants; TIBA, 2,3,5-triiodobenzoic acid; IAA, indole-3-acetic acid; GA, gibberellin; CTK, cytokinin
3.5 P Transport Gene Expression
The expression levels of AhPHT1;3 and AhPHT1;4 involved in P uptake in peanut were examined. As shown in Fig. 5, TIBA, PBZ, and lovastatin strongly downregulated the transcript levels of AhPHT1;3 and AhPHT1;4 in the B3-inoculated group (Fig. 5a, d, h). On this basis, the exogenous replenishment of IAA, GAs, and CKs could restore the expression levels of AhPHT1;3 and AhPHT1;4 (Fig. 5b, e, f, g, i). The application of exogenous GAs had no significant effects on the expression levels of AhPHT1;3 or AhPHT1;4 in the B3-inoculated group treated with TIBA (Fig. 5c) and lovastatin (Fig. 5j), while applications of exogenous IAA and CKs restored the expression levels of AhPHT1;3 and AhPHT1;4 in the B3-inoculated group treated with GAs. These results show that the changing trend of P transport gene expression was similar to that of phytohormone and P content Fig. 6.
The transcriptional levels of phosphorus transporter genes AhPHT 1; 3 and AhPHT 1; 4 in peanuts at 7 days after different treatments. Values are means for three biological replicates with ± SD, and asterisk represents a significant difference between CK and B3 treatments (*, P ≤ 0.05; **, P ≤ 0.01). CK, Ph. liquidambaris-uninfected plants; B3, Ph. liquidambaris-infected plants; TIBA, 2,3,5-triiodobenzoic acid; IAA, indole-3-acetic acid; GA, gibberellin; CTK, cytokinin
A model of endophytic fungus Ph. liquidambaris promoting peanut P uptake by regulating host auxin, gibberellin, and cytokinin signaling cross-talks. Ph. liquidambaris infection could improve the signaling communications among the auxin, gibberellin, and cytokinin in host, which may be contribute to increase the expression levels of phosphorus transporter related genes (AhPHT 1; 3 and AhPHT 1; 4) in root cells of peanut, and then promote host P uptake efficiency. In addition, the potential crosstalk network among IAA, CTK, and GA in promoting peanut P uptake was clarified, and IAA and CTK may serve as the upstream signaling of GA. On the other hand, Ph. liquidambaris infection can also improve the root morphological structure of peanut, which may greatly increase the root’s surface area in contact with soil, thereby helpful in promoting P absorption. Therefore, Ph. liquidambaris infection could increase expression of phosphate transporter gene and improve RSA by regulating phytohormone signaling pathway in peanut, which increase the P absorption efficiency, thereby contribute to promotion of peanut growth and yield. Blue dotted arrows represent a positive effect. Pi, phosphorus; IAA, indole-3-acetic acid; GA, gibberellin; CTK, cytokinin; RSA, root system architecture
4 Discussion
Our results agreed with numerous previous studies (Zhang et al., 2016; Li et al., 2018; Tang et al., 2019), and our findings further indicated that B3 application might improve the agronomic characteristics of peanut by promoting plantlet P acquisition. Furthermore, the total P contents of roots and shoots were improved by B3 colonization, indicating that colonization of B3 promotes the efficiency of P absorption and also increases P transport from roots to shoots. Although many studies have demonstrated that some endophytic fungi can significantly increase plant P accumulation, little information is available concerning the involvement of phytohormone signaling pathways in endophyte-induced P accumulation (Li et al., 2015; Wu et al., 2018). The present study indicates that IAA and CKs may act upstream of GAs and function in B3-induced expression of AhPTH1;3 and AhPTH1;4 and promotion of RSA establishment, which increases P accumulation in peanut under P limitation. In summary, to our knowledge, this is the first study to report the signaling crosstalk involved in B3-induced P accumulation in peanut under P deficiency, particularly at the whole-plant level.
Plants have acquired a range of developmental strategies to improve P accessibility over the course of evolution. Symbiosis of plant roots with fungi creates an extended ‘‘root’’ system that can lead to more far-reaching P foraging. In the plant-AMF model, AMF enlarge the contact area between plant roots and soil by extending dense hyphae into the surrounding soil, upon reaching an organic P patch, transport phosphate solubilizing bacteria (PSB) to the organic P patch, release exudates which stimulate PSB bacterial growth, and further enhance organic P mineralization, in return, PSB stimulates AMF growth and exudates release, thereby promoting nutrient uptake (Ezawa and Saito 2018; Gutjahr and Parniske 2013; Jiang et al. 2021). The mechanism of PSB community to enhance mycorrhizal functioning would be illustrated in further studies. However, in our present study, compared with AMF, B3 resulted in improved RSA and increased the peanut root absorption area, consequently facilitating P uptake efficiency by a more immediate strategy under P limitation. More in-depth research is needed to investigate whether B3-induced changes in root conformation facilitate colonization by mycorrhizal fungi and thus promote recruitment and translocation of PSB, ultimately leading to enhanced phosphorus uptake by the host. Moreover, phytohormones are considered signal molecules to regulate plant physiological and developmental processes, promoting establishment of the AM-plant symbiosis thereby increase P accumulation of host (Pozo et al., 2015; van Overbeek and Saikkonen 2016); therefore, it was assumed that the modulation of the RSA observed in this study was possibly associated with B3-induced phytohormones production thereby actively promote establishment of the AM-plant symbiosis. Additionally, studies in different endophytic-fungus symbionts have indeed shown that endophytic fungi that alter RSA can enhance P uptake and thereby positively influence yield. For example, Mucor sp. was able to induce root development and growth in Arabidopsis arenosa, which was necessary for fungi-induced P uptake promotion (Rozpądek et al., 2018). In addition to the adaptation of RSA, plants also set up symbioses with endophytic fungi that promote the secretion of phosphatase in roots, which can solubilize organic P to reverse the low P condition of soil (Wu et al., 2018; Kapri and Tewari 2010; Ding et al., 2016). However, our results indicate that B3 significantly increased the P accumulation of plants in the trace P, low P, and normal P conditions, suggesting that the promotion of plant growth by fungus is not limited by the growth conditions. Consistent with our result, Rozpądek et al. (2018) reported a similar phenomenon in Mucor sp.-Arabidopsis arenosa symbiosis. In addition, our study showed that B3 significantly improved the establishment of root morphological structure in peanut. However, it remains unclear whether B3-induced morphological changes in roots are related to the enhancement of peanut phosphatase secretion; further research is needed to illustrate the mechanisms underlying the associations between root morphological changes and the enhancement of peanut phosphatase secretion.
Phytohormones play key roles in controlling plant performance during plant development. We found three phytohormones, IAA, CKs, and GAs, that were closely related to B3-induced P accumulation from previous screening experiments (data not shown). Recent studies have reported that exogenous IAA can act as an elicitor that upregulates the expression levels of many BnPHTs in Brassica napus and the transcriptional activities of OsPHT1;8 in rice, suggesting that the action of IAA is necessary for P accumulation in plants (Jia et al., 2017; Yang et al., 2020). Although many studies have reported that endophytic fungi alleviate P stress by synthesizing IAA and mobilizing sparingly soluble soil P, these mobilizations are induced via an IAA-independent mechanism (Priyadharsini and Muthukumar 2017). In this study, the role of IAA in B3-dependent P accumulation in the host was investigated with exogenously applied IAA and TIBA, whereas other reports mainly focused on the implication of IAA in P stress-regulated P uptake in plants. Analogously, CKs are commonly thought to participate in some critical processes during plant growth and development. Recent reports indicate that the endophytic fungus Epichloë sinensis reduces CK levels to stimulate stomatal closure, thereby increasing the host’s chances of survival under drought stress (Xu et al., 2021). In contrast, in our study, the presence of endophytes increased the accumulation and synthesis of CKs. Meanwhile, we also found that lovastatin inhibited B3-induced P accumulation by decreasing the CKs content. Consistent with our results, Cosme et al. (2016) reported that reduced CKs content in plant shoots caused a stronger decline in NtPHT expression and blocked the colonization of AM fungi, suggesting that CKs are a positive regulator of plant P accumulation. In addition, GAs were verified to promote cell growth and the development of leaves and roots. Especially under P limitation, the GAs content of roots was positively correlated with the P utilization efficiency of Sophora davidii (Zhao et al., 2021a, b). Several reports have indicated that many endophytes are capable of producing GAs and promoting plant growth, including Porostereum spadiceum and Aspergillus fumigatus (Hamayun et al., 2017; Bilal et al., 2018). Consistent with our studies, B3 increased the GAs content accompanied by promotion of the P concentration in peanut. However, the concentration of phytohormone detected in our study represented the total concentration of phytohormone in the B3-peanut symbiosis, and the source of the enhanced IAA, GA, and CK levels is not clear. A previous study demonstrated that B3 can produce a small amount of 3-indole acetic acid in vitro, while CKs and GAs were not detected (Chen et al., 2011). Moreover, Zhang et al. (2018) reported that B3 significantly increased the expression of IAA biosynthetic genes in peanuts. Thus, we speculate that B3 colonization can enhance the biosynthesis of phytohormones in peanut, which may be important for increasing P uptake.
Cross-communication of phytohormone signaling pathways provides the plant with a powerful capacity to finely regulate its growth and development. The antagonistic action of CKs and GAs on aerial organ development processes has been widely reported. Zhuang et al. (2019) demonstrated that GAs decreased CKs content and upregulated the expression of CKs degradation genes in tall fescue and controlled tiller bud growth. However, our experiment showed that CKs were localized upstream of GAs in B3-induced P accumulation in peanut and that no obvious antagonistic interplay was found between CKs and GAs. Moreover, elevated IAA and GA concentrations were found to repattern root architectural under P limitation and also upregulate the expression of SiPHT1;1, SiPHT1;2, and SiPHT1;4, thereby conferring foxtail millet tolerance to P deficiency in the soil (Alhmad et al., 2018). Based on further studies, GAs are thought to operate upstream of IAA, stimulating its biosynthesis and transport in the stem of Populus and promoting cambial growth (Björklund et al., 2007). However, our experiment investigated whether IAA and GAs might serve as internal boosts to promote P uptake of peanut and whether IAA may act upstream of GAs and function via GAs signaling in B3-induced increases of AhPHT1;3 and AhPHT1;4 expression. Similarly, Fu and Harberd (2003) reported that GA-induced root elongation in Arabidopsis was inhibited by the removal of the shoot apex, which is a major source of IAA, and root elongation was restored by the application of IAA, indicating that IAA stimulates root elongation by activating the GAs signaling pathway. Furthermore, a mechanism of interaction between GAs and IAA in the regulation of root growth was described by Fu and Harberd (2003), whereby IAA promotes the degradation of DELLA (suppressor of GAs signaling) in root cells and affects transduction of GAs signaling, which is a prerequisite for GA-induced root elongation. Furthermore, Zhang et al. (2018) reported that the IAA signaling pathway responds to the colonization of B3 at the early stage of symbiosis, thereby modulating the root phenotype of the host. These results may explain why IAA is located upstream of GAs signaling. Although different plants stimulate different phytohormone signaling in response to P deficiency, the end-result of the crosstalk between different signaling pathways is always helpful in promoting the acquisition of external P by plants. Generally, phytohormone signal transduction is influenced by external biological and abiotic factors, especially the induction effects of symbiotic microorganisms. Therefore, further biotechnology research is needed to explore the functional mechanisms by which phytohormone interactions in symbiotic microbes increase P uptake of the host.
Based on the results presented in this study, a hypothetical model was proposed. B3 colonization could increase the concentrations of IAA, CKs, and GAs in peanut. IAA and CKs may act as the upstream phytohormone signaling molecules of GAs, while no evident interaction between IAA and CKs was observed in this study. Additionally, increased concentrations of IAA, CKs, and GAs may contribute to upregulating the expression of PHT genes (e.g., AhPHT1;3 and AhPHT1;4) and improving RSA, thereby improving P absorption in peanut. Further experiments with transgenic lines are required to further explore the molecular mechanism by which IAA, GAs, and CKs interact to control the P uptake of peanut. These results provide a theoretical basis for the high P absorption efficiency of plants and further reveal the interactions among peanuts and their endophytes. In summary, this study illustrates the mechanism by which B3 alleviates the obstacles to peanut replanting from the perspective of P uptake.
5 Conclusion
This work, along with our previous studies, demonstrates that the balanced host-endophyte symbiosis serves as a promising means to enhance peanut phosphorus accumulation and growth. Ph. liquidambaris colonization-induced accumulation of phytohormones in the peanut served as an internal boost for phosphorus uptake. Furthermore, auxin and cytokinins signaling molecules may act upstream of gibberellins signaling and function in the Ph. liquidambaris-induced expression of AhPHT1;3 and AhPHT1;4, which significantly increase phosphorus accumulation in peanut, thereby contributing to the growth and yield of peanut. Additionally, our results demonstrate that the root system architecture (RSA) of peanut significantly responded to Ph. liquidambaris colonization, and these changes may more directly improve phosphorus absorption efficiency. Our study highlights the importance of the auxin, cytokinins, and gibberellins signaling pathways in Ph. liquidambaris-induced phosphorus accumulation. This is the first study to report that Ph. liquidambaris acts as an endophytic elicitor to increase peanut phosphorus uptake by regulating the auxin, cytokinins, and gibberellins signaling transduction pathways, alleviating the long-term successive cropping obstacles associated with peanut phosphorus uptake.
References
Ahmad Z, Nadeem F, Wang RF, Diao XM, Han YH, Wang XC, Li XX (2018) A larger root system is coupled with contrasting expression patterns of phosphate and nitrate transporters in foxtail millet [Setaria italica (L.) Beauv.] under phosphate limitation. Front Plant Sci 9: 1367. https://doi.org/10.3389/fpls.2018.01367
Bilal L, Asaf S, Hamayun M, Gul H, Iqbal A, Ullah I, Lee IJ, Hussain A (2018) Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 76:117–127. https://doi.org/10.1007/s13199-018-0545-4
Björklund S, Antti H, Uddestrand I, Moritz T, Sundberg B (2007) Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J 52:499–511. https://doi.org/10.1111/j.1365-313X.2007.03250.x
Chen Y, Peng Y, Dai CC, Ju Q (2011) Biodegradation of 4-hydroxybenzoic acid by Phomopsis liquidambari. Appl Soil Ecol 51:102–110. https://doi.org/10.1016/j.apsoil.2011.09.004
Cosme M, Ramireddy E, Franken P, Schmülling T, Wurst S (2016) Shoot- and root-borne cytokinin influences arbuscular mycorrhizal symbiosis. Mycorrhiza 26:709–720. https://doi.org/10.1007/s00572-016-0706-3
Ding N, Guo HC, Kupper JV, McNear DH (2016) Shoot specific fungal endophytes alter soil phosphorus (P) fractions and potential acid phosphatase activity but do not increase P uptake in tall fescue. Plant Soil 401:291–305. https://doi.org/10.1007/s11104-015-2757-1
Emery RJN, Leport L, Barton JE, Turner NC, Atkins CA (1998) cis-isomers of cytokinins predominate in chickpea seeds throughout their development. Plant Physiol 117:1515–1523. https://doi.org/10.1104/pp.117.4.1515
Ezawa T, Saito K (2018) How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol 220:1116–1121. https://doi.org/10.1111/nph.15187
Fang ZY, Jiang BS, Wu W, Xiang ZC, Ouyang CY, Huang TL, Chen JH, Zeng LW (2013) ELISA detection of semicarbazide based on a fast sample pretreatment method. Chem Commun 49:6164–6166. https://doi.org/10.1039/c3cc42790k
Fu XD, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421:740–743. https://doi.org/10.1038/nature01387
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010. https://doi.org/10.1126/science.1182570
Gutjahr C, Parniske M (2013) Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Bi 29:593–617. https://doi.org/10.1146/annurev-cellbio-101512-122413
Hamayun M, Hussain A, Khan SA, Kim HY, Khan AL, Waqas M, Irshad M, Iqbal A, Rehman G, Jan S, Lee IJ (2017) Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front Microbiol 8:686. https://doi.org/10.3389/fmicb.2017.00686
He GR, Yang PP, Tang YC, Cao YW, Qi XY, Xu LF, Ming J (2020) Mechanism of exogenous cytokinins inducing bulbil formation in Lilium lancifolium in vitro. Plant Cell Rep 39:861–872. https://doi.org/10.1007/s00299-020-02535-x
Jia HF, Zhang ST, Wang LZ, Yang YX, Zhang HY, Cui H, Shao HF, Xu GH (2017) OsPht1;8, a phosphate transporter, is involved in auxin and phosphate starvation response in rice. J Exp Bot 68:5057–5068. https://doi.org/10.1093/jxb/erx317
Jiang CF, Gao XH, Liao L, Harberd NP, Fu XD (2007) Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis1[OA]. Plant Physiol 145:1460–1470. https://doi.org/10.1104/pp.107.103788
Jiang FY, Zhang L, Zhou JC, George TS, Feng G (2021) Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol 230:304–315. https://doi.org/10.1111/nph.17081
Jones HG (1987) Correction for non-specific interference in competitive immunoassays. Physiol Plantarum 70:146–154
Kapri A, Tewari L (2010) Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz J Microbiol 41:787–795. https://doi.org/10.1590/S1517-83822010005000001
Li XG, Ding CF, Zhang TL, Wang XX (2014) Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol Biochem 72:11–18. https://doi.org/10.1016/j.soilbio.2014.01.019
Li RX, Cai F, Pang G, Shen QR, Li R, Chen W (2015) Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE 10:e0130081. https://doi.org/10.1371/journal.pone.0130081
Li X, Zhou J, Xu RS, Meng MY, Yu X, Dai CC (2018) Auxin, cytokinin, and ethylene involved in rice N availability improvement caused by endophyte Phomopsis liquidambari. J Plant Growth Regul 37:128–143. https://doi.org/10.1007/s00344-017-9712-8
Liu Q, Zhou GQ, Xu F, Yan XL, Liao H, Wang JX (2013) The involvement of auxin in root architecture plasticity in Arabidopsis induced by heterogeneous phosphorus availability. Biol Plantarum 57:739–748. https://doi.org/10.1007/s10535-013-0327-z
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
López-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L (2014) Phosphate nutrition: improving low-phosphate tolerance in crops. Annu Rev Plant Biol 65:95–123. https://doi.org/10.1146/annurev-arplant-050213-035949
Mehmood A, Hussain A, Irshad M, Hamayun M, Iqbal A, Khan N (2019) In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis 77:225–235. https://doi.org/10.1007/s13199-018-0583-y
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 26:31–36
Oldroyd GED, Leyser O (2020) A plant’s diet, surviving in a variable nutrient environment. Science 368: eaba0196 https://doi.org/10.1126/science.aba0196
Pang JY, Bansal R, Zhao HX, Bohuon E, Lambers H, Ryan MH, Ranathunge K, Siddique KHM (2018) The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529. https://doi.org/10.1111/nph.15200
Pathak H, Fagodiya RK (2022) Nutrient budget in Indian agriculture during 1970–2018: assessing inputs and outputs of nitrogen, phosphorus, and potassium. J Soil Sci Plant Nut 22:1832–1845. https://doi.org/10.1007/s42729-022-00775-2
Paul S, Rakshit A (2021) Effect of seed bio-priming with Trichoderma viride strain BHU-2953 for enhancing soil phosphorus solubilization and uptake in soybean (Glycine max). J Soil Sci Plant Nut 21:1041–1052. https://doi.org/10.1007/s42729-021-00420-4
Peng Q, Wang HQ, Tong JH, Kabir MH, Huang ZG, Xiao LT (2013) Effects of indole-3-acetic acid and auxin transport inhibitor on auxin distribution and development of peanut at pegging stage. Sci Hortic-Amsterdam 162:76–81. https://doi.org/10.1016/j.scienta.2013.07.027
Péret B, Clément M, Nussaume L, Desnos T (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16:442–450. https://doi.org/10.1016/j.tplants.2011.05.006
Pozo MJ, López-Ráez JA, Azcón-Aguilar C, García-Garrido JM (2015) Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol 205:1431–1436. https://doi.org/10.1111/nph.13252
Priyadharsini P, Muthukumar T (2017) The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol 27:69–77. https://doi.org/10.1016/j.funeco.2017.02.007
Puga MI, Rojas-Triana M, de Lorenzo L, Leyva A, Rubio V, Paz-Are J (2017) Novel signals in the regulation of Pi starvation responses in plants: facts and promises. Curr Opin Plant Biol 39:40–49. https://doi.org/10.1016/j.pbi.2017.05.007
Rawat P, Das S, Shankhdhar D, Shankhdhar SC (2020) Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. J Soil Sci Plant Nut 21:49–68. https://doi.org/10.1007/s42729-020-00342-7
Revalska M, Iantcheva A (2018) Pi-starvation is mitigated in Medicago truncatula plants with upregulated auxin transport through auxin-strigolactone interaction. Plant Cell Tiss Org 133:405–415. https://doi.org/10.1007/s11240-018-1393-x
Rodriguez RJ, White JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330. https://doi.org/10.1111/j.1469-8137.2009.02773.x
Rozpądek P, Domka A, Ważny R, Nosek M, Jędrzejczyk R, Tokarz K, Turnau K (2018) How does the endophytic fungus Mucor sp. improve Arabidopsis arenosa vegetation in the degraded environment of a mine dump? Environ Exp Bot 147:31–42. https://doi.org/10.1016/j.envexpbot.2017.11.009
Sessitsch A, Hardoim P, Döring J, Weilharter A, Krause A, Woyke T, Mitter B, Hauberg-Lotte L, Friedrich F, Rahalkar M, Hurek T, Sarkar A, Bodrossy L, van Overbeek L, Brar D, van Elsas JD, Reinhold-Hurek B (2012) Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol Plant Microbe Interact 25:28–36. https://doi.org/10.1094/MPMI-08-11-0204
Shi QW, Pang JY, Yong JWH, Bai CM, Pereira CG, Song QB, Wu D, Dong QP, Cheng X, Wang F, Zheng JL, Liu YF, Lambers H (2020) Phosphorus-fertilisation has differential effects on leaf growth and photosynthetic capacity of Arachis hypogaea L. Plant Soil 447:99–116. https://doi.org/10.1007/s11104-019-04041-w
Sinharoy S, Saha S, Chaudhury SR, DasGupta M (2009) Transformed hairy roots of Arachis hypogea: a tool for studying root nodule symbiosis in a non-infection thread legume of the aeschynomeneae tribe. Mol Plant-Microbe Interact 22:132–142. https://doi.org/10.1094/MPMI-22-2-0132
Sun K, Xie XG, Lu F, Zhang FM, Zhang W, He W, Dai CC (2021) Peanut preinoculation with a root endophyte induces plant resistance to soil-borne pathogen Fusarium oxysporum via activation of salicylic acid-dependent signaling. Plant Soil 460:297–312. https://doi.org/10.1007/s11104-020-04807-7
Sundberg B, Tuominen H, Little CHA (1994) Effects of the indole-3-acetic acid (IAA) transport inhibitors N-1-naphthylphthalamic acid and morphactin on endogenous IAA dynamics in relation to compression wood formation in 1-year-old Pinus sylvestris (L.) shoots. Plant Physiol 106:469–476. https://doi.org/10.1104/pp.106.2.469
Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39:792–796. https://doi.org/10.1038/ng2041
Tan ZG, Qian YL (2003) Light intensity affects gibberellic acid content in kentucky bluegrass. Hortscience 38:113–116. https://doi.org/10.21273/HORTSCI.38.1.113
Tang MJ, Zhu Q, Zhang FM, Zhang W, Yuan J, Sun K, Xu FJ, Dai CC (2019) Enhanced nitrogen and phosphorus activation with an optimized bacterial community by endophytic fungus Phomopsis liquidambari in paddy soil. Microbiol Res 221:50–59. https://doi.org/10.1016/j.micres.2019.02.005
van Overbeek LS, Saikkonen K (2016) Impact of bacterial-fungal interactions on the colonization of the endosphere. Trends Plant Sci 21:230–242. https://doi.org/10.1016/j.tplants.2016.01.003
Wang ZR, Rahman ABMM, Wang GY, Ludewig U, Shen JB, Neumann G (2015) Hormonal interactions during cluster-root development in phosphate-deficient white lupin (Lupinus albus L.). J Plant Physiol 177:74–82. https://doi.org/10.1016/j.jplph.2014.10.022
Wen ZH, White PJ, Shen JB, Lambers H (2022) Linking root exudation to belowground economic traits for resource acquisition. New Phytol 233:1620–1635. https://doi.org/10.1111/nph.17854
Wu MY, Wei Q, Xu L, Li HZ, Oelmüller R, Zhang WY (2018) Piriformospora indica enhances phosphorus absorption by stimulating acid phosphatase activities and organic acid accumulation in Brassica napus. Plant Soil 432:333–344. https://doi.org/10.1007/s11104-018-3795-2
Wu FL, Qu DH, Tian W, Wang MY, Chen FY, Li KK, Sun YD, Su YH, Yang LN, Su HY, Wang L (2021) Transcriptome analysis for understanding the mechanism of dark septate endophyte S16 in promoting the growth and nitrate uptake of sweet cherry. J Integr Agric 20:1819–1831. https://doi.org/10.1016/S2095-3119(20)63355-X
Xie XG, Zhang FM, Wang XX, Li XG, Dai CC (2019b) Phomopsis liquidambari colonization promotes continuous cropping peanut growth by improving the rhizosphere microenvironment, nutrient uptake and disease incidence. J Sci Food Agr 99:1898–1907. https://doi.org/10.1002/jsfa.9385
Xie XG, Zhang FM, Yang T, Chen Y, Li XG, Dai CC (2019a) Endophytic fungus drives nodulation and N2 fixation attributable to specific root exudates. mBio 10: e00728–19. https://doi.org/10.1128/mBio.00728-19
Xu WB, Li MM, Lin WH, Nan ZB, Tian P (2021) Effects of Epichloë sinensis endophyte and host ecotype on physiology of Festuca sinensis under different soil moisture conditions. Plants-Basel 10:1649. https://doi.org/10.3390/plants10081649
Yang J, Zhou J, Zhou HJ, Wang MM, Liu MM, Ke YZ, Li PF, Li JN, Du H (2020) Global survey and expressions of the phosphate transporter gene families in Brassica napus and their roles in phosphorus response. Int J Mol Sci 21:1752. https://doi.org/10.3390/ijms21051752
Zhang W, Wang HW, Wang XX, Xie XG, Siddikee MA, Xu RS, Dai CC (2016) Enhanced nodulation of peanut when co-inoculated with fungal endophyte Phomopsis liquidambari and bradyrhizobium. Plant Physiol Bioch 98:1–11. https://doi.org/10.1016/j.plaphy.2015.11.002
Zhang W, Sun K, Shi RH, Yuan J, Wang XJ, Dai CC (2018) Auxin signalling of Arachis hypogaea activated by colonization of mutualistic fungus Phomopsis liquidambari enhances nodulation and N2-fixation. Plant Cell Environ 41:2093–2108. https://doi.org/10.1111/pce.13170
Zhang Y, Yu XX, Zhang WJ, Lang DY, Zhang XJ, Cui GC, Zhang XH (2019) Interactions between endophytes and plants: beneficial effect of endophytes to ameliorate biotic and abiotic stresses in plants. J Plant Biol 62:1–13. https://doi.org/10.1007/s12374-018-0274-5
Zhang FM, He W, Wu CY, Sun K, Zhang W, Dai CC (2020a) Phomopsis liquidambaris inoculation induces resistance in peanut to leaf spot and root rot. Biocontrol 65:475–488. https://doi.org/10.1007/s10526-020-10013-2
Zhang W, Li XG, Sun K, Tang MJ, Xu FJ, Zhang M, Dai CC (2020b) Mycelial network-mediated rhizobial dispersal enhances legume nodulation. ISME J 14:1015–1029. https://doi.org/10.1038/s41396-020-0587-5
Zhao X, Zhao LL, Huang LJ, Sun XF, Wang PC (2021a) Response of growth characteristics and endogenous hormones of Sophora davidii to low-phosphorus stress. Acta Physiol Plant 43:118. https://doi.org/10.1007/s11738-021-03284-4
Zhao YY, Jiang HJ, Xu FJ, Zhang W, Sun K, Xie XG, Dai CC (2021b) Soil acidification negatively affects Arachis hypogeae L. growth by inhibiting nodule initiation and nitrogen fixation. J Soil Sci Plant Nut 22:571–584. https://doi.org/10.1007/s42729-021-00669-9
Zhou JY, Li X, Zhao D, Deng-Wang MY, Dai CC (2016) Reactive oxygen species and hormone signaling cascades in endophytic bacterium induced essential oil accumulation in Atractylodes lancea. Planta 244:699–712. https://doi.org/10.1007/s00425-016-2536-0
Zhu YL, Zhang MQ, Wang LS, Mei YZ, Dai CC (2022) Overexpression of chitinase in the endophyte Phomopsis liquidambaris enhances wheat resistance to Fusarium graminearum. Fungal Genet Biol 158:103650. https://doi.org/10.1016/j.fgb.2021.103650
Zhuang LL, Ge Y, Wang J, Yu JJ, Yang ZM, Huang BR (2019) Gibberellic acid inhibition of tillering in tall fescue involving crosstalks with cytokinins and transcriptional regulation of genes controlling axillary bud outgrowth. Plant Sci 287:110168. https://doi.org/10.1016/j.plantsci.2019.110168
Acknowledgements
We thank anonymous reviewers and editor for their invaluable comments on the manuscript. We are grateful for the technical help of Jie Zhang and Xue Luo.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 31870478), the Program for Jiangsu Excellent Scientific and Technological Innovation team (17CXTD00014), and a project funded by the Priority Academic Program Development (PAPD) of the Jiangsu Higher Education Institutions of China.
Author information
Authors and Affiliations
Contributions
Chuan-Chao Dai and Xing-Guang Xie put forward the hypothesis. Hui-Jun Jiang, Xing-Guang Xie, and Chuan-Chao Dai designed the experiments, analyzed the data, and wrote and revised the manuscript. Hui-Jun Jiang, Yuan-Yuan Zhao, Yi-Tong Pan, and Kai Sun performed the experiments. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, HJ., Zhao, YY., Pan, YT. et al. The Endophytic Fungus Phomopsis liquidambaris Promotes Phosphorus Uptake by Arachis hypogaea L. by Regulating Host Auxin, Gibberellins, and Cytokinins Signaling Pathways. J Soil Sci Plant Nutr 22, 4913–4927 (2022). https://doi.org/10.1007/s42729-022-00970-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00970-1