Abstract
Chest radiographs and CT scans have been the cornerstone of pulmonary imaging given their advantages of being rapid and easily available techniques. However, a significant concern with their use in the pediatric population is the associated ionisation radiation. The use of magnetic resonance imaging (MRI) in pulmonary imaging has lagged behind its adoption in other organ systems. Previously, the lung parenchyma was considered difficult to evaluate by magnetic resonance due to low proton density in the pulmonary tissue, susceptibility artefacts within the lungs, and respiratory motion artefacts. However, in recent years, there have been a multitude of technical advancements to overcome these limitations. MRI can be an excellent radiation-free alternative in patients who require protracted follow-up like in cases such as cystic fibrosis, complicated pneumonias, tuberculosis and mediastinal neoplasms. An added advantage of MRI is that it can provide functional information in addition to the structural information provided by traditional imaging techniques. One of the major reasons of limited use of MRI despite its established utility is the lack of clarity regarding its indications, and a paucity of data on tailored MRI protocols customised to clinical needs. This article aims to review the basic MRI techniques, indications and terminologies used in chest imaging, with special emphasis on imaging findings of common pathologies in the pediatric population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chest radiograph remains the primary imaging modality for imaging respiratory disorders in children and adults. Ultrasonography (USG) has emerged as a useful complimentary modality especially in emergency, out-patient clinics and bedside settings. However cross-sectional imaging is often required in complicated cases and also for pre-surgical planning. Chest computed tomography (CT) has formed the corner stone of pulmonary imaging. Given its rapid acquisition, superior spatial resolution and easy accessibility, it has been the investigation of choice for cross -sectional pulmonary imaging over the last few decades. However a major drawback of CT is the associated ionisation radiation. The radiation issues are even more serious in the pediatric population where the risk of antecedent radiation related malignancies has been shown to be significantly high [1].
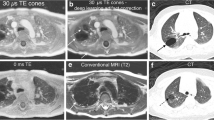
A radiation free alternative that has superseded the value of CT in other parts of the body is magnetic resonance imaging (MRI). MRI offers superior contrast resolution, high spatial resolution as well as functional and chemical information about tissues. Its use in pulmonary imaging has however been limited by a number of factors. First, MRI is based on the precession of hydrogen protons. The signal is directly proportional to the proton density of tissues. As the predominant content of lungs is air, the proton density of lung parenchyma is low. Another significant impediment in the use of MR in pulmonary imaging is the susceptibility different between air and tissues. Due to the large difference in susceptibility between air and tissues, there is rapid decay in signal. In addition, there is constant motion of the lungs and the beating heart to contend with. Current generation CT machines have the ability of covering the entire lung parenchyma within seconds. MR scanners do not offer the same speed. Every sequence in MR takes a few minutes to acquire and hence cardiac and respiratory motion causes artefacts to appear in the images [2]. However, recently advances have been made in MR imaging such as parallel imaging, shared echo-technique and rotating phase encoding which have helped circumvent some of these issues. Currently, there are breath-hold sequences available which can be acquired in few seconds. The pros and cons of CT and MRI are listed in Table 1 and depicted in Fig. 1.
Pros and cons of CT and MRI. CT (a – lung window b–mediastinal window) provides better spatial resolution and better delineation of the intraparenchymal airways (arrows in a) without any artefacts. MRI however offers superior contrast resolution with a T1 hyperintense (c), T2 hypointense (d) mucous plug (asterisk) in the right middle lobe (RML) with diffusion restriction at places (arrows in e) s/o superadded allergic bronchopulmonary aspergillosis (ABPA) /inspissated secretions. Bronchial wall thickening is also well seen esp on the STIR images (arrowheads in f)
MR Techniques
Basic MRI protocol consists of a fast breath-hold T1, T2 weighted images and a free breathing steady state free precession (SSFP) sequence (Fig. 2). This bouquet of sequences provides the basic anatomic and pathologic information usually required within minimum scan duration. Additional sequences can be added to this mix to improve the diagnostic confidence and provide functional information if required. Contrast enhanced/post gadolinium images can provide vital information about enhancement of lymph nodes/lung masses in addition to delineating the pulmonary vasculature.
In patients with respiratory difficulties, in infants and small children, respiratory triggered T2 weighted sequences can be used instead of breath-hold sequences. Short tau inversion recovery (STIR) sequence can be used to suppress the background fat and hence increase the contrast between the pathology and normal surrounding tissues. At this time, role of diffusion weighted imaging (DWI) is still under the scanner, though it may have a role in predicting invasiveness of malignancies and separating a mass from atelectasis. It can also help demarcate lesions adjacent to the pleura, however its utility in differentiating benign from malignant lymph nodes or lung lesions is still controversial [3]. The extended MRI protocol is shown in Fig. 3.
Sequences included in the extended MRI protocol. (a) Axial T1PG, (b) STIR, (c) DWI and (d) coronal T1PG images. The diffusion images highlighting the nodule in the RML and the mediastinal lymph nodes including a periesophageal node not well seen on routine sequences (asterisk). The lymph nodes show peripheral contrast enhancement s/o necrosis
Indications
Indications for Chest MRI can be broadly divided into mediastinal and pulmonary ones. Although MRI can be used for most pathologies where a CT is indicated, there are a few indications where MRI scores over CT (Table 2), and vice versa. The main pathologies encountered where MRI is being utilised are discussed below.
Indications where MRI offers tangible advantages over CT includes characterisation of mediastinal masses and for the evaluation of their extent (mediastinal/chest wall/spinal invasion) and for evaluation of respiratory mechanics (e.g., in cases of tracheobronchomalacia). In patients with deranged renal function tests, use of gadolinium based contrasts is now restricted, similar to iodinated contrast in CT. However, due to its superior contrast resolution, MRI can demonstrate majority of mediastinal and pulmonary lesions well even without contrast. Indications where CT still scores over MRI include evaluation of pulmonary micronodules (< 3 mm) and interstitial lung disease (ILD) [3, 4].
Role of MRI in Mediastinal Pathologies
The utility of MRI in the evaluation of mediastinal lesions is better established compared to its lung applications, and its role in common scenarios is discussed below.
Congenital Masses
MRI is superior to CT in demonstrating the spinal communication in cases with neurenteric cysts. In bronchogenic cysts and esophageal duplication cysts, MRI demonstrates the lesions as well as CT. Cervico-thoracic lesions (e.g., lymphatic malformations) and thoraco-abdominal lesions (e.g., few duplication cysts) are well mapped out on MRI [5].
Thymic Lesions
It is well recognised that the differentiation between normal thymus, thymic hyperplasia and tumors involving thymus (e.g., lymphoma, thymoma) can be challenging. USG is useful as it shows the typical ‘speckled/starry sky’ pattern of normal thymus. MRI also allows confident differentiation amongst these entities on chemical shift MRI; normal thymus and hyperplasia show drop in signal in out-of-phase images, unlike tumors. Also tumors demonstrate restriction on diffusion weighted images. In clinical scenarios such as patients of lymphoma receiving chemotherapy; with treatment the tumor regresses while thymus may show a rebound thymic hyperplasia. In such situations, MRI is immensely useful in making the differentiation between recurrence of lymphoma and thymic hyperplasia [6]. Benign lesions like thymolipomas are also well demonstrated by using fat saturation images (Fig. 4).
Tumors
In posterior mediastinal tumors, the superiority of MRI over CT is acknowledged. This is due to its ability to demonstrate neural foraminal/spinal involvement in neurogenic tumors. Its role in thymic mass lesions has already been alluded to above. For evaluation of germ cell tumors, both CT and MRI are equally accurate (Fig. 5). Another application of MRI in aggressive round cell tumors (e.g., neuroblastoma), is the use of whole body MRI concurrently for detecting metastases [7]. At times, the nature of mediastinal tumors may not be obvious. MRI can be a problem solving tool in such cases with the T1/T2 contrast, characteristics of the mass on other sequences such as diffusion imaging providing a clue to the diagnosis (Fig. 6).
Anterior mediastinal mass. A young boy presented with progressive shortness of breath. CT images showed a low density homogenous mass in the anterior mediastinum with encasement of the great vessels (a). MRI showed STIR hyperintense lobular soft tissue in the retroperitoneum (arrows in b) which showed patchy areas of mild to moderate diffusion restriction (c, d), features odd for lymphoma. Biopsy proved the mass to be kaposiform hemangioendothelioma
Infections
Mediastinal collections/abscesses and associated osseous involvement are well delineated on MRI. Its role in tubercular nodes will be discussed subsequently in the article.
Role of MRI in Pulmonary Imaging
There have been several recent publications on the use of MRI for diverse pulmonary pathologies, especially using ‘rapid-lung MRI’ protocols [8].
Cystic Fibrosis (CF)
CF is one of the most frequent indications for repeated thoracic imaging in children. This is because imaging is critical to the assessment of disease progression. While compared to CT, MRI definitely suffers from the limitation of not being able to demonstrate fine anatomic details such as early peripheral bronchiectasis and mosaic attenuation. However it is felt that the detection of these changes may not really alter management strategies [9,10,11].
On the other hand, contrast enhanced MRI offers the advantage of being able to demonstrate inflamed bronchial walls even in the presence of mucous plugging while it is not possible on CT [9]. Superadded infection in airways or parenchyma is well appreciated on both CT and MRI. In case of superadded allergic bronchopulmonary aspergillosis (ABPA), CT shows high attenuation material (HAM), while these appear hypointense on T2 WI. MRI thus has the limitation of underestimating the disease in these situations. The presence of mucus with both high T1 and low T2 signal intensities, described as the inverted mucoid impaction signal (IMIS) sign has been shown to have 94% sensitivity and 100% specificity in diagnosing ABPA in CF patients, hence awareness of this sign is important while interpreting MRI in CF patients [12].
The structural changes on MRI in CF patients are described in Table 3 and Figs. 1 and 7. [9]
Pneumonia
While CXR remains the primary imaging modality in children with suspected lower respiratory tract infections (LRTI), its limitations in this clinical setting are well recognised [13]. It should hence be supplemented with ultrasound (USG) in case of large areas of consolidation that are likely to reach up to chest wall, suspected pleural effusion/empyema and for upper mediastinal lymph nodes. However, cross-sectional imaging is required in several situations where USG is unable to provide complete answers. Lung MRI has the potential to replace CT in this clinical setting [14, 15]. MRI may play a role even in atypical pulmonary infections like hydatid cyst where repeated imaging may be necessary for follow-up and surgical planning (Fig. 8).
Hydatid cyst. A 15-y-old patient who was operated case of pulmonary hydatid presented with acute onset hemoptysis. CT at the time of presentation showed complete collapse of the right lung (a). Post contrast MR images done at a later date for surgical planning show a well defined peripherally enhancing hydatid cyst in the medial basal segment of the right lower lobe with heterogeneous contents within (b)
Imaging findings in pneumonia are summarised in Table 4 and shown in Figs. 9 and 10.
Tuberculosis
While CXR remains the primary imaging modality for the diagnosis and follow- up of children with thoracic TB, several patients require a further CT scan. CT scan is performed in cases with equivocal diagnosis on CXR, complications and non-response/partial response to treatment. In those patients requiring second-line drugs or MDR regimes, the treatment is prolonged, and more number of follow-up scans are required. In these instances, radiation risk of CT scan becomes a major limitation. MRI has the potential of being the preferred diagnostic tool in this group of patients. Studies have demonstrated that MRI is distinctly superior to CXR in the delineation of pulmonary and pleural abnormalities as well as nodes [16].
Compared to CT, MRI shows comparable sensitivity and specificity in detection of most abnormalities except small nodules and ground glass nodules, wherein CT is superior to MRI. However missing small or soft nodules may not impact management in most cases [16]. Areas of consolidation and cavitation are well visualised on MRI (Fig. 11).
Active nodes in TB show central non-enhancing areas on contrast enhanced magnetic resonance (CEMR) imaging. On T2WI, liquefied areas appear hyperintense, however, nodes can display intermediate/low T2 signal (Fig. 12). While assessment of activity of nodes requires contrast administration, follow-up of size of nodes and most parenchymal abnormalities can be done reasonably even without contrast. Hence, every follow-up scan need not be contrast enhanced. As in CT, homogenous nodes may also be seen in active TB. However if necrotic nodes become homogeneous on follow-up especially if accompanied by appearance of perinodal fat, it is indicative of response [17].
Tuberculosis: Enlarged prevascular and upper paratracheal lymph nodes (arrows showing areas of STIR hyperintensity (a), peripheral contrast enhancement (b) and diffusion restriction (c,d) within s/o necrosis. Coronal BTFE images (e) are useful in visualising enlarged subcarinal and right hilar lymph nodes. Incidentally multiple splenic granulomas were seen in the abdominal sections [(f) axial BTFE]
Immunocompromised Host
Patients with primary/acquired immunodeficiencies comprise another group of patients requiring repeated chest imaging [18]. A study by Ozcan et al. in children with iatrogenic immunosuppression and febrile neutropenia showed that MRI had a diagnostic accuracy of 80.9% in detecting abnormalities in these patients. MRI was equivalent/nearly equivalent to CT in demonstrating consolidation, atelectasis, nodules more than 5 mm in size, mediastinal nodes and pleural effusion. MRI however showed lower sensitivity in detecting small nodules and ground glass opacities [19]. More than iatrogenic immunosuppression, the potential of MRI lies in the evaluation of children with HIV/AIDS and primary immunodeficiency disorders (PIDD).
Congenital Anomalies
Congenital anomalies of the thorax (bronchopulmonary foregut malformation) are a complex group of disorders requiring evaluation of pulmonary artery, pulmonary veins and aorta; in addition to the lung parenchyma and airway. CT angiography is the optimal imaging modality for complete assessment of these disorders. However, often when children undergo CT scans with symptoms such as recurrent infections or hemoptysis, CT angiography protocols are not followed. Reviews of these CT scans suggest the diagnosis but do not provide sufficient information for surgical planning/confident diagnosis. Instead of performing a repeat CT scan; MRI offers a good alternative for further evaluation of affected children. MR angiography demonstrates pulmonary arterial hypoplasia/agenesis, aortic arch anomalies, as well as anomalous systemic arterial supply (Figs. 13 and 14)and [20]. Also, diaphragmatic defects are well delineated in patients with smaller diaphragmatic defects who often present at an older age [21].
Pleural Pathologies
Pleural effusion can be easily seen on MRI. Simple pleural effusions can be seen as T2 hyperintense, T1 hypointense fluid in the pleural space without any septae or loculation (Fig. 15). The appearance of empyema on CEMR is similar to that on CT with the split pleura sign. There is thickening and enhancement of the parietal and visceral pleura with T2 bright fluid separating them. Septations and debris may be seen within the pleural space which leads to heterogeneity within the fluid [14].
Chest Wall Pathologies
Common chest wall pathologies include abscesses and tumors. Chest wall abscesses can be pyogenic, fungal or tubercular. Abscesses show peripheral enhancement with central diffusion restriction. Surrounding soft tissues show T2 hyperintensity and heterogeneity. Presence of central fluid signal (T2 hyperintense/T1 hypointense) indicates drainable component within a collection. Common sites of tubercular involvement include sternoclavicular joint, costochondral junctions and ribs. Early joint and marrow changes are better depicted on MRI than CT.
Chest wall tumors can be benign or malignant. Although there are features which may help in making a distinction between the two but radiologic manifestations of benign and malignant chest wall tumors can overlap. Well defined margins, presence of fat and calcification generally indicate benignity whereas infiltrative margins, bony destruction and pleural effusion may indicate malignant potential of the mass. MRI, given its superior contrast resolution can identify fat within benign masses like dermoid cysts and lipomas, targetoid appearance of neurofibromas as well as blood fluid levels of aneurysmal bone cysts. Malignant lesions like Ewing’s sarcoma show a soft tissue mass with adjacent bony destruction. Again, being a radiation free modality, MRI can help in follow-up and response evaluation in these masses.
Conclusions
Hence, MRI is perhaps an underutilized tool in pediatric chest imaging; comparable or even superior to CT in several situations. CT, however, retains its superiority in providing fine details of lung parenchyma and airways. Critical to chest MRI is the planning and tailoring of protocols, to minimize procedure times and optimise the use of contrast.
References
Sodhi KS, Lee EY. What all physicians should know about the potential radiation risk that computed tomography poses for paediatric patients. Acta Paediatr. 2014;103:807–11.
Wild JM, Marshall H, Bock M, et al. MRI of the lung (1/3): methods. Insights Imag. 2012;3:345–53.
Biederer J, Beer M, Hirsch W, et al. MRI of the lung (2/3). Why ... When ... How? Insights Imag. 2012;3:355–71.
Biederer J, Mirsadraee S, Beer M, et al. MRI of the lung (3/3)-current applications and future perspectives. Insights Imaging. 2012;3:373–86.
Jeung MY, Gasser B, Gangi A, et al. Imaging of cystic masses of the mediastinum. Radiographics. 2002;22:S79–93.
McInnis MC, Flores EJ. Shepard J-AO, Ackman JB. Pitfalls in the imaging and interpretation of benign thymic lesions: how thymic MRI can help. Am J Roentgenol. 2015;206:W1–8.
Ackman JB. MR imaging of mediastinal masses. Magn Reson Imaging Clin North Am. 2015;23:141–64.
Sodhi KS, Khandelwal N, Saxena AK, et al. Rapid lung MRI in children with pulmonary infections: time to change our diagnostic algorithms. J Magn Reson Imaging. 2016;43:1196–206.
Nagle SKPM, Eichinger M, Altes TA. Magnetic resonance imaging of the lung: cystic fibrosis. In: Kauczor HU, Wielpütz MO, editors. MRI of the Lung.Medical radiology. Berlin, Heidelberg: Springer; 2017. p. 1–15.
Sileo C, Corvol H, Boelle PY, et al. HRCT and MRI of the lung in children with cystic fibrosis: comparison of different scoring systems. J Cystic Fibrosis. 2013;13:198–204.
Teufel M, Ketelsen D, Fleischer S, et al. Comparison between high-resolution CT and MRI using a very short echo time in patients with cystic fibrosis with extra focus on mosaic attenuation. Respiration; Int Rev Thoracic Dis. 2013;86:302–11.
Dournes G, Berger P, Refait J, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis: MR imaging of airway mucus contrasts as a tool for diagnosis. Radiology. 2017;285:261–9.
Yikilmaz A, Koc A, Coskun A, Ozturk MK, Mulkern RV, Lee EY. Evaluation of pneumonia in children: comparison of MRI with fast imaging sequences at 1.5T with chest radiographs. Acta Radiol. 2011;52:914–9.
Liszewski MC, Gorkem S, Sodhi KS, Lee EY. Lung magnetic resonance imaging for pneumonia in children. Pediatr Radio. 2017;47:1420–30.
Peltola V, Ruuskanen O, Svedstrom E. Magnetic resonance imaging of lung infections in children. Pediatr Radiol. 2008;38:1225–31.
Sodhi KS, Sharma M, Saxena AK, Mathew JL, Singh M, Khandelwal N. MRI in thoracic tuberculosis of children. Indian J Pediatr. 2017;84:670–6.
Bhalla A, Goyal A, Guleria R, Gupta A. Chest tuberculosis: radiological review and imaging recommendations. Indian J Radiol Imaging. 2015;25:213–25.
Sodhi KS, Khandelwal N, Saxena AK, et al. Rapid lung MRI - paradigm shift in evaluation of febrile neutropenia in children with leukemia: a pilot study. Leuk Lymphoma. 2016;57:70–5.
Ozcan HN, Gormez A, Ozsurekci Y, et al. Magnetic resonance imaging of pulmonary infection in immunocompromised children: comparison with multidetector computed tomography. Pediatr Radiol. 2017;47:146–53.
Liszewski MC, Hersman FW, Altes TA, et al. Magnetic resonance imaging of pediatric lung parenchyma, airways, vasculature, ventilation, and perfusion: state of the art. Radiol Clin North Am. 2013;51:555–82.
Tiddens HAWM, Kuo W, Van Straten M, Ciet P. Paediatric lung imaging: the times they are a-changin'. Eur Respir Rev. 2018;27. https://doi.org/10.1183/16000617.0097-2017.
Author information
Authors and Affiliations
Contributions
SK: Literature search, manuscript preparation; ASB: Concept, framework of the article and will act as guarantor for the paper; MJ: Concept and proof reading. ASB will act as guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kapur, S., Bhalla, A.S. & Jana, M. Pediatric Chest MRI: A Review. Indian J Pediatr 86, 842–853 (2019). https://doi.org/10.1007/s12098-018-02852-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-018-02852-w