Abstract
A facile method based on high performance liquid chromatography coupled with electrospray ionization tandem triple quadrupole mass spectrometry (HPLC-ESI-QqQ-MS) has been established to investigate the production of the oxidized scytonemin by Nostoc commune Vauch in different environmental conditions. In optimized HPLC-ESI-QqQ-MS conditions, the oxidized scytonemin can be effectively detected in selected reaction monitoring (SRM) mode. After regression, high linearity of the calibration curve was achieved with a correlation coefficient (r 2) at 0.99. The limit of detection and limit of quantification were 0.03 and 0.10 ng mL−1, respectively. The recovery of the oxidized scytonemin was at 76.90 %, and the relative standard deviations of inter- and intraday precisions were lower than 8.95 % (n = 4). Subsequently, the production of the oxidized scytonemin from N. commune has been investigated in different culture conditions. High culture temperature, strong illumination intensity, and light–dark cycle (12:12 h) were found to be good for producing the oxidized scytonemin by N. commune. In short, the HPLC-QqQ-SRM-MS method is a powerful tool for comprehensive analysis of the oxidized scytonemin from N. commune, owing to its excellent selectivity and sensitivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photosynthetic cyanobacteria have evolved a variety of strategies to prevent the damages caused by exposure to solar UV radiation, including DNA repair processes, UV avoidance behavior, and the synthesis of radiation-absorbing pigments (Balskus and Walsh 2008, 2010; Bebout and Garcia-Pichel 1995; Castenholz et al. 2000; Cockell and Knowland 1999; Gao and Garcia-Pichel 2011; Levine and Thiel 1987). UV-absorbing/screening pigments were first found in terrestrial cyanobacteria. The cyanobacterium, Nostoc commune Vauch, can retain viability for over 100 years during desiccation (Cameron 1962; Lipman 1941). As the water content decreases during desiccation, photosynthetic activity decreases, and no photosynthetic activity is detected in desiccated colonies (Sakamoto et al. 2009). This cessation of photosynthetic electron transport during desiccation is thought to be a protective response to avoid light-induced damage by suppressing reactive oxygen species production (Fukuda et al. 2008; Hirai et al. 2004). However, photosynthetic activity of the cyanobacterium recovers rapidly upon rehydration (Tamaru et al. 2005; Sakamoto et al. 2009; Satoh et al. 2002). With the UV-absorbing pigment of scytonemin in its extracellular matrices, N. commune is thought to be able to adapt to terrestrial environments with high levels of solar radiation (Matsui et al. 2011).
Scytonemin, a hydrophobic yellow-brown pigment, absorbs UV-A radiations from 320 to 400 nm and is found exclusively in the cyanobacterial sheath of around 300 cyanobacterial species (Geitler 1932; Desikachary 1959; Sinha and Häder 2008). It has been reported that the amount of scytonemin in cyanobacterial sheaths may be sufficient to prevent about 85–90 % of UV-A radiation from entering the cells (Garcia-Pichel and Castenholz 1991; Garcia-Pichel et al. 1992). Scytonemin is thought to be synthesized from metabolites of aromatic amino acid biosynthesis and can be induced by high photon fluence rate, and scytonemin can protect cyanobacteria when other ultraviolet-protective mechanisms, such as active repair of damaged cellular components, are ineffective.
Various analytical techniques have been widely used to study cyanobacteria and scytonemin; Raman spectrometry (Edwards et al. 1999), UV absorbance (Garcia-Pichel and Castenholz 1991; Dillon and Castenholz 1999), nuclear magnetic resonance (Proteau et al. 1993), HPLC, and mass spectrometry (MS) (Sinha et al. 1998; Squier et al. 2004a, b; Hunsucker et al. 2001; Jones et al. 2011) have been used to qualitatively or quantitatively analyze scytonemin. However, to our knowledge, a method based on high performance liquid chromatography tandem triple quadrupole coupled with selected reaction monitoring mass spectrometry (HPLC-QqQ-SRM-MS) to study the production of scytonemin by N. commune in different conditions has not been reported. HPLC-MS is a widely used technique for both qualitative and quantitative analyses of ingredients, which combines the efficient separation capability of HPLC with powerful structural characterization of MS. Moreover, the use of MS in the SRM mode results in high selectivity, as well as improved sensitivity compared to UV and evaporative light-scattering detection.
In this study, we developed a novel HPLC-QqQ-SRM-MS method for separation and determination of trace amount of the oxidized scytonemin in N. commune. Different culture conditions, such as irradiance, culture temperature, and light–dark cycle, were used to investigate the production of the oxidized scytonemin by N. commune.
Materials and methods
The reference compound, oxidized scytonemin (91 %), was obtained from Merck (Germany). HPLC-MS grade dimethyl sulfoxide (DMSO) and acetonitrile were supplied by Merck, and analytical grade trifluoroacetic acid was obtained from Sigma-Aldrich. Deionized water from a Milli-Q system (Millipore, USA) was used in all procedures.
The HPLC-MS analysis was carried out in Finnigan Surveyor and TSQ Quantum Access system (Thermo Fisher Scientific, USA) equipped with electrospray ionization (ESI) interfaced triple quadrupole mass spectrometer. All the operations, the acquiring and analysis of data, were processed by Xcalibur (Thermo Fisher Scientific).
HPLC-MS conditions
In all cases, analyses were carried out at 30 °C on a reversed phase Hypersil Gold C18 (100 × 2.1 mm, 1.7 μm); A linear gradient elution of 0.1 % trifluoroacetic acid (A) and acetonitrile (B) was used with the gradient procedure as follows: 0–8 min, 10 to 50 % B; 8–15 min, 50–80 % B; 15–20 min, 80–90 % B; 20–25 min, 90–100 % B. The flow rate was 0.3 mL min−1, and the injection volume was 10 μL. The ionization conditions were adjusted as follows: sheath gas pressure (N2) flow rate, 30 L min−1; aux gas pressure (N2) flow rate, 10 arb; spray voltage, 2.8 KV; vaporizer temperature, 300 °C; and capillary temperature, 350 °C; argon was introduced into the trap with an estimated pressure 6 × 10−6 mbar to improve trapping efficiency to act as the collision gas for the selected reaction mode data, and collision gas pressure was set at 1.5 mTorr. The HPLC-MS was operated in the positive ionization mode with data acquisition mode of SRM for quantification. The transitions monitored of the oxidized scytonemin in SRM mode were m/z 545.1 to 517.2 (28 V) and m/z 545.1 to 488.8 (29 V). Both product ions at m/z 517.2 and 488.8 were utilized as quantitative ions.
Materials and sample preparation
Nostoc commune was collected from sandy soil in Ningbo University. Fresh N. commune was cleaned and then soaked in 75 % ethanol for 40 s. Subsequently, the N. commune was immersed in a combination of ampicillin (300 μg mL−1), kanamycin (100 μg mL−1), and gentamicin (100 μg mL−1) for 2 h. Finally, the N. commune was irradiated under UV light for 20 min.
The treated N. commune (0.2 g) was weighted and transferred into a sterile flask with 150-mL BG11-C liquid nutrient medium (shown in Table 1). Then, the flask was placed on a shaker and shaking at 35 rpm for 7 days. The culture conditions, such as illumination intensity, culture temperature, and light–dark cycle, were used to culture N. commune. The culture conditions are shown in Table 2.
After culture, the samples were dried at 37 °C for 24 h. Then, they were soaked and ground in acetone at 4 °C for 12 h. After extraction of the oxidized scytonemin from the treated samples by a 75 % acetone water solution under ultrasonication for 10 min, the acetone water solution with the oxidized scytonemin was separated by centrifugation at 10,000 rpm for 5 min. The extraction and centrifuge procedures were repeated for several times until the extract became colorless. After evaporation of the solvent, the concentrate was dissolved in 1.5 mL acetone, membrane filtered (0.45 μm), and analyzed by HPLC-MS.
Standard and solution preparation
To prepare stock solutions, the oxidized scytonemin was dissolved in the mixture of methanol and DMSO (1:1) to give a concentration of 1 mg mL−1. Working solutions were obtained by dilution of the stock solution with the mixture of methanol and DMSO (1:1).
The recovery rate (in percent) was calculated as the ratio of the peak area for a spiked amount of the oxidized scytonemin standard (10 μg mL−1, 50 μL) in N. commune that contained no detectable oxidized scytonemin passing through the sample preparation procedure to the peak area for the same amount of the spiked standard in a neat solution, as analyzed by HPLC-QqQ-SRM-MS.
In order to evaluate the ion suppression of the complexity samples, the experiment has been achieved as following: the 0.1 g N. commune with additional known amount of oxidized scytonemin (10 μg mL−1, 1 mL) was used as spiked sample, and original sample was obtained from the extract of 0.1 g N. commune. Both spiked and original samples were enriched and purified according to the sample preparation procedure. Then, the spiked and original samples were both analyzed by HPLC-QqQ-SRM-MS.
Results and discussion
Optimization of SRM/MS conditions
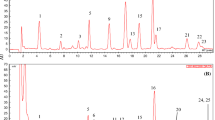
HPLC-QqQ-SRM-MS was performed to analyze the oxidized scytonemin in N. commune. According to the retention times and mass spectra of the corresponding standard, oxidized scytonemin (t R 13.8 min) and reduced scytonemin (t R 12.8 min) were identified in HPLC-ESI-QqQ-MS from the protonated molecules (m/z 545 and 547, respectively). In this paper, only oxidized scytonemin was analyzed by HPLC-QqQ-SRM-MS, and the SRM mode provided better sensitivity than the full scan mode. The results indicated that the SRM mode had more advantages in the quantification of low content analysis and separating overlapped constituents in complicated mixtures. Figure 1 shows the total ionization chromatogram (TIC) and SRM chromatogram of the extract of N. commune.
The total ionization chromatogram and SRM chromatogram of the extract of N. commune. a The SRM chromatogram of the oxidized scytonemin in N. commune. b The transitions monitored of the oxidized scytonemin in SRM mode. c The TIC chromatogram of the extract of N. commune. d The extract ion chromatogram of the oxidized scytonemin. e The extract ion chromatogram of the reduced scytonemin
In the majority of MS2 scan taken across the scytonemin peak, the ions at m/z 517.2 and 488.8 were generated by neutral losses of 28 and 56 Da, which were assigned to elimination of one and two CO from cyclopentyl carbonyl groups, respectively (Fig. 2). These two fragment ions were the base peak and had the high relative abundances in MS2 spectrum, thus they were chosen to be the fragment ions at SRM mode. The transitions monitored of the oxidized scytonemin in SRM mode were m/z 545.1 to 517.2 (28 V) and m/z 545.1 to 488.8 (29 V).
Method validation
Reliable and robust quantitative analyses utilizing HPLC-QqQ-SRM-MS were achieved with the oxidized scytonemin standard solutions ranging from 0.5, 1, 5, 10, 25, and 50 μg mL−1. The calibration curve of the oxidized scytonemin showed good linear regression with an R 2 = 0.99 within relatively wide test ranges. Table 3 summarizes the result of the calibration curve of scytonemin.
The relative standard deviations (RSDs) evaluated with the oxidized scytonemin were taken once a day and tested for five consecutive days. The RSDs for the intra- and interday precisions were achieved in three different concentrations (0.5, 5, and 25 μg mL−1). The area peak reproducibility (A r) was acceptable with RSD values ranging from 1.48 to 4.46 % for intraday precision and 4.19 to 8.95 % for interday precision by analyses of HPLC-QqQ-SRM-MS. The data suggested that the method showed good reproducibility in terms of peak area. The results from the quantitative analysis of the precision of this method could achieve the desired requirements.
The limit of detection (LOD) and limit of quantification (LOQ) values were determined by first dilution to 100 ng mL−1 standard solution, and an aliquot of 10 μL was injected. The peak signal to noise ratio (S/N in accordance with 3/1 for LOD and 10/1 for LOQ) was obtained. The results demonstrated that the method was very sensitive with LOD (0.03 ng mL−1) and LOQ (0.11 ng mL−1) for the oxidized scytonemin.
The recovery was used to evaluate the accuracy of the method. The N. commune without oxidized scytonemin was used as blank matrix spiking with known amounts of the standard (10 μg mL−1, 50 μL). Sample was enriched and purified according to the method in “Standard and solution preparation.” The recovery was obtained as 76.9 % (RSD = 9.2 %, n = 5) by the above method. The result showed that the method had acceptable accuracy and repeatability for the oxidized scytonemin.
The spiked and original samples for the ion suppression evaluation were both analyzed by HPLC-QqQ-SRM-MS. The results showed that the original sample contained 21.8 μg oxidized scytonemin, and in the spiked sample, the amount of the oxidized scytonemin was 32.7 μg close to the expected 31.8 μg oxidized scytonemin in the spiked sample, indicating that the ion suppression effect did not obviously affect the accuracy of the quantitative analysis.
The influence of culture condition in the oxidized scytonemin from N. commune
This study had the advantage of allowing comparison of several culture conditions using environmental samples rather than reconstructed lab samples, in order to assess organisms in their natural state, including adaptation to environmental conditions and also possibly damaged or slow replicating organisms. Different culture conditions, such as irradiance, culture temperature, and light–dark cycle, were studied. According to the culture condition, nine samples were divided into three groups. Group 1 (assigned as A, B, and C) was defined as the culture condition of a light–dark cycle of 12:12 h, irradiance 25 μmol photons m−2 s−1, and the culture temperature was changed from 25 to 35 °C, by steps of 5 °C. Group 2 (assigned as D, E, and F) was defined with a light–dark cycle of 12:12 h, with an irradiance of 50 μmol photons m−2 s−1, and the culture temperature was changed from 25, 30, to 35 °C. Group 3 (assigned as G, H, and I) was defined as a light–dark cycle of 24:0 h, with an irradiance of 50 μmol photons m−2 s−1, and the culture temperature was changed from 25 to 35 °C, by steps of 5 °C. All of the samples of groups 1 to 3 were obtained by sunlight irradiation for 7 days. The scytonemin in these three groups of samples with different culture conditions were identified by comparing the retention time, the online UV, and MS information with authentic standard. The oxidized scytonemin of three groups were simultaneously determined, and the amounts of the scytonemin in the samples are listed in Table 2. The results showed that there were significant differences in the contents of three classes of the oxidized scytonemin.
The influence of culture temperature
Figure 3a shows that the oxidized scytonemin content changed according to the different culture temperatures. In groups 1 and 2, the contents of the oxidized scytonemin increased when the temperature was changed from 25, 30, to 35 °C. However, in the samples of group 3 which was different with a 24:0-h light–dark cycle, the content of scytonemin increased when the culture temperature changed from 25 to 30 °C. As the culture temperature rises to 35 °C, the content of the oxidized scytonemin increased steadily. The data presented here clearly suggest that the accumulation of the oxidized scytonemin was correlated to the culture temperature increasing with increasing temperature.
The influence of irradiance
The irradiance also influenced in the amount of oxidized scytonemin (Fig. 3b). The samples of groups 1 and 2 were utilized, with the culture conditions differing only in the irradiance. The content of oxidized scytonemin increased with increasing irradiance. Furthermore, the higher the culture temperature, the larger the increment in the amount of oxidized scytonemin. According to these results, it can be suggested that, at high temperature and strong solar irradiation, N. commune would produce a number of UV-protecting pigments (the oxidized scytonemin) for adapting this environment.
The influence of light–dark cycle
The light–dark cycle also plays an important role in the stimulation of oxidized scytonemin in N. commune as shown in Fig. 3c. The samples of groups 2 and 3 were used, which only differed with respect to light–dark cycle. Here, the amount of oxidized scytonemin was reduced as the light–dark cycle was changed from 12:12 to 24:0 h. Furthermore, the decrease in the oxidized scytonemin content was enhanced at higher culture temperature. There results suggest that, in photosynthesis, the carbon cycle worked in coordination with light reaction in generating the UV pigment—scytonemin. Thus, the 12:12-h light–dark cycle stimulated the production of oxidized scytonemin.
In conclusion, a simple and rapid quantitative HPLC-QqQ-SRM-MS method was developed for the comprehensive analysis of the oxidized scytonemin in N. commune. According to this method, the amount of the oxidized scytonemin was definitely identified based on SRM mode. The HPLC-QqQ-SRM-MS method was also successfully applied to monitoring of the amount of the oxidized scytonemin in N. commune and screening the best culture conditions (temperature, light–dark cycle, and irradiance). The results presented here are intended to provide information for helping to monitor and investigate oxidized scytonemin from N. commune.
References
Balskus EP, Walsh CT (2008) Investigating the initial steps in the biosynthesis of cyanobacterial sunscreen scytonemin. J Am Chem Soc 130:15260–15261
Balskus EP, Walsh CT (2010) The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 329:1653–1656
Bebout BM, Garcia-Pichel F (1995) UV B-Induced vertical migrations of cyanobacteria in a microbial mat. Appl Env Microbiol 61:4215–4222
Cameron RE (1962) Species of Nostoc Vaucher occurring in the Sonoran Desert in Arizona. Trans Am Microsc Soc 81:379–384
Castenholz RW, Garcia-Pichel F, (2000) Cyanobacterial responses to UV-radiation. In Whitton BA, Potts M (eds) The ecology of cyanobacteria. Kluwer, Dordrecht, pp 591–611
Cockell CS, Knowland J (1999) Ultraviolet radiation screening compounds. Biol Rev 74:311–345
Desikachary TV (1959) Cyanophyta. Indian Council of Agriculture Research, New Delhi, India, pp 222–223
Dillon JG, Castenholz RW (1999) Scytonemin, a cyanobacterial sheath pigment, protects against UVC radiation: implications for early photosynthetic life. J Phycol 35:673–681
Edwards HGM, Garcia-Pichel F, Newton M, Wynn Williams DD (1999) Vibrational Raman spectroscopic study of scytonemin, the UV-protective cyanobacterial pigment. Spectrochim Acta A Mol Biomol Spectrosc 56:193–200
Fukuda S, Yamakawa R, Hirai M, Kashino Y, Koike H, Satoh K (2008) Mechanisms to avoid photoinhibition in a desiccation-tolerant cyanobacterium, Nostoc commune. Plant Cell Physiol 49:488–492
Gao QJ, Garcia-Pichel F (2011) Microbial ultraviolet sunscreens. Nat Rev Microbiol 9:791–802
Garcia-Pichel F, Castenholz RW (1991) Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J Phycol 27:395–409
Garcia-Pichel F, Sherry ND, Castenholz RW (1992) Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem Photobiol 56:17–23
Geitler L (1932) Cyanophyceae (Blaualgen). Kryptogamen-Flora von Deutschland, Österreich und der Schweiz, vol 14. Akademische Verlagsgesellschaft, Leipzig, pp 1–1196
Hirai M, Yamakawa R, Nishio J, Yamaji T, Kashino Y, Koike H, Satoh K (2004) Deactivation of photosynthetic activities is triggered by loss of a small amount of water in a desiccation-tolerant cyanobacterium. Nostoc commune. Plant Cell Physiol 45:872–878
Hunsucker SW, Tissue BM, Potts M, Helm RF (2001) Screening protocol for the ultraviolet-photoprotective pigment scytonemin. Anal Biochem 288:227–230
Jones CS, Esquenazi E, Dorrestein PC, Gerwick WH (2011) Probing the in vivo biosynthesis of scytonemin, a cyanobacterial ultraviolet radiation sunscreen, through small scale stable isotope incubation studies and MALDI-TOF mass spectrometry. Bioorg Med Chem 19:6620–6627
Levine E, Thiel T (1987) UV-inducible DNA repair in the cyanobacteria Anabaena spp. J Bacteriol 169:3988–3993
Lipman CB (1941) The successful revival of Nostoc commune from a herbarium specimen 87 years old. Bull Torrey Bot Club 68:664–666
Matsui K, Nazifi E, Kunita S, Wada N, Matsugo S, Sakamoto T (2011) Novel glycosylated mycosporine-like amino acids with radical scavenging activity from the cyanobacterium Nostoc commune. J Photochem Photobiol B 105:81–89
Proteau PJ, Gerwick WH, Garcia-Pichel F, Castenholz R (1993) The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 49:825–829
Sakamoto T, Yoshida T, Arima H, Hatanaka Y, Takani Y, Tamaru Y (2009) Accumulation of trehalose in response to desiccation and salt stress in the terrestrial cyanobacterium Nostoc commune. Phycol Res 57:66–73
Satoh K, Hirai M, Nishio J, Yamaji T, Kashino Y, Koike H (2002) Recovery of photosynthetic systems during rewetting is quite rapid in a terrestrial cyanobacterium, Nostoc commune. Plant Cell Physiol 43:170–176
Sinha RP, Häder DP (2008) UV-protectants in cyanobacteria. Plant Sci 174:278–289
Sinha RP, Klisch M, Gröniger A, Häder DP (1998) Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J Photochem Photobiol B 47:83–94
Squier AH, Airs RL, Hodgson DA, Keely BJ (2004a) Atmospheric pressure chemical ionisation liquid chromatography/mass spectrometry of the ultraviolet screening pigment scytonemin: characteristic fragmentations. Rapid Commun Mass Spectrom 18:2934–2938
Squier AH, Hodgson DA, Keely BJ (2004b) A critical assessment of the analysis and distributions of scytonemin and related UV-screening pigments in sediments. Org Geochem 35:1221–1228
Tamaru Y, Takani Y, Yoshida T, Sakamoto T (2005) Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl Env Microbiol 71:7327–7333
Acknowledgments
The work was supported by the Science Technology Department of Zhejiang Province (2011C37016), Zhejiang Marine Biotechnology Innovation Team (ZMBIT, 2012R10029-1), Ningbo Marine Algae Biotechnology Team (2011B81007) and K. C. Wong Magna Fund of Ningbo University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Zhao, L., Xu, J. et al. Determination of oxidized scytonemin in Nostoc commune Vauch cultured on different conditions by high performance liquid chromatography coupled with triple quadrupole mass spectrometry. J Appl Phycol 25, 1001–1007 (2013). https://doi.org/10.1007/s10811-012-9914-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9914-1