Abstract
Purpose
FDG PET is an established tool in presurgical epilepsy evaluation, but it is most often used selectively in patients with discordant MRI and EEG results. Interpretation is complicated by the presence of remote or multiple areas of hypometabolism, which leads to doubt as to the true location of the seizure onset zone (SOZ) and might have implications for predicting the surgical outcome. In the current study, we determined the sensitivity and specificity of PET localization prospectively in a consecutive unselected cohort of patients with focal epilepsy undergoing in-depth presurgical evaluation.
Methods
A total of 130 patients who underwent PET imaging between 2006 and 2015 matched our inclusion criteria, and of these, 86 were operated on (72% with a favourable surgical outcome, Engel class I). Areas of focal hypometabolism were identified using statistical parametric mapping and concordance with MRI, EEG and intracranial EEG was evaluated. In the surgically treated patients, postsurgical outcome was used as the gold standard for correctness of localization (minimum follow-up 12 months).
Results
PET sensitivity and specificity were both 95% in 86 patients with temporal lobe epilepsy (TLE) and 80% and 95%, respectively, in 44 patients with extratemporal epilepsy (ETLE). Significant extratemporal hypometabolism was observed in 17 TLE patients (20%). Temporal hypometabolism was observed in eight ETLE patients (18%). Among the 86 surgically treated patients, 26 (30%) had hypometabolism extending beyond the SOZ. The presence of unilobar hypometabolism, included in the resection, was predictive of complete seizure control (p = 0.007), with an odds ratio of 5.4.
Conclusion
Additional hypometabolic areas were found in one of five of this group of nonselected patients with focal epilepsy, including patients with “simple” lesional epilepsy, and this finding should prompt further in-depth evaluation of the correlation between EEG findings, semiology and PET. Hypometabolism confined to the epileptogenic zone as defined by EEG and MRI is associated with a favourable postoperative outcome in both TLE and ETLE patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most frequent chronic neurological disorders, with an estimated prevalence in the general population of 6.38 per 1,000 persons [1]. Approximately one-third of the patients are drug-resistant, and in these patients, surgery is the only treatment that offers the possibility of cure. Localizing the epileptogenic zone in presurgical patients requires a dedicated protocol of electrophysiological, structural and functional examinations, which often includes positron emission tomography (PET) imaging [2]. [18F]Fluorodeoxyglucose PET (FDG PET) using positron-emitting isotopes is usually performed as an interictal study. FDG PET contributes to the definition of the functional deficit zone [3] by revealing the area of reduced glucose metabolism. This zone can be larger than the epileptogenic cortex and can extend to remote areas [3, 4]. For example, in patients with a presumed seizure onset zone (SOZ) limited to the mesial temporal lobe due to hippocampal sclerosis (HS), the hypometabolism often involves the whole temporal lobe and even extends beyond [5,6,7]. The epileptogenic zone is localized by PET in up to 90% of patients with temporal lobe epilepsy (TLE) and in up to 60% of patients with extratemporal lobe epilepsy (ETLE) [8].
FDG PET is particularly useful in TLE without magnetic resonance imaging (MRI) abnormalities: patients with hypometabolism on PET concordant with EEG findings benefit from surgery as much as patients with HS identified on MRI [9, 10]. In patients with ETLE, FDG PET has been used as a guide in the search for subtle cortical dysplasia or to inform the placement of intracranial electrodes [11]. However, FDG PET can be misleading, as in some patients with ETLE, the hypometabolism is more prominently seen in the temporal lobe [8]. Factors reported to be associated with a poorer prognosis are both hypometabolism remote from the epileptogenic zone [12] and a less severe hypometabolism of the epileptogenic zone [13]. However, in most studies PET was used only in selected patients, e.g. those negative on MRI; therefore, the presence of remote hypometabolic areas could be due to the complexity of the case or lack of knowledge of seizure status on EEG before or during tracer injection.
The present study had two main aims:
-
1.
To compare patients with hypometabolism confined to the lobe of the SOZ and patients with ambiguous results, i.e. those with hypometabolism extending to other adjacent lobes or only in remote structures, or those with incorrectly normal findings.
-
2.
To determine the predictive value of the hypometabolic pattern observed, including a subset of patients treated surgically with a follow-up of at least 12 months.
Since all our patients underwent PET as part of the standard presurgical work-up, our series presents a unique opportunity to determine the yield of PET in an unselected group of patients with drug-resistant TLE or ETLE.
Materials and methods
Data collection and subjects
This study was based on a prospectively collected database at a single institution, including a total of 346 consecutive patients attending our epilepsy unit and undergoing PET imaging between 2006 and 2015. Informed consent was obtained from all individual participants included in the study and also from legally authorized representatives when the participant was a minor. This study was approved by the Geneva Cantonal Ethics Commission, and was in accordance with the principles of the Declaration of Helsinki and its further amendments.
We selected patients matching the following criteria:
-
Inclusion criteria:
-
1.
More than 6 years of age (younger children have a more extended diffuse hypometabolism, which could lead to false-positive findings [14, 15])
-
2.
Pharmacoresistant (persistence of seizures after treatment with at least two antiepileptic drugs at the maximal tolerated doses) and unifocal epilepsy based on clinical history, semiology and laboratory tests described below
-
3.
Presurgical evaluation consisting of at least MRI, FDG PET and long-term video-EEG recording
-
4.
PET scan acquired with a standardized protocol on a Siemens Biograph PET/CT tomograph (in our hospital since January 2006)
-
5.
No previous brain surgery
-
1.
-
Exclusion criteria: multilobar lesions visible on MRI, generalized or multifocal epilepsy, posttraumatic epilepsy, progressive tumour, seizures during the 24 h before tracer injection as determined by continuous monitoring before, during and after tracer injection [16]
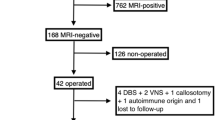
A total of 130 patients (86 with TLE, 44 with ETLE) matched the criteria and were included in the present analysis (Fig. 1), and 216 patients were excluded for the following reasons: not meeting inclusion criteria (n = 48), having exclusion criteria that could be associated with false-positive findings, namely multilobar lesions visible on MRI, including HS in patients with ETLE, generalized or multifocal nonlesional epilepsy, posttraumatic epilepsy and progressive tumour and seizures within 24 h before tracer injection (n = 109), or having incomplete data (n = 59).
Definition of hypometabolism
All PET images were analysed visually by one expert reader (V.G.) who was not aware of the presumed location of the epileptogenic zone based on clinical, EEG and MRI findings. All images were reanalysed after coregistration of the PET images to the patient’s individual MRI images on a Leonardo 3D reconstruction workstation (Siemens Healthineers). Statistical parametric mapping (SPM) using version 8 (SPM8; Wellcome Department of Cognitive Neurology, London, UK) was performed by comparing each individual PET image with a reference set of brain PET scans from 38 young healthy controls (20 men; mean age 35 years, range 18–53 years) obtained from CERMEP (Lyon; copyright CERMEP and the authors, 2014; all rights reserved) to identify areas with significantly reduced glucose metabolism, as previously described [17] and detailed in the section Statistical analysis. The PET images of the controls were acquired on an ECAT EXACT HR+ scanner, which has physical properties comparable to those of the Siemens Biograph scanner used in the patients [18].
EEG recordings
Conventional long-term video-EEG recording was performed in all patients with the standard clinical EEG set-up of 31–37 electrodes (10/10 system). The placement of additional inferior longitudinal temporal electrodes was extremely important because of their selectivity to temporal lobe activity. Impedances were kept below 10 kΩ, the sampling rate was 256 Hz and bandpass filters were set to 0.1 Hz and 120 Hz, with a vertex contact as the reference electrode. The recordings were obtained 24 h before tracer injection and included in the current analysis if no clinical or subclinical seizures occurred during EEG monitoring, which also included uptake of the tracer.
Magnetic resonance imaging
All patients had an MRI scan as part of presurgical evaluation. The scans were acquired on a 3-T Trio scanner (Siemens AG, Germany) and were performed according to the following standardized epilepsy protocol [19] using a 32-channel brain coil: (a) axial T2-weighted fast spin-echo, repetition time (TR) 7,520 ms, echo time (TE) 114 ms, voxel size 0.5 × 0.4 × 3 mm (slice thickness); (b) 3D FLAIR (fluid-attenuated inversion recovery), TR 5,000 ms, TE 419 ms, inversion time (TI) 1,800 ms, isotropic voxel size 0.9 × 0.9 × 0.9 mm; (c) 3D gradient echo T1 or T2, TR 1,750 ms, TE 2.29 ms, isotropic voxel size 0.7 × 0.7 × 0.7 mm; (d) diffusion-weighted imaging, TR 8,000 ms, TE 84 ms, 30 directions; and (e) axial arterial spin labelling, TR 4,000 ms, TE 12 ms, voxel size 3.4 × 3.4 × 4 mm (slice thickness). 3D images were analysed in the axial, coronal and sagittal planes. In 101 of 130 patients, the structural MRI scan showed a pathological result, indicating an epileptogenic lesion, specifically HS in 45 patients with TLE; the other 29 patients had normal MRI findings (12 with a final diagnosis of TLE).
Positron emission tomography
FDG PET was performed using 2-[18F]fluoro-2-deoxy-d-glucose in all patients in the interictal phase (at least 24 h after the last seizure). After fasting for at least 6 h, the patients received an injection of 200 MBq (or a weight-adapted dose in children, according to the EANM paediatric card; http://www.eanm.org/content-eanm/uploads/2017/01/EANM_Dosage_Card_040214.pdf) of [18F]FDG while resting in a quiet dimly lit room. In all patients, the EEG was continuously monitored during the 30 min uptake time to exclude subclinical seizures, that could have led to false-negative or false-positive findings. PET images were acquired on tomographs equipped with lutetium oxyorthosilicate crystals (Siemens Biograph Hi-Rez, TruePoint or mCT PET/CT systems) in 3D mode. The scan duration was 20 min.
Temporal lobe epilepsy and extratemporal lobe epilepsy
Localization of the epileptogenic zone was based on anatomoelectroclinical correlations (i.e. correlations between ictal semiology, electrophysiological activity and the structural lesion) [20] by a neurologist (F.P.) blinded to the results of the PET examination. By definition, patients with an epileptic focus in the mesial temporal lobe and/or temporal pole were considered to have TLE. The remaining patients were considered to have ETLE, that included lateral and posterior temporal, parietal, occipital and frontal lobe epilepsy.
Surgery
All cases were discussed in a weekly interdisciplinary case conference. Of the 130 patients, 86 (66%) underwent temporal or extratemporal surgical intervention, while 44 were not operated upon for different reasons: epileptic focus in eloquent areas with unclear cost–benefit from surgery (30 patients), severe psychiatric comorbidities interfering with the patient’s decision making (10 patients), and patient refusal of surgery after considering the risks (4 patients).
Performance of PET as a test
The epileptogenic zone was defined on the concordance of clinical information (semiology), and EEG and MRI findings. Intracranial EEG was performed in 30 patients (23% of the total sample; 29 operated) and was considered in the present analysis. Of the 29 patients with intracranial EEG, 14 were implanted with depth electrodes only, two were implanted with subdural electrodes only, and 12 were implanted with both depth and subdural electrodes. Bilateral explorations were performed in 20 of these patients, and were limited to one hemisphere in 9 patients.
Using standardized PET analysis with SPM, as described above, the degree of concordance between the PET results and the presumed SOZ was described as follows:
-
Concordant (C): hypometabolism concordant and confined to the lobe of the presumed SOZ
-
Concordant plus (C+): hypometabolism extends from the epileptogenic zone to adjacent lobes
-
Multifocal (M): multiple clusters of hypometabolism in nonadjacent lobes
-
Remote (R): hypometabolism located outside the presumed SOZ lobe
-
Normal (N): no significant hypometabolism, either at the presumed SOZ or elsewhere
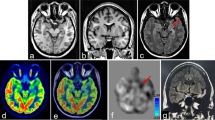
Analyses were performed by dichotomizing between patients with metabolic pattern C and those with the other metabolic patterns. The different paterns are shown in example TLE and ETLE patients in Fig. 2.
Metabolic patterns identified in temporal lobe epilepsy (TLE) and extratemporal lobe epilepsy (ETLE) in the study participants (colour bars indicate T values, individual maps are thresholded at p < 0.01, k > 40, R indicates right hemisphere, L indicates left hemisphere). The images indicate the presumed seizure onset zone (SOZ) location and the surgical outcome (Engel class) in surgically treated patients
Seizure outcome after epilepsy surgery
All 86 surgically treated patients were seen postoperatively by the neurosurgeon and neurologist or neuropaediatrician. The mean follow-up period was 30.5 ± 22.9 months (range 12–108 months). Seizure outcomes were classified according to the classification proposed by Engel et al. [21]:
-
Class I
Patients free of disabling seizures, specifically including:
-
Ia
Patients seizure-free after surgery
-
Ib
Patients with nondisabling simple partial seizures only since surgery
-
Ic
Patients with some disabling seizures after surgery, but free of disabling seizures for at least 2 years
-
Id
Patients with generalized convulsions with antiepileptic drug discontinuation only
-
Ia
-
Class II
Patients with rare disabling seizures (“almost seizure-free”, i.e. seizure decrease >90%)
-
Class III
Patients with worthwhile improvement (seizure decrease 50–90%)
-
Class IV
Patients with no worthwhile improvement (seizure decrease <50%)
In the present study, patients free of disabling seizures (Engel class I) were compared with all other patients (Engel classes II–IV).
Statistical analysis
PET images were processed using SPM8. After normalization to the CERMEP FDG PET template (copyright CERMEP and the authors, 2014; all rights reserved), with an isotropic voxel size of 2 mm and smoothing (8 mm), images from individual patients and controls were compared using a two-sample t test with proportional scaling to global activity and age as covariate. The resulting statistical parametric map was thresholded at p < 0.01 (uncorrected), and a minimum cluster extent of 40 voxels was considered, taking into account only true hypometabolic clusters, i.e. abnormalities with cortical locations and concordant with subsequent visual analysis of fused PET/MRI data [17]. Hypometabolic clusters were described quantitatively in terms of their size (number of voxels above the threshold) and severity (highest Z score in the cluster). The correlation between cluster size and severity of hypometabolism was also evaluated with different clinical variables using Spearman’s correlation.
Logistic regression analysis was used to predict the presence of a favourable outcome (Engel class I), including the type of epilepsy (TLE and ETLE) and the type of hypometabolism (metabolic pattern C versus the others) as variables in the analysis. The benefits of gender, age at onset, age at PET and lesional epilepsy as predictors were also tested.
Results
The main characteristics and demographic data of the 130 patients (66 women) are shown in Table 1. The mean age of the patient group was 27.4 ± 13.5 years (range 6–60 years), the mean duration of epilepsy was 15.0 ± 11.5 years (range 0–53 years), and the mean age at onset was 12.8 ± 10.5 years (range 0–46 years).
The epileptic lesions included HS (in 45 patients), cortical dysplasia (in 34, of whom 25 were treated surgically, including 15 with focal dysplasia type II, 3 with focal dysplasia type I, 3 with cortical dysplasia not otherwise specified, and 4 with mild malformations of cortical development), nonprogressive tumours (in 14, of whom 10 were treated surgically including 6 with a dysembryoplastic neuroepithelial tumour, 2 with oligodendroglioma WHO grade II, and 2 with ganglioglioma WHO grade I), polymicrogyria (in 1), hemimegalencephaly (in 1), posttraumatic lesion (in 2), vascular lesions (in 3) and postinfectious lesions (in 1).
Localizing value of PET
In TLE patients, including those treated and not treated surgically, both the sensitivity and specificity of interictal PET were 95%. In ETLE patients, the sensitivity was 80% and the specificity was 95%. Table 2 shows the distribution of metabolic patterns among TLE patients (with and without HS) and ETLE patients. Hypometabolism only at remote sites (metabolic pattern R) was found in only one patient with TLE and one with ETLE (1.5%; one lesional left temporal epilepsy with right frontal hypometabolism only, one with nonlesional right frontal epilepsy but bilateral temporal hypometabolism), i.e. this pattern is rarely observed. PET was entirely normal, i.e. without any hypometabolism, in 3 of 86 TLE patients (3%) and 8 of 44 ETLE patients (18%; p = 0.007), and in 5 of 101 patients (5%) with lesional and 6 of 29 patients (21%) with nonlesional epilepsy (p = 0.015).
There were no significant differences in the distribution of metabolic patterns, namely C, C+, M, R and N, between ETLE and TLE patients, nor between TLE patients with and without HS (p = 0.08 and p = 0.31, respectively). Analysis of the different hypometabolic regions in TLE patients showed that five patients had extratemporal hypometabolism in more than one area (22 hypometabolic foci in total). These were noted in the frontal, insular, parietal/temporal posterior and occipital cortex. Overall, remote hypometabolism was more frequent in the frontal lobe (12 of 22, 55%) and ipsilateral to the side of the temporal focus (18 of 22, 82%; an example is shown in Fig. 2). Among patients with HS, additional hypometabolism was observed outside the SOZ lobe in 14 of 45 patients with HS, most commonly contralateral temporal hypometabolism (7 patients). In ETLE patients, additional temporal hypometabolism was observed in 8 of 44 patients (18%), 5 of 8 (62%) ipsilateral and 3 of 8 (38%) bilateral. Temporal hypometabolism was associated with frontal epilepsy in three patients (one bilateral), with parietal epilepsy in two (one bilateral), and occipital epilepsy in three (one bilateral; Fig. 2).
No differences in age of onset, age at evaluation and duration of epilepsy were found in either TLE or ETLE patients between those with metabolic pattern C and those with the other patterns. There was no significant difference between TLE and ETLE patients regarding the cluster size of the hypometabolism corresponding to the SOZ (p = 0.245).
Predictive value of PET in surgically treated patients
A total of 86 patients were treated surgically (62 with TLE, 24 with ETLE), allowing the value of PET in predicting the postoperative outcome to be determined. Freedom from disabling seizures after surgery was achieved in 53 of 62 TLE patients (85%) and in 19 of 24 ETLE patients (79%; p = 0.477). Among surgically treated TLE patients, the hypometabolic patterns were distributed as follows: 43 pattern C (69%), 7 pattern C+ (11%), 11 pattern M (18%) and 1 pattern R (2%). Extratemporal hypometabolic areas (16 patients) were most frequently observed in the ipsilateral frontal lobe (7 patients, 44%). Among the ETLE patients, the hypometabolic patterns were distributed as follows: 16 pattern C (67%), 1 pattern C+ (4%), 5 pattern M (21%) and 2 pattern N (8%) Hypometabolic sites beyond the SOZ (8 patients) were most frequently seen in the ipsilateral temporal anterior region (5 patients, 62%). There were no significant differences between the frequency of fully concordant and other hypometabolic patterns between the TLE and ETLE patients.
The presence of unifocal hypometabolism limited to the epileptogenic zone (pattern C) had a more favourable predictive value than the remaining metabolic patterns (p = 0.007), with a high odds ratio of 5.4 (95% confidence interval 1.6–18.3; 6.2 for the TLE patients and 4.2 for the ETLE patients analysed separately). The distribution of metabolic patterns in relation to postoperative outcome is shown in Table 3. The odds ratio estimate was unchanged (5.4, 95% confidence interval 1.5–19.3) when gender, age at onset, age at PET and lesional vs. nonlesional epilepsy were included in the model.
Discussion
This prospective study in a large consecutive cohort showed two main findings: (1) hypometabolism beyond the presumed SOZ is not rare and occurs with a similar frequency in both TLE and ETLE patients who are surgical candidates, and (2) hypometabolism outside the SOZ has significant predictive value for surgical outcome.
Localizing value of PET abnormalities
In previous studies, PET has been found to provide localizing information in 60–90% of patients with TLE and in 30–60% of the patients with ETLE [8, 22,23,24,25,26,27,28,29]. Additional areas of hypometabolism are frequently reported and are seen in 10–43% of patients. However, these studies used PET in selected patient groups, so the true frequencies of additional hypometabolic foci and nonconcordant findings are not known.
In our study of nonselected patients, temporal hypometabolism was found in 18% of ETLE patients and extratemporal hypometabolism, mostly in the ipsilateral frontal lobe, in 20% of TLE patients [30,31,32]. These values are at the lower end of the frequencies of nonlocalizing findings previously reported, that range from 20% to 67% [8, 30]. Apart from the selection criteria, the studies differed in analysis tools and patient populations (e.g. number of children or ETLE patients). We stress that in contrast to the present study, many previous studies did not offer EEG monitoring before and during cerebral tracer absorption [15, 24, 27], and thus the presence of subclinical seizures is unknown and could have added to the variability of the findings.
The occurrence of additional and remote abnormalities in focal epilepsy represents a diagnostic dilemma, and occurs in approximately one in five patients. This calls for meticulous comparison of the site of the PET abnormality with the semiology, structural anomalies, and ictal and interictal EEG findings. It is of note that even in our 130 patients with “simple” lesional focal epilepsy, 5 (4%) had an entirely normal PET scan.
The precise relationship between SOZ and PET hypometabolism has been studied in patients with intracranial electrodes, and a complex association has been shown. Two studies in paediatric patients undergoing subdural EEG monitoring showed that the ictal onset electrodes were located over the most hypometabolic area as well as within 2 to 3 cm of adjacent regions. However, a large proportion of hypometabolic regions had no involvement in early seizures [33, 34]. A previous study in cavernous angioma suggested deafferentation as a possible mechanism [35].
Predictive value of remote metabolic abnormalities for surgical outcome
The number of patients without seizures after surgery found in this study compares favourably with the numbers found in previous studies [36, 37]. In our series, the pattern of hypometabolism carried predictive information for surgical outcome, in agreement with the majority of previous studies, which are summarized in Table 4. Overall, the majority of studies in patients with TLE concordantly showed that patterns of hypometabolism on PET carry predictive information, with more severe hypometabolism in the SOZ and the absence of hypometabolism beyond the SOZ associated with a better prognosis [13, 25, 30, 38,39,40,41,42,43]. In contrast, the results in ETLE patients are discordant, with four studies showing significant predictive value of a positive localization on PET [12, 26, 44, 45] and two studies failing to show predictive value [46, 47].
In this study, hypometabolism confined to the lobe of the SOZ (pattern C), assessed using a combined visual and semiquantitative approach, was a significant predictor of a seizure-free postoperative outcome in both TLE and ETLE patients. Importantly, in our series, gender, age at onset, age at PET and the presence of a lesion on MRI had no additional predictive value.
Possible explanations for a worse outcome if remote and nonconcordant hypometabolism are present could include diffuse seizure spreading or incorrect presurgical localization of the resected region. Fast propagation to other areas, as determined by EEG, usually indicates a more pathological and widespread network, with a lower chance of benefiting from surgery. This might be due to the type of preexisting epilepsy or a consequence of longstanding epilepsy. Additional areas of hypometabolism might then reflect these constitutional or acquired conditions. We are not aware of prospective studies using follow-up PET in patients with chronic epilepsy to distinguish between these two conditions. Alternatively, additional or remote hypometabolism represents the true focus, despite its smaller size, and harbours subtle structural anomalies. Independent of the underlying pathophysiological mechanism, our results suggest that incongruent findings between the PET localization and SOZ as determined by EEG and semiology should be considered as “red flags”. They call for further investigations if there is no epileptogenic lesion on MRI with concordant semiology, including electric source localization, ictal SPECT or intracranial EEG.
Non-FDG PET tracers as predictors of surgical outcome
PET tracers other than FDG have been tested, mainly in research settings, in the presurgical evaluation of epilepsy, targeting other pathophysiological and neurotransmission pathways, for example GABAergic, glutamatergic, serotonergic and dopaminergic neurotransmission or neuroinflammation, as recently reviewed [48, 49]. Only a minority of studies, however, have specifically tested the predictive value of these tracers for surgical outcome, mainly in TLE. An association was found between a worse postoperative outcome and an increase in binding of [11C]flumazenil, a PET radioligand targeting GABA-A, in the periventricular white matter in patients with TLE and HS, possibly due to the presence of an increased concentration of heterotopic neurons [50, 51]. The probability of TLE patients being seizure-free has also been associated with reduced serotonin 1A receptor density, as measured with 18F-FCWAY, and reduced metabotropic glutamate receptor type 5 density, as measured with 11C-ABP688 [52, 53]. A study with 11C-alpha-methyl-tryptophan, a tracer of serotonin synthesis which helps identify epileptogenic tubers in patients with tuberous sclerosis, showed that the resection of tubers with a higher uptake is a good predictor of surgical outcome [52, 54]. Finally, one study investigated the expression of P-glycoprotein (Pgp), which can be associated with drug-resistant epilepsy, using the tracer [11C]verapamil: an optimal surgical outcome was associated with higher Pgp function before surgery and larger Pgp reduction postoperatively [55].
Conclusion
Extratemporal hypometabolism was found in 20% of TLE patients and temporal hypometabolism in 18% of ETLE patients, i.e. additional hypometabolic areas were found in about one in five patients, and were more frequently observed in the same hemisphere (82% for ipsilateral extratemporal hypometabolism in TLE patients, 73% for ipsilateral temporal hypometabolism in ETLE patients). These abnormalities are not only diagnostically challenging but also predict a negative outcome in TLE and ETLE patients, as they are associated with a greater risk of disabling seizures after surgery. Our prospective study confirms previous retrospective observations in TLE and ETLE suggesting that careful analysis of the metabolic pattern, also beyond the presumed SOZ, has predictive value, even in patients with “simple” lesional epilepsy.
Abbreviations
- FDG:
-
18F-Fluorodeoxyglucose
- PET:
-
Positron emission tomography
- MRI:
-
Magnetic resonance imaging
- TLE:
-
Temporal lobe epilepsy
- ETLE:
-
Extratemporal lobe epilepsy
- EEG:
-
Electroencephalography
- SOZ:
-
Seizure onset zone
- SPM:
-
Statistical parametric mapping
References
Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303.
Pittau F, Grouiller F, Spinelli L, Seeck M, Michel CM, Vulliemoz S. The role of functional neuroimaging in pre-surgical epilepsy evaluation. Front Neurol. 2014;5:31.
Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124(Pt 9):1683–700.
Engel J Jr, Brown WJ, Kuhl DE, Phelps ME, Mazziotta JC, Crandall PH. Pathological findings underlying focal temporal lobe hypometabolism in partial epilepsy. Ann Neurol. 1982;12(6):518–28.
Henry TR, Roman DD. Presurgical epilepsy localization with interictal cerebral dysfunction. Epilepsy Behav. 2011;20(2):194–208.
Nelissen N, Van Paesschen W, Baete K, Van Laere K, Palmini A, Van Billoen H, et al. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2006;32(2):684–95.
Foldvary N, Lee N, Hanson MW, Coleman RE, Hulette CM, Friedman AH, et al. Correlation of hippocampal neuronal density and FDG-PET in mesial temporal lobe epilepsy. Epilepsia. 1999;40(1):26–9.
Rathore C, Dickson JC, Teotónio R, Ell P, Duncan JS. The utility of 18F-fluorodeoxyglucose PET (FDG PET) in epilepsy surgery. Epilepsy Res. 2014;108(8):1306–14.
LoPinto-Khoury C, Sperling MR, Skidmore C, Nei M, Evans J, Sharan A, et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia. 2012;53(2):342–8.
Carne RP, O'Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127(Pt 10):2276–85.
Chassoux F, Landré E, Mellerio C, Turak B, Mann MW, Daumas-Duport C, et al. Type II focal cortical dysplasia: electroclinical phenotype and surgical outcome related to imaging. Epilepsia. 2012;53(2):349–58.
Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, et al. Relationship between preoperative hypometabolism and surgical outcome in neocortical epilepsy surgery. Epilepsia. 2012;53(8):1333–40.
Guedj E, Bonini F, Gavaret M, Trébuchon A, Aubert S, Boucekine M, et al. 18FDG-PET in different subtypes of temporal lobe epilepsy: SEEG validation and predictive value. Epilepsia. 2015;56(3):414–21.
Kumar A, Juhász C, Asano E, Sood S, Muzik O, Chugani HT. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med. 2010;51(12):1901–7.
Lee JJ, Kang WJ, Lee DS, Lee JS, Hwang H, Kim KJ, et al. Diagnostic performance of 18F-FDG PET and ictal 99mTc-HMPAO SPET in pediatric temporal lobe epilepsy: quantitative analysis by statistical parametric mapping, statistical probabilistic anatomical map, and subtraction ictal SPET. Seizure. 2005;14(3):213–20.
Leiderman DB, Albert P, Balish M, Bromfield E, Theodore WH. The dynamics of metabolic change following seizures as measured by positron emission tomography with fludeoxyglucose F 18. Arch Neurol. 1994;51(9):932–6.
Archambaud F, Bouilleret V, Hertz-Pannier L, Chaumet-Riffaud P, Rodrigo S, Dulac O, et al. Optimizing statistical parametric mapping analysis of 18F-FDG PET in children. EJNMMI Res. 2013;3(1):2.
Trebossen R, Comtat C, Brulon V, Bailly P, Meyer ME. Comparison of two commercial whole body PET systems based on LSO and BGO crystals respectively for brain imaging. Med Phys. 2009;36(4):1399–409.
Vargas MI, Becker M, Garibotto V, Heinzer S, Loubeyre P, Gariani J, et al. Approaches for the optimization of MR protocols in clinical hybrid PET/MRI studies. MAGMA. 2013;26(1):57–69.
Bancaud J. Surgery of epilepsy based on stereotactic investigations – the plan of the SEEG investigation. Acta Neurochir Suppl (Wien). 1980;30:25–34.
Engel J Jr, Van Ness P, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr (editor) Surgical treatment of the epilepsies. 2nd ed. New York: Raven Press; 1993. p. 609–21.
Theodore WH, Sato S, Kufta C, Balish MB, Bromfield EB, Leiderman DB. Temporal lobectomy for uncontrolled seizures: the role of positron emission tomography. Ann Neurol. 1992;32(6):789–94.
Kim SK, Lee DS, Lee SK, Kim YK, Kang KW, Chung CK, et al. Diagnostic performance of [18F]FDG-PET and ictal [99mTc]-HMPAO SPECT in occipital lobe epilepsy. Epilepsia. 2001;42(12):1531–40.
Meyer PT, Cortés-Blanco A, Pourdehnad M, Levy-Reis I, Desiderio L, Jang S, et al. Inter-modality comparisons of seizure focus lateralization in complex partial seizures. Eur J Nucl Med. 2001;28(10):1529–40.
Salanova V, Markand O, Worth R. Focal functional deficits in temporal lobe epilepsy on PET scans and the intracarotid amobarbital procedure: comparison of patients with unitemporal epilepsy with those requiring intracranial recordings. Epilepsia. 2001;42(2):198–203.
Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58(4):525–32.
Uijl SG, Leijten FS, Arends JB, Parra J, van Huffelen AC, Moons KG. The added value of [18F]-fluoro-D-deoxyglucose positron emission tomography in screening for temporal lobe epilepsy surgery. Epilepsia. 2007;48(11):2121–9.
Kim SK, Na DG, Byun HS, Kim SE, Suh YL, Choi JY, et al. Focal cortical dysplasia: comparison of MRI and FDG-PET. J Comput Assist Tomogr. 2000;24(2):296–302.
Mendes Coelho VC, Morita ME, Amorim BJ, Ramos CD, Yasuda CL, Tedeschi H, et al. Automated online quantification method for (18)F-FDG positron emission tomography/CT improves detection of the epileptogenic zone in patients with pharmacoresistant epilepsy. Front Neurol. 2017;8:453.
Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, et al. The topography and significance of extratemporal hypometabolism in refractory mesial temporal lobe epilepsy examined by FDG-PET. Epilepsia. 2010;51(8):1365–73.
Takaya S, Hanakawa T, Hashikawa K, Ikeda A, Sawamoto N, Nagamine T, et al. Prefrontal hypofunction in patients with intractable mesial temporal lobe epilepsy. Neurology. 2006;67(9):1674–6.
Lieb JP, Dasheiff RM, Engel J Jr. Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991;32(6):822–37.
Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87(1):77–87.
Jeong JW, Asano E, Kumar Pilli V, Nakai Y, Chugani HT, Juhász C. Objective 3D surface evaluation of intracranial electrophysiologic correlates of cerebral glucose metabolic abnormalities in children with focal epilepsy. Hum Brain Mapp. 2017;38:3098–112.
Ryvlin P, Mauguière F, Sindou M, Froment JC, Cinotti L. Interictal cerebral metabolism and epilepsy in cavernous angiomas. Brain. 1995;118(Pt 3):677–87.
Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525–37.
Tellez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2-3):310–8.
Radtke RA, Hanson MW, Hoffman JM, Crain BJ, Walczak TS, Lewis DV, et al. Temporal lobe hypometabolism on PET: predictor of seizure control after temporal lobectomy. Neurology. 1993;43(6):1088–92.
Knowlton RC, Laxer KD, Ende G, Hawkins RA, Wong ST, Matson GB, et al. Presurgical multimodality neuroimaging in electroencephalographic lateralized temporal lobe epilepsy. Ann Neurol. 1997;42(6):829–37.
Dupont S, Semah F, Clémenceau S, Adam C, Baulac M, Samson Y. Accurate prediction of postoperative outcome in mesial temporal lobe epilepsy: a study using positron emission tomography with 18fluorodeoxyglucose. Arch Neurol. 2000;57(9):1331–6.
Struck AF, Hall LT, Floberg JM, Perlman SB, Dulli DA. Surgical decision making in temporal lobe epilepsy: a comparison of [(18)F]FDG-PET, MRI, and EEG. Epilepsy Behav. 2011;22(2):293–7.
Higo T, Sugano H, Nakajima M, Karagiozov K, Iimura Y, Suzuki M, et al. The predictive value of FDG-PET with 3D-SSP for surgical outcomes in patients with temporal lobe epilepsy. Seizure. 2016;41:127–33.
Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, et al. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003;30(4):581–7.
Kim DW, Lee SK, Moon HJ, Jung KY, Chu K, Chung CK. Surgical treatment of nonlesional neocortical epilepsy: long-term longitudinal study. JAMA Neurol. 2017;74(3):324–31.
Desarnaud S, Mellerio C, Semah F, Laurent A, Landre E, Devaux B, et al. (18)F-FDG PET in drug-resistant epilepsy due to focal cortical dysplasia type 2: additional value of electroclinical data and coregistration with MRI. Eur J Nucl Med Mol Imaging. 2018;45(8):1449–60.
Kim DW, Lee SK, Yun CH, Kim KK, Lee DS, Chung CK, et al. Parietal lobe epilepsy: the semiology, yield of diagnostic workup, and surgical outcome. Epilepsia. 2004;45(6):641–9.
Kogias E, Klingler JH, Urbach H, Scheiwe C, Schmeiser B, Doostkam S, et al. 3 tesla MRI-negative focal epilepsies: presurgical evaluation, postoperative outcome and predictive factors. Clin Neurol Neurosurg. 2017;163:116–20.
Sarikaya I. PET studies in epilepsy. Am J Nucl Med Mol Imaging. 2015;5(5):416–30.
Galovic M, Koepp M. Advances of molecular imaging in epilepsy. Curr Neurol Neurosci Rep. 2016;16(6):58.
Hammers A, Koepp MJ, Brooks DJ, Duncan JS. Periventricular white matter flumazenil binding and postoperative outcome in hippocampal sclerosis. Epilepsia. 2005;46(6):944–8.
Yankam Njiwa J, Bouvard S, Catenoix H, Mauguiere F, Ryvlin P, Hammers A. Periventricular [(11)C]flumazenil binding for predicting postoperative outcome in individual patients with temporal lobe epilepsy and hippocampal sclerosis. Neuroimage Clin. 2013;3:242–8.
Theodore WH, Martinez AR, Khan OI, Liew CJ, Auh S, Dustin IM, et al. PET of serotonin 1A receptors and cerebral glucose metabolism for temporal lobectomy. J Nucl Med. 2012;53(9):1375–82.
Lam J, DuBois JM, Rowley J, González-Otárula KA, Soucy JP, Massarweh G, et al. In vivo metabotropic glutamate receptor type 5 abnormalities localize the epileptogenic zone in mesial temporal lobe epilepsy. Ann Neurol. 2019;85(2):218–28.
Kagawa K, Chugani DC, Asano E, Juhász C, Muzik O, Shah A, et al. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with alpha-[11C]methyl-L-tryptophan positron emission tomography (PET). J Child Neurol. 2005;20(5):429–38.
Bauer M, Karch R, Zeitlinger M, Liu J, Koepp MJ, Asselin MC, et al. In vivo P-glycoprotein function before and after epilepsy surgery. Neurology. 2014;83(15):1326–31.
Acknowledgments
J.T. was supported by the “Egas Moniz” grant of the Neurology Portuguese Society. M.S. was supported by SNF 113766, SNF 180365, SNF 163398. Statistical help was provided by the Clinical Research Center, Geneva University Hospitals (Prof. Thomas Perneger). The reference database was provided by the CERMEP-Imagerie du vivant, Lyon, France.
Author information
Authors and Affiliations
Contributions
J.T., M.S. and V.G. contributed to the concept and study design. M.S. and F.Picard. were responsible for patient management. J.T., F.Pittau, F.Picard, M.I.V., M.S. and V.G. contributed to data acquisition and analysis. J.T. and V.G. drafted the manuscript, tables and figures. F.Pittau, A.H., S.B., F.Picard, M.I.V., F.S. and M.S. contributed to critical revision of the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
This study was approved by the Geneva Cantonal Ethics Commission, and was in accordance with the principles of the Declaration of Helsinki and its further amendments.
Conflicts of interests
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology.
Rights and permissions
About this article
Cite this article
Tomás, J., Pittau, F., Hammers, A. et al. The predictive value of hypometabolism in focal epilepsy: a prospective study in surgical candidates. Eur J Nucl Med Mol Imaging 46, 1806–1816 (2019). https://doi.org/10.1007/s00259-019-04356-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04356-x