Abstract
Studies of the altitudinal distributions of soil microorganisms are rare or have led to contradictory results. Therefore, we studied archaeal and bacterial abundance and microbial-mediated activities across an altitudinal gradient (2700 to 3500 m) on the southwestern slope of Mt. Schrankogel (Central Alps, Austria). Sampling sites distributed over the alpine (2700 to 2900 m), the alpine-nival (3000 to 3100 m), and the nival altitudinal belts (3200 to 3500 m), which are populated by characteristic plant assemblages. Bacterial and archaeal abundances were measured via quantitative real-time PCR (qPCR). Moreover, microbial biomass C, microbial activity (dehydrogenase), and enzymes involved in carbon (CM-cellulase), nitrogen (protease), phosphorus (alkaline phosphatase), and sulfur (arylsulfatase) cycling were determined. Abundances, microbial biomass C, and activities almost linearly decreased along the gradient. Archaeal abundance experienced a sharper decrease, thus pointing to pronounced sensitivity toward environmental harshness. Additionally, abundance and activities were significantly higher in soils of the alpine belt compared with those of the nival belt, whereas the alpine-nival ecotone represented a transitional area with intermediate values, thus highlighting the importance of vegetation. Archaeal abundance along the gradient was significantly related to soil temperature only, whereas bacterial abundance was significantly related to temperature and dissolved organic carbon (DOC). Soil carbon and nitrogen concentrations explained most of the variance in enzyme activities involved in the cycling of C, N, P, and S. Increasing temperature could therefore increase the abundances and activities of microorganisms either directly or indirectly via expansion of alpine vegetation to higher altitudes and increased plant cover.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Serving as “natural laboratories,” altitudinal gradients offer the possibility of studying the distribution of organisms in response to changing environmental conditions that typically occur over short geographical distances in the alpine life zone [1]. Research on altitudinal gradients does not only result in the formation of ecological theories but also allows the evaluation of effects of future global climatic changes (e.g., temperature increase) since mountain ecosystems are expected to be particularly vulnerable [2, 3]. As shown in previous studies along altitudinal gradients, organisms most frequently exhibited either a monotonically declining or a unimodal pattern with increasing altitude [4]. To explain these trends, four major contributing factors including climate, space, evolutionary history as well as biotic properties (e.g., competition, mutualism) are proposed [5]. These results are, however, largely based on studies on the diversity and abundance of plant and animal taxa during the last 200 years, while soil microorganisms remained understudied.
Available investigations on microorganisms along altitudinal transects are on the one hand rare compared to those targeting plants and animals and on the other hand led to contradictory results. For instance, Bryant et al. [6] revealed that decreasing diversity of the Acidobacteria phylum along with increasing altitude in the Rocky Mountains was mainly driven by soil pH. Another recent study showed that more bacterial than archaeal taxonomic groups exhibited decreasing trends along with altitude and thus concluded that bacteria were more sensitive toward changing abiotic properties [7]. By contrast, on an altitudinal gradient in forests of the eastern Peruvian Andes ranging from 200 to 3400 m above sea level, no clear diversity patterns of bacteria sampled in the phyllosphere as well as the organic and mineral soil layers were detected, although plant and animal taxa did exhibit clear trends [8]. In some cases, even increasing [9] and unimodal [10] diversity distributions were detected. With respect to these contradictions, it is of particular interest to add data from other mountain systems and to extend the study gradient to the limits of vascular plant life.
Moreover, knowledge on the diversity and abundance distributions of soil archaea in harsh high-alpine environments is rare. Since the first discovery of archaea [11] and their recognition as a domain of life in the 1990s [12], these microbes were long considered to be extremophilic. However, this opinion changed when archaea were discovered in diverse environments including temperate soils, sediments of lakes and rivers, and the oceans [13]. The percentage of archaea relative to the entire prokaryotic population ranges from 0.5 to 3.0 % in moderate terrestrial environments [14] and therefore points to the ubiquity and ecological role of archaea. Since putative archaeal ammonia monooxygenase (amoA) gene sequences are widespread in soil ecosystems including cold alpine soils [15–17], a possible role of archaea as ammonia oxidizers was suggested [18] and confirmed by 13CO2 stable isotope probing [19–21]. However, there are indications that some of these non-extremophilic archaea might exhibit a heterotrophic or mixotrophic lifestyle [22] and could consequently be involved in soil organic matter decomposition. Nevertheless, data on archaeal abundance in high altitudinal soil ecosystems is limited [7, 16, 17, 23–26]. Furthermore, some of the biochemical and genetic properties of archaea, such as membrane structure and the genes encoding ammonia oxidation pathway, are distinctly different from those found in bacteria [27]. Hence, it is possible that both domains respond differently to environmental changes as found on altitudinal gradients.
According to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, surface temperatures worldwide are expected to increase by 1.1 to 6.4 °C by the end of the twenty-first century [28]. The mean air temperatures in the European Alps have increased by 1.1 to 1.3 °C since the end of the nineteenth century [29], twice as much as the global average [30]. In particular, on the southern slope system of Mount Schrankogel (Eastern Central Alps, Austria) a Global Observation Research Initiative in Alpine Environments (GLORIA) master site was established in 1994 to monitor changes of the vegetation on a regular basis. The alpine and nival altitudinal belts were characterized by typical plant species such as Carex curvula and Androsace alpina, respectively. In between, a transitional area (termed the alpine-nival ecotone) harboring both alpine and nival species was distinguished [31]. So far, repeated investigations have indicated an expansion of pioneer plants of alpine grassland to higher altitudes and a simultaneous contraction of subnival and nival plant species as a consequence of climate warming [32]. Alterations of vegetation composition and the amount and quality of plant litter associated with increasing temperatures may in turn induce changes in the composition and activity of microbial decomposers, which are mainly responsible for processing of soil organic matter and might have consequences for the nutrient cycles of ecosystems [33, 34]. It is for instance suspected that warming leads to a positive feedback of carbon cycling by decoupling the amount of CO2 released by microbes and the amount of CO2 sequestered by plants [35].
The objectives of the present study were to (1) determine altitudinal changes of the population sizes of bacteria and archaea on Mt. Schrankogel (Eastern Central Alps, Austria); (2) assess the correspondent microbial activities engaged in C-, N-, S-, and P-cycling in the alpine altitudinal belt, the alpine-nival transitional ecotone and the nival altitudinal belt; (3) and relate these changes to environmental drivers.
Materials and Methods
Study area Description and Soil Sampling
This study was conducted at Mount Schrankogel, Eastern Central Alps, Tyrol, Austria (11° 05′ 58″ E, 47° 02′ 41″ N; 3498 m) in August 2014. The northern and eastern sides of this mountain are surrounded by receding glaciers and the respective forelands. Mt. Schrankogel is mainly comprised of siliceous bedrocks [36] and typical soil types are leptosols and cambisols. The altitudinal gradient investigated was located at the southwest-facing slope of the mountain and covered altitudes ranging from 2700 to approximately 3500 m above sea level (a.s.l.) (Fig. S1). Altitudes ranging from 2700 to 2900 m a.s.l. are alpine grasslands dominated by C. curvula and Oreochloa disticha [37], whereas nival plant species such as Androsace alpina, Poa laxa, Ranunculus glacialis, and Saxifraga bryoides occur at 3200 m a.s.l. [31, 38]. The alpine-nival ecotone from 3000 to 3100 m a.s.l. represents a transitional area in which alpine grassland and patchy and open nival vegetation co-occur [38].
In August 2014, nine sites were sampled along the southwestern slope at intervals of 100 m (2700, 2800, 2900, 3000, 3100, 3200, 3300, 3400, and 3500 m a.s.l.). The slope of the sampling sites was relatively uniform and ranged between 20° and 30° (Fig. S1). At each site, three replicate sampling plots of 1 m × 1 m were set up. Soil was collected from the first 5 cm below the surface at three randomly selected spots per plot and afterward merged to a composite sample. The samples were immediately transported to the laboratory, sieved at 2 mm, and stored at 4 °C for physicochemical analyses as well as determination of microbial activity and biomass. For DNA extraction, soil samples were stored at −20 °C. The sampling plots were located near permanently installed GeoPrecision Mlog5W loggers (GeoPrecision, Germany) and Tinytag loggers (Gemini Data Loggers, UK), which were used to measure soil temperatures at depths of 10 cm below ground once per hour. Based on all data points recorded from August 2013 to July 2014, mean annual temperatures (MAT) were calculated.
For assessing the effect of vegetation cover and plant litter on microbial abundance and activity, vegetation samples from across the southwest-facing slope were recorded during the recent years. Vegetation plots were positioned at the same elevations, where soil samples were taken and soil temperature was measured, i.e., five plots of 1 m2 at each 100-m elevational step from 2700 to 3400 m. Vegetation data were divided into three groups: alpine (2700 to 2900 m), alpine-nival ecotone (3000 to 3100 m), and nival belt (3200 m upwards).
Physicochemical Analyses
Soil moisture was determined gravimetrically by drying 5 g of sieved soil at 105 °C overnight. pH was measured in a 1:2.5 (w/v) soil to CaCl2 (0.01 M) mixture. Maximum water holding capacity (MWHC) was determined by weighing 10 g of sieved soil into glass cylinders that were perforated on one side. The cylinders were placed in deionized water for 1 h. After water saturation, the samples were placed on 10 cm quartz sand for 3 h. The wet samples were weighed and dried for 2 days at 105 °C. Subsequent weighing allowed determination of MWHC. Determination of organic matter (OM) was carried out using the loss on ignition method. Oven-dried (105 °C, overnight) soils were incinerated for 4 h at 430 °C for this analysis [39]. The total carbon (Ct) and total nitrogen (Nt) contents of the soils were measured on a CN analyzer (Truspec CHN Macro, Leco, MI, USA) using oven-dried soil. Dissolved organic carbon (DOC) was quantified on a TOC-L analyzer (Shimadzu Co, Japan) after extraction using soil and distilled water at a mixing ratio of 1:5 (w/v). NH4 +-N and NO3 −-N was extracted from fresh soil using 2 M KCl and determined by photometry at 660 and 210 nm, respectively [39].
Microbial Biomass and Activities
Basal soil respiration and microbial biomass (Cmic) were measured on an infrared gas analyzer (IRGA) [40] at 22 ± 0.5 °C as described previously [23]. Cmic was calculated using the formula of Anderson and Domsch, who recognized that 1 ml CO2 g−1 dry soil h−1 produced after glucose addition correspond to 40.04 mg microbial biomass C. Assuming the average fraction of C to be 45 % based on microbial dry mass, this results in the following equation [41]:
As a measure of general microbial activity, intracellular dehydrogenases (DHAs) were quantified as described by Schinner et al. [39]. To measure DHA, 2.5 ml of 0.75 % (w/v) triphenyl tetrazolium chloride (TTC) solution was mixed with 2.5 g of sieved soil and incubated in darkness at 25 °C for 16 h. During incubation, TTC is metabolized into triphenyl formazan (TPF) by intracellular dehydrogenases. Extraction of TPF was performed using acetone. TPF concentrations in the filtered samples were measured using photometry at 546 nm, and DHA was expressed as μg TPF g−1 dry soil 16 h−1. Carboxymethyl-cellulase activity (CM-cellulase) was also determined according to standard methods [39]. For determination of protease activity (PR), 5 ml of a 2 % (w/v) sodium-caseinate solution and 5 ml of Tris buffer (0.05 M, pH 8.1) were added to 1 g of sieved soil. Incubation was performed at 50 °C for 2 h on a shaker. After filtration, 5 ml of the sample was mixed with 7.5 ml of alkali reagent and 5 ml of Folin-Ciocalteau’s reagent, incubated at room temperature for 1.5 h and measured at 700 nm on a photometer. PR was expressed as μg tyrosine equivalents g−1 dry soil 2 h−1 [39]. Arylsulfatase activity (AS) was determined by mixing 1 g of sieved soil samples with 4 ml of acetate buffer (0.5 M, pH 5.8), and 1 ml of a 0.02 M K-p-nitrophenyl sulfate solution, which served as the substrate for the enzyme. Incubation time was 1 h at 37 °C. The produced nitrophenol was stained with 0.5 M sodium hydroxide solution and quantified at 420 nm. Phosphatase activity was measured after incubating 1 g of sieved soil with p-nitrophenyl phosphate for 1 h at 37 °C. The released p-nitrophenol was stained using 0.5 M NaOH and quantified at 400 nm on a photometer [39].
DNA Extraction and qPCR
Genomic DNA was extracted from 0.5 g of sieved soil using a commercially available kit (NucleoSpin Soil, Macherey-Nagel, Germany). The quality and quantity of the DNA was checked by UV/VIS spectroscopy using a Nanodrop 2000c (PEQLAB, Germany). Quantification of archaeal 16S rRNA gene copies was conducted on a Corbett Life Science (Qiagen, Netherlands) Rotor-Gene Q system using the primers 787 F and 1059 R [42] as described previously [23]. Bacterial 16S rRNA gene copies were quantified using the primer pair 338 F/518 R [43]. Reactions containing a total volume of 20 μl were setup as follows: 10 μl of SensiMix SYBR no-ROX kit (Bioline, UK), 0.2 μM of each primer, 5 mM MgCl2, 0.04 % (v/v) bovine serum albumin, and 2 μl of 1:10 diluted DNA template. Quantitative PCR targeting bacteria was preceded by an initial denaturing step of 10 min at 95 °C and included 35 cycles of 95 °C hold for 20 s, 53 °C hold for 20 s, and 72 °C hold for 20 s. Non-template DNA and non-template controls (UltraPure DNase/RNase-free distilled water, Invitrogen, USA) were included in each run. DNA standards for construction of the calibration curves were derived from Methanosarcina acetivorans (DSM2834) and Methylosinus sporium (DSM17706) for the assays targeting archaea and bacteria, respectively. Run efficiencies were 86 % (R 2 = 0.997) and 94 % (R 2 = 0.999) for the runs targeting archaea and bacteria, respectively. The relation of bacteria/archaea was calculated based on the Log10-transformed gene copy numbers. Ratios equaling 1 indicate that archaeal and bacterial 16S rRNA genes are equally abundant, whereas ratios <1 and >1indicate the dominance of archaeal and bacterial genes, respectively.
Statistical Analyses
All statistical analyses were conducted using STATISTICA version 9 (Stat Soft Inc., USA). One-way analysis of variance was applied to test for significant impacts of the investigated vegetation zones (alpine, alpine-nival, nival) on archaeal and bacterial abundance, microbial biomass, and activities. Because of the unequal numbers of observations per vegetation zone (alpine = 9; alpine-nival = 6; nival = 12), type III-ANOVA was chosen as suggested in Quinn and Keough [44]. Post hoc analyses were performed using Tukey’s honestly significant difference test. Correlations between altitude and the abundances, microbial biomass, activities, and physicochemical soil properties as well as between abundances (bacteria and archaea) and activities were calculated using Pearson’s correlation coefficients in order to highlight the spatial structure of these parameters along the gradient.
Multiple linear regression analysis was used to relate bacterial and archaeal abundances, microbial biomass, and activities to possible environmental drivers (abiotic soil properties and temperature) and to determine their relative importance by comparing the standardized regression coefficients and the decomposition of the variance. Initially, all measured environmental variables were fitted to the dependent variables in multiple linear regressions. However, not surprisingly, some abiotic predictor variables were strongly autocorrelated (e.g., organic matter, Ct, and Nt; water content and MWHC) and thus did not meet the assumption of independence. According to Quinn and Keough [44], we excluded those variables which caused multicollinearity problems prior to selection of the final models. As one would expect, soil temperature is linked to altitude in an almost linear relationship (r = −0.97; ***P < 0.001). Hence, altitude and temperature can be seen as statistically exchangeable variables. For our models, we used temperature, because it is biologically more meaningful than altitude. Best-fit models were chosen based on backward selection as recommended in Quinn and Keough [44]. The differences between groups, the correlation coefficients, and the regression models were regarded as significant when P values were below 0.05.
Results
Soil Properties, Temperature and Vegetation Data
Along the investigated altitudinal transect, soil moisture (r = −0.71; ***P < 0.001), MWHC (r = −0.75; ***P < 0.001), OM (r = −0.60; ***P < 0.001), DOC (r = −0.57; **P < 0.01), Ct (r = −0.60; ***P < 0.001), and Nt (r = −0.63; ***P < 0.001) decreased along with increasing altitude. Soil pH ranged from 4.1 to 5.2 (Table 1) and slightly increased (r = 0.39; *P < 0.05) with altitude. The pools of NH4 +-N and NO3 −-N did not follow any consistent trend. Both nutrient pools remained relatively constant. Soil temperature data indicated that the altitudinal gradient is strongly linked (r = −0.97; ***P < 0.001) to a temperature gradient. Within the studied altitudinal gradient (2900 to 3500 m) mean annual temperature belowground decreased by 1.2 °C per 100 m of altitudinal increase (Table 1). Both percentage plant cover and plant litter were significantly influenced (***P < 0.001) by altitude and decreased along the gradient (Fig. 1).
Percentage of vascular plant cover (a) and litter (b) at study plots of 1 m2distributed among the alpine (2700 to 2900 m; n = 15), the alpine-nival (3000 to 3100 m; n = 10), and the nival vegetation belt (3200 to 3400 m; n = 15) at Mt. Schrankogel (Eastern Central Alps, Tyrol, Austria). The boxes show means ± SE; the spreads depict SD. Significant differences (*P < 0.05) between groups are indicated by different letters
Microbial Biomass and Activities
Microbial biomass (Cmic) linearly decreased (r = −0.65, ***P < 0.001) along the altitudinal gradient with values ranging from 70.6 ± 4.06 μg g−1 dry soil at 2700 m and 5.9 ± 3.04 μg g−1 dry soil at 3500 m. Cmic was significantly higher (***P < 0.001) in soils covered by alpine vegetation (2700 to 2900 m) compared to those covered by nival plants (3200 to 3500 m). Cmic of sites that are characterized by a mixture of alpine and nival vegetation was neither different from the values measured at alpine sites nor nival sites and thus represented a transitional stage (Fig. 2). Variance partitioning pointed out that soil water content and total carbon content explained more of the variation in Cmic than mean annual soil temperature (which corresponds to altitude) (Table 3). Although other abiotic factors (e.g., pH, DOC) were also taken into consideration, they could not be significantly related to Cmic. General microbial activities as determined by DHA and basal soil respiration decreased with increasing altitude as indicated by significant negative correlations (Table 2) and ranged between 2.5 ± 0.40 and 0.3 ± 0.21 μg CO2 g−1 dry soil h−1 as well as between 173.9 ± 18.11 and 69.6 ± 35.64 μg TPF g−1 dry soil 16 h−1, respectively. DHA was significantly higher (*P < 0.05) in soils under alpine plant cover compared to those dominated by a mixture of alpine and nival plants as well as to those covered by nival plants only (Fig. 3a) and was to a similar extent affected by soil temperature and OM exclusively (Table 3). Basal soil respiration (Fig. 3b) and microbial enzyme activities involved in C (CM-cellulase), N (protease), S (arylsulfatase), and P cycling (phosphatase) exhibited the same trends as Cmic (Figs. 4a–d) and decreased along with altitude (Table 2). The extracellular enzymes showed similar patterns with temperature being a significant predictor variable of CM-cellulase, protease, and arylsulfatase. However, temperature was in no case more important than soil C and N concentrations (Table 3). Although bacterial and archaeal abundances were initially considered as predictors of the measured activities, both abundances did not contribute to explain the variations in the final model. Nevertheless, significant correlations (based on Pearson’s r) of archaeal and bacterial abundance with each of the microbial activities (Table S1, supplementary material) at least indicates some influence. CM-cellulase, protease, arylsulfatase, and alkaline phosphatase showed a positive linear relationship with vascular plant cover and plant litter, whereas basal respiration was positively related to vascular plant cover only (Table S2, supplementary material).
Microbial biomass (Cmic) of soils sampled at the alpine belt (2700 to 2900 m; n = 9), the alpine-nival ecotone (3000 to 3100 m; n = 6), and the nival belt (3200 to 3500 m; n =12) at Mt. Schrankogel (Eastern Central Alps, Tyrol, Austria). The boxes show means ± SE, and the spreads depict SD. Significant differences (*P < 0.05) between groups are indicated by different letters
Activities of dehydrogenases (DHAs) as described by the concentration of triphenyl formazan (TPF) (a) and basal soil respiration (b) in soils of the alpine belt (2700 to 2900 m; n = 9), the alpine-nival ecotone (3000 to 3100 m; n = 6), and the nival belt (3200 to 3500 m; n = 12) at Mt. Schrankogel (Eastern Central Alps, Tyrol, Austria). The boxes show means ± SE, the spreads depict SD. Significant differences (*P < 0.05) between groups are indicated by different letters
Microbial activities related to carbon (a), phosphorus (b), sulfur (c), and nitrogen cycle (d) detected in soils of the alpine belt (2700 to 2900 m; n = 9), the alpine-nival ecotone (3000 to 3100 m; n = 6), and the nival belt (3200 to 3500 m; n = 12) at Mt. Schrankogel (Eastern Central Alps, Austria). The boxes show means ± SE, and the spreads depict SD. Significant differences (*P < 0.05) between groups are indicated by different letters
All microbial activities were additionally expressed as specific activities (based on the biomass proxy Cmic) to obtain insights into possible microbial responses to the changing environmental conditions along the gradient. The ratio of basal respiration and Cmic (metabolic quotient, qCO2) was highest in the nival soils and there was no significant difference between qCO2 of alpine and alpine-nival soils (Fig. S1a, supplementary material). Specific DHA and the specific activities of CM-cellulase and protease did not show any consistent trend along the gradient (Fig. S1b–d, supplementary material), whereas specific arylsulfatase and alkaline phosphatase activities were highest in the nival soils (Fig. S1e–f, supplementary material).
Bacterial and Archaeal Abundances
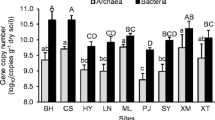
Archaeal abundance (as determined from Log10 16S rRNA gene copy numbers) ranged from 8.7 ± 0.29 to 6.2 ± 0.65 Log10 copies g−1 dry soil and was much lower than bacterial abundance, which ranged between 9.9 ± 0.04 and 8.3 ± 0.45 Log10 copies g−1 dry soil from the lowest sampling site at 2700 m to the highest site located at 3500 m. Archaeal abundance accounted for 0.3 to 12 % of the abundance of prokaryotes (calculated from the sum of bacterial and archaeal 16S rRNA gene copies g−1 dry soil). Bacterial abundance was significantly higher (***P < 0.001) in the alpine soils compared to the nival soils, while alpine-nival soils represented a transitional stage in terms of gene copy numbers (Fig. 5a). Archaea were significantly more abundant in soils of the alpine (***P < 0.001) and alpine-nival (*P < 0.05) ecotones compared to soils sampled within the nival ecotone (Fig. 5b). The Log10 of the ratio bacterial/archaeal abundance was significantly lower (***P < 0.001) in soils located at the alpine belt and the alpine-nival ecotone compared to the ones sampled at the nival belt (Fig. 5c).
Bacterial (a) and archaeal (b) Log10 16S rRNA gene abundance and the Log10 ratio of bacteria/archaea (c) in soils of the alpine belt (2700 to 2900 m; n = 9), the alpine-nival ecotone (3000 to 3100 m; n = 6), and the nival ecotone (3200 to 3500 m; n = 12) at Mt. Schrankogel (Eastern Central Alps, Tyrol, Austria). The boxes show means ± SE, and the spreads depict SD. Significant differences (*P < 0.05) between groups are indicated by different letters
Significantly negative correlations between bacterial (r = −0.84) and archaeal (r = −0.88) abundance and altitude could be detected (Fig. 6a), while the Log10 ratio of bacteria/archaea was positively correlated (r = 0.80) to altitude (Table 2) (Fig. 6b). Archaeal abundance was significantly correlated with vascular plant cover (r = 0.93) and plant litter (r = 0.81), while bacterial abundance was correlated with vascular plant cover (r = 0.83) only (Table S2, supplementary material) . Temperature explained most of the variation in bacterial and archaeal abundance. Contrarily, physicochemical soil properties such as DOC, OM, and water content either explained less of the observed variations in bacterial and archaeal abundance or were not even significant (Table 3). The remaining abiotic factors we measured did not contribute to explain the observed variations.
Discussion
In this study, we examined the prokaryotic abundance and microbial soil functions (nutrient cycling) along an altitudinal gradient located on Mt. Schrankogel, which is among the highest peaks in Austria. The studied gradient extends over three vertical zones populated by characteristic plant communities, i.e., the alpine altitudinal belt (2700 to 2900 m), the alpine-nival ecotone (3000 to 3100 m), and the nival altitudinal belt (3200 to 3500 m).
Microbial Abundances in Relation to Altitude, Plant Cover and Soil Properties
Both bacterial and archaeal abundance linearly decreased along with altitude and is to at least two thirds explained by models including only temperature and DOC. This observation is in stark contrast to the results of Wang et al. [7], who could not detect a consistent pattern of abundances of both bacteria and archaea along an altitudinal gradient on Mt. Shegyla. A possible reason for this discrepancy might be the different representative aboveground vegetation along the slopes of Mt. Shegyla and Mt. Schrankogel. The entire vegetation along the southwest-facing slope of Mt. Schrankogel is solely composed of herbaceous plant species, while the vegetation on Mt. Shegyla is dominated by woody plants [7]. Likewise, Djukic et al. [33] could not detect any consistent trend of bacterial biomass (as estimated from PLFAs) across an altitudinal gradient located in the Austrian Northern Limestone Alps, which covered the typical alpine vegetation zones, albeit at lower elevations. However, bacterial PLFAs decreased according to altitude on a gradient ranging from 1500 to 2530 m in the Austrian Central Alps [45]. Although Zhang et al. [16] restricted themselves to the investigation of ammonia-oxidizing archaea along an altitudinal gradient on Mt. Everest, they found a linear decrease of abundance similar to the archaeal abundance patterns we observed across Mt. Schrankogel.
We observed a stronger decline of archaeal abundance (4.8 ± 0.65 % per 100 m) compared to bacterial abundance (2.0 ± 0.12 % per 100 m) along the altitudinal gradient, which suggests that the bacterial population is more tolerant toward harsh environmental conditions, i.e., cold temperature. This view is supported by the regression results, which show that temperature decrease exerts a stronger effect on archaea and is in agreement with the results of Zhang et al. [16]. This trend was reflected by the Log10 ratio bacteria/archaea, which linearly increased along with increasing altitude from 1.3 ± 0.27 at 2700 m to 2.2 ± 0.46 at 3500 m pointing to the fact that nival soils were dominated by bacteria, while the soils of the alpine belt were more balanced, though archaea were still less abundant than bacteria. The negative correlation of the Log10 ratio bacteria/archaea and Log10 archaeal abundance (r = −0.91; ***P < 0.001) indicates that the sharp decrease of archaea with altitude is the reason for the development toward increasing dominance of bacteria at high altitudes. Our finding partly coincides with previously observed dominance of ammonia oxidizing archaea over their bacterial counterparts in lower altitudes of an elevational gradient, which developed toward bacterial dominance with increasing altitude [16].
Bacteria and archaea experience the same environmental conditions along the studied gradient but react differently, most likely because of different adaptation to cold conditions [27]. The climatic conditions along the altitudinal gradient play an important role in structuring plant diversity and plant cover [46] and may in turn directly or indirectly affect microbial communities. The analysis of variance indicated that both bacterial and archaeal abundances were significantly affected (***P < 0.001) by the vegetation types occurring along the studied slope on Mt. Schrankogel. The measured abundances were significantly higher in soils of the alpine belt and alpine-nival ecotone compared to those originating from the nival altitudinal belt. Aboveground, vegetation is the most important source of litter introduced into soils and provides nutrients for soil microorganisms. Along the studied gradient, vascular plant cover and therefore the amount of plant litter input declines with increasing altitude, which is consistent with other reports [31, 37]. Consequently, we observed that organic matter contents were decreasing. Moreover, qualitative differences in the introduced litter depending on the altitude can also be expected due to the different vegetation compositions of the alpine, the alpine-nival, and the nival belts [31, 37]. As indicated by positive relationships of bacterial abundance with DOC, the amount of plant litter input and root exudation may at least partly explain the observed pattern. However, our data leads us to suppose that the different vegetation types and thus quality of the introduced litter and root exudates may be also an important driver of bacterial and archaeal abundance. This assumption is supported by Djukic et al. [33], who found higher microbial biomass in alpine grasslands and beech forests compared to coniferous forests and sites covered by acidophilic vegetation. Since mean annual soil temperature was identified to be a strong driver of bacterial and archaeal abundance at the sampling sites on Mt. Schrankogel, this climatic factor is a likely reason for the decreasing trend of their population sizes along the altitudinal gradient. However, it remains unclear whether abundance is directly or indirectly impacted by temperature. On the one hand, decreasing temperatures would directly limit microbial growth by constraining reproduction and metabolic activity but, on the other hand, affect plant growth and vegetation composition and in turn the quantity and quality of the substrate on which microorganisms depend.
So far, high-alpine environments have been insufficiently studied especially regarding prokaryotic abundances. The Log10 population size of bacteria ranged from 8.3 to 9.9 and was thus lower than the values detected at Mt. Shegyla on the Tibetan Plateau at elevations between 3118 and 4477 m. Archaeal abundance covered a similar range compared to that observed at Mt. Shegyla with values between 6.2 and 8.7 Log10 16S rRNA gene copies g−1 dry soil but were higher compared to the abundances measured in alpine glacier foreland soils of different ages (5.4 to 7.6) and in non-grazed subalpine grassland soil [7, 23, 24].
Surveys conducted in moderate terrestrial ecosystems including pristine forests and grasslands revealed that the relative abundance of non-thermophilic Crenarchaeaota (now Thaumarchaeota) ranged between 0.5 and 3 % (of total prokaryotes) in the aerobic layers of the soils [14]. A temperate mixed deciduous forest yielded relative abundances of soil archaea between 12 and 17 % of total prokaryotes [47] at soil depths comparable to those sampled along the gradient investigated in the present study. The altitudinal gradient at Mt. Schrankogel yielded relative abundances of archaea between 6 and 12 % (relative to total prokaryotes) in soils of the alpine belt and alpine-nival ecotone as well as relative abundances of 0.3 to 1 % in the nival belt. These results might suggest a greater ecological importance of archaea in high-alpine soils, at least compared with moderate grassland soils, in spite of the prevailing environmental harshness.
Microbial Activities in Relation to Altitude, Plant Cover, and Soil Properties
The microbiota of soils plays a crucial role in the biogeochemical cycling of carbon, nitrogen, sulfur, and phosphorus. Slight changes in the quality of the introduced organic material because of climatic changes such as temperature increase may impact these soil functions [48]. Since alpine environments are expected to be among the areas most heavily impacted by global climate warming [29], it is of particular importance to assess microbial soil functions and their environmental drivers.
The turnover rates of the intracellular dehydrogenases showed a decrease along with increasing altitude at Mt. Schrankogel. Our results were consistent with the results of Margesin et al. [45] who observed that DHA was significantly higher in subalpine (1500 to 1900 m) compared with alpine soils (2300 to 2530 m) but not in agreement with the inconsistent pattern of microbial activity found along an altitudinal gradient ranging from 900 to 1900 m in the Northern Limestone Alps [33]. The DHA activities across the gradient on Mt. Schrankogel ranged from 2.1 ± 0.22 to 10.9 ± 1.13 μg TPF g−1 dry soil h−1 and were thus lower compared to the rates observed in alpine glacier foreland soils in Austria [23] and Switzerland [49]. The utilized substrate serves as an electron acceptor for dehydrogenases of bacteria but also of archaea [50] and hence, the activities of both groups can be targeted simultaneously, which was also confirmed by the significant positive correlations of bacterial (r = 0.59; *P < 0.05) and archaeal abundances (r = 0.45; *P < 0.05) with DHA in our study. The intracellular activity of dehydrogenases per unit biomass proxy slightly increased along the altitudinal gradient, which was not consistent with the same activity expressed on a dry mass basis.
Basal soil respiration represents a proxy for the entire C mineralization mediated by soil microorganisms. The decreasing trend found at Mt. Schrankogel coincides with the development of this parameter at another location in the Austrian Central Alps [51]. Respiration ranged between 2.5 ± 0.40 μg CO2 g−1 dry soil h−1 at 2700 m and 0.3 ± 0.11 μg CO2 g−1 dry soil h−1 at 3500 m and was therefore comparable with the rates found in high-alpine glacier foreland soils, although these soils were located at lower altitudes (KH, unpublished). Carbon mineralization per microbial biomass unit (as expressed by the metabolic quotient qCO2) was highest in the nival soils. The low metabolic efficiency might indicate a microbial community that invests in high reproduction (r strategy) rather than maintenance (K strategy) at nival locations and is consistent with other studies [52, 53].
In addition to overall mineralization process, we focused on extracellular enzymes usually produced by soil microbes. These enzymes mediate the decomposition of soil organic matter into simple compounds (e.g., sugars, amino acids, NH4 +, PO4 3−) that can be assimilated by microbes and plants [54]. Across the altitudinal gradient studied here, the soil microbial extracellular enzyme activities engaged in decomposition of the plant polysaccharide cellulose (CM-cellulase) and in N (protease), P (alkaline phosphatase), and S (arylsulfatase) cycling exhibited a linearly declining pattern along with increasing altitude, which was only partly in agreement with other investigations. On Changbai Mountain, vertical vegetation zones, including mixed forest (540 to 750 m), coniferous forest (1100 to 1700 m), deciduous forest (1700 to 2000 m), and alpine tundra (above 2000 m) enzyme activities, were significantly higher in the mixed forest stand located between 540 and 750 m a.s.l compared to the other elevations. However, at altitudes above 1000 m, hardly any differences between microbial activities were observed [55]. Schinner [51] reported cellulase activity and basal respiration to clearly decrease with increasing altitude, whereas in the Italian Alps, the opposite was found [56].
We assume these contradictions to be explained by differences in the organic matter contents of the soils, which are known to strongly control the levels and activities of soil enzymes [57, 58]. On Mt. Schrankogel, we found most microbial activities to be significantly higher in soils of the alpine vegetation belt (2700 to 2900 m) compared to those of the nival vegetation belt (3200 to approximately 3500 m), while the activities were intermediate in the alpine-nival ecotone (3000 to 3100 m). Together with the observed significant positive relationships of CM-cellulase with C content, protease with N content, arylsulfatase and alkaline phosphatase with OM, this indicates that both quantity and quality of organic matter play a role in structuring these soil functions along the gradient studied in the present investigation. Hence, our results correspond to those of Sinsabaugh [59], who reported enzyme activities to be limited by the availability of C, N, S, and P. However, microbial activities along the gradient on Mt. Schrankogel were at least to some extent explained by mean annual soil temperature, although not predominately. Generally, the turnover rate of enzymes increases with rising temperatures as long as their structural stability is maintained. The positive relationship between enzyme activities and temperature shown in our study was confirmed by similar reports published earlier [60, 61]. Decreasing activities of arylsulfatase and phosphatase per biomass unit suggests low turnover at the nival locations and high turnover at the lower locations. Similarly, Siles et al. [56] found the highest specific activities of arylsulfatase at the lowest study locations (545 to 570 m a.s.l.). Phosphatase activities of soils were previously shown to be inhibited by inorganic P [62, 63], and phosphate inhibited the expression of PHO genes [64]. The differences of specific phosphatase activities along the slope of Mt. Schrankogel might be caused by these mechanisms.
Conclusions
Climate warming is predicted to be in the range of 1.1 to 6.4 °C by the end of the twenty-first century and is expected to severely affect plants, animals, and microorganisms in high-alpine environments. Our study shed light on the controversially discussed spatial distribution of archaeal and bacterial abundances as well as microbial activities involved in the main biogeochemical cycles. Different environmental factors were found to be correlated with abundance and activity. Abundances were most strongly correlated with soil temperature, whereas the enzyme activities were most strongly correlated with organic matter or C and N concentrations. Moreover, we observed that soil temperature had a greater impact on the abundance of archaea. Our results indicate that the abundance and activities related to the decomposition of organic matter might be increased with ongoing climate warming, either directly or indirectly, through alterations of plant cover and communities, which in turn could change the quantity and quality of nutrients released to the soil. The multiple linear regression models presented here may provide first insights into the extent to which future changes might influence the microbiota in soils of the European Alps.
References
Körner C (2007) The use of altitude in ecological research. Trends Ecol Evol 22:569–574
Diaz HF, Grosjean M, Graumlich L (2003) Climate variability and change in high elevation regions: past, present and future. Clim Change 59:1–4
Schröter D, Cramer W, Leemans R, Prentice IC, Araujo MB, Arnell NW, Bondeau A, Bugmann H, Carter TR, Gracia CA, de la Vega-Leinert AC, Erhard M, Ewert F, Glendining M, House JI, Kankaanpää S, Klein RJT, Lavorel S, Lindner M, Metzger MJ, Meyer J, Mitchell TD, Reginster I, Rounsevell M, Sabate S, Sitch S, Smith B, Smith J, Smith P, Sykes MT, Thonicke K, Thuiller W, Tuck G, Zaehle S, Zier (2005) Ecosystem service supply and vulnerability to global change in Europe. Sci 310:1333–1337
Rahbek C (2005) The role of spatial scale and the perception of large-scale species-richness patterns. Ecol Letters 8:224–239
McCain CM, Grytnes JA (2010) Elevational gradient in species richness. In: Encyclopedia of life sciences. John Wiley & Sons Ltd, Chichester. doi:10.1002/9780470015902.a0022548
Bryant JA, Lamanna C, Morlon H, Kerkhoff AJ, Enquist BJ, Green JL (2008) Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci U S A 105:05–11
Wang JT, Cao P, Hu HW, Li J, Han LL, Zhang LM, Zheng YM, He JZ (2015) Altitudinal distribution patterns of soil bacterial and archaeal communities along Mt. Shegyla on the Tibetan Plateau. Microb Ecol 69:135–145
Fierer N, McCain CM, Meir P, Zimmermann M, Rapp JM, Silman MR, Knight R (2011) Microbes do not follow the elevational diversity patterns of plants and animals. Ecol 92:797–804
Wang J, Soininen J, Zhang Y, Wang B, Yang X, Shen J (2011) Contrasting patterns in elevational diversity between microorganisms and macroorganisms. J Biogeogr 38:595–603
Singh D, Takahashi K, Kim M, Chun J, Adams JM (2012) A hump-backed trend in bacterial diversity with elevation on Mount Fuji, Japan. Microb Ecol 63:429–437
Woese CR, Fox GE (1977) Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A 74:5088–5090
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A 87:4576–4579
Timonen S, Bomberg M (2009) Archaea in dry soil environments. Phytochem Rev 8:505–518
Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C (2003) Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ Microbiol 5:787–797
Nicol GW, Tscherko D, Embley TM, Prosser JI (2005) Primary succession of soil Crenarchaeota across a receding glacier foreland. Environ Microbiol 7:337–347
Zhang LM, Wang M, Prosser JI, Zheng YM, He JZ (2009) Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. FEMS Microbiol Ecol 70:208–217
Siles JA, Margesin R (2016) Abundance and diversity of bacterial, archaeal. and fungal communities along an altitudinal gradient in alpine forest soil: what are the driving factors? Microb Ecol. doi:10.1007/s00248-016-0748-2
Pester M, Schleper C, Wagner M (2011) The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology. Curr Opin Microbiol 14:300–306
Pratscher J, Dumont MG, Conrad R (2011) Ammonia oxidation coupled to CO2 fixation by archaea and bacteria in an agricultural soil. Proc Natl Acad Sci U S A 108:4170–4175
Xia W, Zhang C, Zeng X, Feng Y, Weng J, Lin X, Zhu J, Xiong Z, Xu J, Cai Z, Jia Z (2011) Autotrophic growth of nitrifying community in an agricultural soil. ISME J 5:1226–1236
Zhang L-M, Offre PR, He J-Z, Verhamme DT, Nicol GW, Prosser JI (2010) Autotrophic ammonia oxidation by soil Thaumarchaeota. Proc Natl Acad Sci U S A 107:17240–17245
Berg IA, Kockelhorn D, Ramon-Vera WH, Say RF, Zarzycki J, Hügler M, Alber BE, Fuchs G (2010) Autotrophic carbon fixation in archaea. Nat Rev Microbiol 8:447–460
Hofmann K, Reitschuler C, Illmer P (2013) Aerobic and anaerobic microbial activities in the foreland of a receding glacier. Soil Biol Biochem 57:418–426
Prem E, Reitschuler C, Illmer P (2014) Livestock grazing on Alpine soils causes changes in abiotic and biotic soil properties and thus in abundance and activity of methanogenic Archaea. Eur J Soil Biol 62:22–29
Brankatschk R, Töwe S, Kleineidam K, Schloter M, Zeyer J (2010) Abundances and potential activities of nitrogen cycling microbial communities along a chronosequence of a glacier forefield. ISME J 5:1025–1037
Hofmann K, Illmer P (2015) Temporal patterns of prokaryotic abundance, community structure and microbial activity in glacier foreland soils. Antonie Van Leeuwenhoek 108:793–799
He JZ, Hu HW, Zhang LM (2012) Current insights into the autotrophic Thaumarchaeal ammonia oxidation in acidic soils. Soil Biol Biochem 55:146–154
Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (2013) Climate change 2013—the physical science basis (contribution of working group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change). Cambridge University Press, Cambridge
Böhm R, Auer I, Brunetti M, Maugeri M, Nanni T, Schöner W (2001) Regional temperature variability in the European Alps: 1760–1998 from homogenized instrumental time series. Int J Climatol 21:1779–1801
Jones PD, Moberg A (2003) Hemispheric and large-scale surface air temperature variations: an extensive revision and an update to 2001. J Climate 16:206–223
Pauli H, Gottfried M, Grabherr G (1999) Vascular plant distribution patterns at the low-temperature limits of plant life—the alpine-nival ecotone of Mount Schrankogel (Tyrol, Austria). Phytocoenologia 29:297–325
Pauli H, Gottfried M, Reiter K, Klettner C, Grabherr G (2007) Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994–2004) at the GLORIA*master site Schrankogel, Tyrol, Austria. Glob Change Biol 13:147–156
Djukic I, Zehetner F, Mentler A, Gerzabek MH (2010) Microbial community composition and activity in different Alpine vegetation zones. Soil Biol Biochem 42:155–161
Djukic I, Zehetner F, Watzinger A, Horacek M, Gerzabek M (2013) In situ carbon turnover dynamics and the role of soil microorganisms therein: a climate warming study in an Alpine ecosystem. FEMS Microbiol Ecol 83:112–124
Bradford MA (2013) Thermal adaptation of decomposer communities in warming soils. Front Microbio 4:333. doi:10.3389/fmicb.2013.00333
Hammer W (1929) Der granitische Kern der Stubaier Gruppe und seine Beziehungen zum Bau der Ötztaler Alpen. Jahrbuch der Kaiserlich-Königlichen Geologischen Reichsanstalt 79:87–128
Gottfried M, Pauli H, Grabherr G (1998) Prediction of vegetation patterns at the limits of plant life: a new view of the alpine-nival ecotone. Arctic Alpine Res 30:207–221
Gottfried M, Pauli H, Reiter K, Grabherr G (1999) A fine-scaled predictive model for changes in species distribution patterns of high mountain plants induced by climate warming. Divers Distrib 5:241–251
Schinner F, Öhlinger R, Kandeler E, Margesin R (1996) Methods in soil biology. Springer, Berlin Heidelberg
Heinemeyer O, Insam H, Kaiser EA, Walenzik G (1989) Soil microbial biomass measurements: an automated technique based on infra-red gas analysis. Plant Soil 116:191–195
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Yu Y, Lee C, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89:670–679
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microb 71:4117–4120
Quinn GP, Keough MJ (2011) Experimental design and data analysis for biologists., p 11
Margesin R, Jud M, Tscherko D, Schinner F (2009) Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol Ecol 67:208–218
Grabherr G, Gottfried M, Pauli H (2010) Climate change impacts in alpine environments. Geogr Compass 4:1133–1153
Kemnitz D, Kolb S, Conrad R (2007) High abundance of Crenarchaeota ina temperate acidic forest soil. FEMS Microbiol Ecol 60:442–448
Lucas RW, Casper BB, Jackson JK (2007) Soil microbial communities and extracellular enzyme activity in the New Jersey Pinelands. Soil Biol Biochem 39:2508–2519
Sigler WV, Zeyer J (2002) Microbial diversity and activity along the forefields of two receding glaciers. Microb Ecol 43:397–407
Ottow JCG (2011) Mikrobiologie von Böden. Springer, Berlin Heidelberg
Schinner F (1982) Soil microbial activities and litter decomposition related to altitude. Plant Soil 65:87–94
Insam H, Haselwandter K (1989) Metabolic quotient of the soil microflora in relation to plant succession. Oecologia 79:174–178
Sigler WV, Crivii S, Zeyer J (2002) Bacterial succession in glacial forefield soils characterized by community structure, activity and opportunistic growth dynamics. Microb Ecol 44:306–316
Burns RG, Dick RP (2002) Enzymes in the environment: activity, ecology and applications. Marcel Dekker, New York
Xu Z, Yu G, Zhang X, Ge J, He N, Wang Q, Wang D (2014) The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter ecomposition along the northern slope of Changbai Mountain. Appl Soil Ecol 86:19–29
Siles JA, Cajthaml T, Minerbi S, Margesin R (2016) Effect of altitude and season on microbial activity, abundance and community structure in Alpine forest soils. FEMS Microbiol Ecol 92:1–12
Stursova M, Baldrian P (2011) Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant Soil 338:99–110
Wallenius K, Rita H, Mikkonen A, Lappi K, Lindström K, Hartikainen H, Raateland A, Niemi RM (2011) Effects of land use on the level, variation and spatial structure of soil enzyme activities and bacterial communities. Soil Biol Biochem 43:1464–1473
Sinsabaugh RL (1994) Enzymic analysis of microbial pattern and process. Biol Fertil Soils 17:69–74
Niklinska M, Klimek B (2007) Effect of temperature on the respiration rate of forest soil organic layer along an elevation gradient in the Polish Carpathians. Biol Fertil Soils 43:511–518
Couteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Tree 10:63–66
Moscatelli MC, Lagomarsino A, De Angelis O, Grego S (2005) Seasonality of soil biological properties in a poplar plantation growing under elevated atmospheric CO2. Appl Soil Ecol 30:162–173
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochem 49:175–190
Oshima Y, Ogawa N, Harashima S (1996) Regulation of phosphatase synthesis in Saccharomyces cerevisiae—a review. Gene 179:171–177
Acknowledgments
We thank S. Farbmacher, N. Praeg, and A. O. Wagner for their help with the field work. KH thanks M. Huber and P. Wischounig for helpful discussions about statistics. This study was supported by the Austrian Climate Research Program (Project GZ B368633).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
ᅟ (DOCX 1970 kb)
Rights and permissions
About this article
Cite this article
Hofmann, K., Lamprecht, A., Pauli, H. et al. Distribution of Prokaryotic Abundance and Microbial Nutrient Cycling Across a High-Alpine Altitudinal Gradient in the Austrian Central Alps is Affected by Vegetation, Temperature, and Soil Nutrients. Microb Ecol 72, 704–716 (2016). https://doi.org/10.1007/s00248-016-0803-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0803-z