Abstract
Background
Ultrasound (US) has been used in the adult trauma population with reported moderate to high sensitivities, but data are scarce in the pediatric trauma population.
Objective
The purpose of this study was to specifically examine the sensitivity and specificity of one lung US methodology (single-point anterior exam) in the pediatric trauma population when compared to chest radiography or CT.
Materials and methods
We conducted a retrospective review of pediatric trauma patients who received lung US as an extension of the focused assessment with sonography for trauma (FAST) exam. We compared lung US findings with chest radiography and CT scans.
Results
Two hundred twenty-six pediatric trauma patients underwent lung US exam with confirmatory exams; 11 pneumothoraces (4.8%) were observed. Of those 11, 6 were evaluated as false negatives on the lung US. Analyses resulted in 45.5% sensitivity, 98.6% specificity and 96.0% accuracy. Pneumothoraces undetected by lung US were small and apical and were likely not observed because of their size and location. None of the false negatives required intervention. All true positives were associated with lung contusions.
Conclusion
Pneumothorax is less common in the pediatric than the adult trauma population, and when encountered in children pneumothorax is often occult and might be associated with lung contusions. Existing evidence supports the usefulness of chest US in detecting pneumothorax in adults and suggests that it can be translated to injured children. However, our findings suggest that the sensitivity of lung US as a single-point anterior exam extension of the FAST exam might not be as reliable in the pediatric trauma population as in adults. Other methodologies using lung US might improve sensitivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the United States, trauma leads to greater mortality than all other causes combined in children older than 1 year [1]. Mortality from thoracic injuries, which can result from blunt and penetrating mechanisms, ranges 6–20% [2]. Thoracic injuries commonly present as rib fractures, pulmonary contusions and pneumothoraces [3]. Advanced Trauma Life Support [4] teaches that chest radiography is an important adjunct following the primary survey of the injured child, to aid in detection of traumatic pneumothorax, a preventable cause of death.
Chest radiography, typically performed early in the course of evaluating trauma patients, is often obtained in an anteroposterior manner in the supine patient undergoing initial assessment and in whom potential spine injury is a concern. It is rapid, almost universally available, does not require moving the patient to a diagnostic suite, allows ready assessment of lungs, pleura, bones, soft tissues and mediastinum, and allows for venous access lines and tubes. This technique is known to have high specificity for detecting pneumothorax; however, sensitivity is variable (58.9% to 98.2%) [5, 6]. Thus the reference standard for pneumothorax diagnosis has become chest CT [7, 8] because the small and asymptomatic pneumothorax can evolve into life-threatening complications, especially in people requiring positive-pressure ventilation, or those with limited cardiopulmonary reserve capacity [3]. However, CT exposes people to more radiation than chest radiography and requires transport to a diagnostic suite.
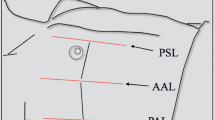
Lung ultrasound (US) was first used for detecting pneumothorax in humans in 1987 [9]. This diagnostic test is portable, non-radiating, repeatable, noninvasive and relatively inexpensive, thus potentially useful in detecting pneumothorax. The lung US technique typically involves using B-mode with or without M-mode to evaluate the pleura at the most anti-dependent portion of the thorax in a supine child, generally the 2nd–4th intercostal space anteriorly at the midclavicular line. With breathing, the visceral–parietal interface can be visualized as a sliding echogenic stripe below the intercostal space [10]. The presence of sliding pleura essentially excludes pneumothorax in the region that is scanned [11] (Fig. 1, Supplementary online material 1 [12]). Because absence of sliding pleura is highly suggestive but not diagnostic of pneumothorax [12], findings are generally reported in our institution as presence or absence of sliding pleura (Fig. 2, Supplementary online material 2).
Ultrasound in a 14-year-old boy with sliding pleura. Sagittal lung ultrasound image shows B-lines (“lung rockets;” arrows), which are considered comet tail artifact, extending from the pleural edge and observed in the normal aerated lung. In addition, as shown on Supplementary online material 1, the pleura is seen sliding below the ribs
Sonographically detecting pneumothorax has been studied among cohorts of trauma patients [5, 8, 13, 14]; however, each of these studies excluded patients younger than 18 years. While US has been used to assess lungs in the pediatric critical care environment [15], few studies have examined the use of US in managing pediatric chest trauma with potential for pneumothorax. Brook et al. [16] and Knudtson et al. [17] included some pediatric patients in their mixed study samples.
Alternative imaging that decreases exposure to ionizing radiation is increasingly important to the pediatric population [18]. Thus the purpose of this study was to examine the sensitivity and specificity of a single-point lung US exam when compared to chest radiographs and CT scans in a pediatric trauma cohort at a community-based Level II pediatric trauma center.
Materials and methods
Our institutional review board approved this retrospective study. We searched the trauma registry and medical records of pediatric patients who received a lung US exam in conjunction with focused assessment with sonography for trauma (FAST) scan [8]. The study sample included children (ages 0–17 years) identified in the trauma registry and treated between May 1, 2016, and Sept. 21, 2017. We excluded children from analysis if they: (1) did not have a lung US/FAST assessment, (2) did not have complete data in the dataset or (3) did not have confirmatory chest radiography or CT scans.
Each trauma patient underwent a FAST exam and the US was extended to a single-point exam of each lung. The US exams were performed in the trauma bay by US technicians who had completed standard training, using a 2- to 5-MHz curved transducer on a GE Logiq E (GE Healthcare, Boston, MA) US unit preset to abdominal imaging. The imaging depth was reduced to 4–6 cm and the gain was adjusted to accentuate the hyperechoic pleural stripe. A single-point chest US was performed on each side with the probe placed in an anterior sagittal position perpendicular to the ribs at the 2nd–3rd intercostal space at the mid-clavicular line. The child was preferably in supine position, although semi-upright position was acceptable in children who were unable to maintain a supine position. In those cases, the probe was placed below the clavicles. Live interpretation was performed by a radiology resident or attending physician. The sliding pleura was the only parameter evaluated and was described in a binary fashion. Other findings described in the literature such as B-lines, M-mode, lung point and lung pulse were not evaluated.
Lung US exams with positive findings (absence of pleural slide) were recorded as “positive” and exams with negative findings (presence of pleural slide) were recorded as “negative.” Lung US findings were confirmed as true or false by chest radiography or CT scans. The size of the pneumothorax (PTX) was retrospectively calculated by measuring the interpleural distance using PTX=4.1 + [4.7 x (A+B+C)] [19, 20]. For the purpose of this study, children who did not have confirmatory follow-up tests were considered as negative per clinical outcome [8, 21] and evaluated to determine whether there were demographic and clinical differences from those who did receive a confirmatory exam; they were excluded from other analyses.
We summarized descriptive statistics using frequencies (percentages) and means (standard deviations) where appropriate. Mean comparison was evaluated using an independent samples t-test with P<0.05 defined as statistically significant. We performed statistical analyses using SPSS for Windows, version 23 (IBM, Armonk, NY). We confirmed sensitivity, specificity and accuracy analyses using MedCalc (MedCalc Software, Ostend, Belgium). We conducted a sub-analysis restricted to children whose pneumothoraces extended below the clavicle (excluding those with only apical pneumothoraces that were not interrogated by the US transducer). We calculated positive/negative likelihood ratios and positive/negative predictive values using MedCalc Software.
Results
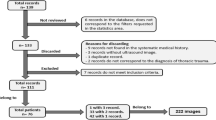
We identified 750 pediatric trauma patients in the trauma registry during the study time period. Three hundred thirty-six (44.8%) of patients were excluded because they did not have lung US/FAST exam, leaving 414 eligible for the study. Of those, 11 (2.6%) children were excluded for missing/incomplete data. Of the remaining 403 study patients, 177 (44%) children whose lung US findings were not confirmed with either a chest radiograph or a CT scan were excluded from sensitivity, specificity and accuracy analyses (clinical observation only group). These children had statistically significantly lower Injury Severity Scores, lower incidence of motor vehicle collisions, and lower hospital and intensive care lengths of stay than those with confirmatory exams. The remaining 226 (56%) were included in the sensitivity and specificity analysis. Patient flow is illustrated in Fig. 3. The mean age was 9.4 years (standard deviation [SD] = 5.8 years) and over half (59.7%) were boys. Patient demographics are listed in Table 1 [22].
Table 2 displays the clinical characteristics of the study population. The mean Injury Severity Score for the total study population was 6.8 (SD=7.4); however those who had follow-up confirmatory tests had a statistically significant higher Injury Severity Score (8.3) than those who were observed (4.9). The median Glasgow Coma Scale for the total study population was 15, the same as each sub-group — those with confirmatory tests (15) and those who were observed (15). The mean length of stay for both hospital and intensive care unit was statistically higher for children who received confirmatory tests than those who were just observed clinically.
We calculated sensitivity, specificity and accuracy analyses (Table 3) for only those who had confirmatory tests (chest radiograph or CT scan; n=226). Sensitivity was low (using only single-point evaluation) in this population, with the lung US only identifying 45% of the pneumothoraces. Specificity and accuracy were high: 98.6% and 96.0%, respectively. When the two apical pneumothorax patients were removed from analysis, sensitivity was only improved to 55%. Those children whose lung US were negative (sliding pleura noted) and observed with no confirmatory imaging were discharged without adverse event. For 15 children who received a CT scan, the determination results were 1 true positive, 11 true negatives and 3 false negatives for 25% sensitivity, 100% specificity and 80% accuracy.
Table 4 describes the characteristics of the children with confirmed pneumothoraces. There were 11 confirmed pneumothoraces out of the entire pediatric trauma population in the study timeframe (1.5%; 11/750). The pneumothorax sizes ranged 5–18% overall. The average size of the pneumothorax of the true positives was 11% vs. 8% for false negatives. Half of false-negative exams demonstrated apical pneumothoraces above the level of the clavicle, whereas all true-positive exams demonstrated loss of the visceral–parietal apposition along the mid-anterior chest. All of the true positives, but none of the false negatives, had pulmonary contusions. None of the false negatives required intervention. Three children were readmitted within 30 days. Of those, two were deemed true negatives confirmed with chest radiographs. One child with a false-negative finding was readmitted within 30 days with a retained hemothorax. There was no difference in the body mass index between the true-positive group (21.8 kg/m2, SD=6.5 kg/m2) and the false-negative group (21.8 kg/m2, SD=2.7 kg/m2).
Discussion
Chest US has been gaining popularity because of reports of superior sensitivity compared to the chest radiograph and ultrasonography’s desirable safety profile. Despite the growing body of evidence supporting its use in adults, the use of US for detecting pneumothoraces in the pediatric trauma population is not as well evidenced. Thus we conducted this single-institution retrospective analysis of one technique (single-point exam extended from FAST exam) in using lung US in the pediatric trauma population. Our findings suggest that pneumothorax in the pediatric trauma population is uncommon (1.5%) and associated with chest injury (evidenced by lung contusions in the true positives). Further, the findings of this single-point exam study demonstrated lower sensitivity of lung US than previously published findings.
The superior sensitivity of lung US over chest radiography in the detection of traumatic pneumothorax has been demonstrated in prospective studies [5, 8, 22, 23], with reported sensitivities ranging from 58.9% to 98.2%. Published evidence-based guidelines give recommendations for using lung US for ruling in the diagnosis of pneumothorax and for ruling it out, compared to the use of supine anterior chest radiography [24]. A recent systematic review and meta-analysis suggested that chest sonography is useful for detecting pneumothorax in injured adults [25]; however, none of these prospective studies [5, 8, 26] involved children younger than 16 years. Knudtson et al. [17] studied patients from 6 months to 94 years of age and observed a sensitivity of 92.3% in lung US for detecting pneumothorax when compared to chest radiograph; however none of the children younger than 17 years received chest CT (J. Knudtson, personal communication), thus it is possible the actual sensitivity of lung US could be lower in their study when compared to the gold standard of chest CT. Brook et al. [16] prospectively studied lung US in trauma patients ranging from 6 months to 88 years. While their study also demonstrated the superior sensitivity of lung US over chest radiography, when compared to the gold standard of chest CT the sensitivity of lung US was 47%. In both of these studies, the pediatric experience is difficult to tease out. A 2011 review of pediatric critical care sonography recognized the potential role of lung US in pediatric trauma [27], as did a large prospective study of pediatric occult pneumothorax in 2014 [3]. Marin et al. [15] recently reviewed the evidence undergirding point-of-care US. Extrapolating mostly from adult literature concerning US use in critical care in addition to neonatal intensive care unit studies of sonographic pneumothorax detection [28, 29], these authors formulated curricula and guidelines for reporting the implementation of US in pediatric emergency medicine. Even more recently, the American College of Emergency Physicians guidelines advocated use of lung US for pneumothorax detection in injured children [30].
It seems important to recognize that existing evidence indicates that lung US is employed for the exclusion of significant or large pneumothorax in the supine trauma patient because of its consistently high specificity [31]. Exclusion of pneumothorax only requires one sonographic finding: detection of pleural slide [24, 31]. Conversely, lack of pleural sliding alone does not establish the diagnosis of pneumothorax; rather, all the sonographic signs entailed in the full lung US exam are required: absent lung sliding, detection of B-lines (“lung rockets”), lung pulse and lung point [32]. The small pneumothorax not seen on initial supine chest radiography, known as the occult pneumothorax, remains an entity of uncertain clinical significance [33], especially in the pediatric patient [3]. However, the potential for deterioration of even the small pneumothorax makes detecting these desirable, despite our findings and those of others [3], suggesting that observation for the asymptomatic patient appears to be safe. The apical or trace pneumothorax is more readily detectable by CT scan compared to lung US, so a false-negative US for pneumothorax can be expected to result when occult pneumothorax is present because the US probe only reflects conditions at the site directly underneath the probe; this could be especially true in the focused lung US single-point examinations, as performed in this study.
Most of our false-negative results were from trace pneumothoraces: two were apical to probe placement; two were small and medial to the location of probe placement (Figs. 2 and 4). The remaining two were small to moderate in size, and these were located lateral to where the probe was placed. False negatives might also be a limitation of exam methodology (single probe placement per institutional protocol), which has been noted in other studies [13, 17] (Figs. 5 and 6, Supplementary online material 3). It has been suggested that a more comprehensive exam could yield increased sensitivity [11]. Toward this end, a two-stage lung US scan might achieve this. The initial sonographic assessment would serve to rapidly rule out large pneumothorax during the primary survey; clinical conditions permitting, the subsequent detailed secondary survey would include an increased number of chest areas surveilled [34].
Bilateral pneumothoraces with associated pulmonary contusion (arrows) in a 14-year-old boy. a Axial CT image of the upper chest shows a small right pneumothorax with preserved pleural contact, illustrating a potential pitfall when using single-point lung US evaluation (arrowheads). b Axial CT image of the lower chest reveals the pleura is displaced, demonstrating absence of pleural sliding in the US (arrowheads). In this case the lung ultrasound was positive
False-negative lung ultrasound in a 16-year-old girl. The pleural line was sliding, as demonstrated in Online supplementary material 3. Sagittal ultrasound image shows lines that represent an artifact of normal lung parenchema (arrows). The corresponding CT (Fig. 6), however, demonstrated a tiny apical pneumothorax
CT in a 16-year-old girl with false-negative lung ultrasound (by single-point exam), the same girl as in Fig. 5. a Axial CT image of the chest demonstrates trace pneumothorax (arrow) in the sonographically occult posterior lower chest. b Axial CT image shows a left apical pneumothorax isolated medially in the upper chest in the same girl (arrowhead). These findings were unlikely to be detected by the single-point lung ultrasound (arrows)
Pitfalls for lung US detection of pneumothorax in trauma are known. Failure to detect lung sliding can result from inappropriate technique, probe choice or dynamic filter settings [31]. Contusions can present with a lack of pleural slide [5, 16]; interestingly, all of our true-positive lung US exams had lung contusions present. Subcutaneous emphysema [17, 35] and contraction of chest muscles during spontaneous respirations [36] can interfere with analysis of pleural lines, as can mainstem intubation [37], which is common among pediatric patients [38] and seems to occur more commonly in smaller patients [39].
There is also variability in the performance of the examination for pneumothorax, the practitioner, and choice of US probe type and frequency. Examining the two studies involving injured children, Knudtson et al. [17] employed a 2.5- to 4-MHz phased-array probe to examine one site on the anterior chest. In contrast, Brook et al. [16] used a 3.5-MHz sector array to interrogate two sites per hemithorax. An overview of trauma ultrasonography recommends a 10-MHz probe [40]. In their review of the literature, Alrajab et al. [41] documented the variability found in published studies with regard to operator, US signs sought, and probe type used. It is interesting to note that the US operator in most of the studies in their meta-analysis were bedside clinicians.
Our study has several limitations. US technicians were at varying levels of experience. Radiologists interpreting comparative exams were not necessarily unaware of US findings. This was an initial experience in evaluating lung US in injured children for our trauma program, which is appropriate for evaluating institutional practices for quality assurance. Further, the incidence of occult pneumothorax in our population was lower than that found in the literature [3].
Currently, no established guidelines for the use of lung US in the detection of pediatric traumatic pneumothorax exist, despite its promotion [3, 15, 27] and reported adoption [16, 17]. Future study of the current practice of pediatric trauma centers involving lung US for pneumothorax is needed to address the deficits in the literature (such as multi-point exams or differences between ultrasound technicians and bedside clinicians). Other unanswered questions are the curriculum and training required, and whether US should be used in a standardized fashion in pediatric trauma and placed in the hands of bedside clinicians — which in the United States increasingly include advanced practice practitioners — and even pre-hospital personnel.
Conclusion
Pneumothorax is uncommon in the pediatric trauma population and when encountered, is often occult. It can be associated with lung contusions. Chest US has been increasingly demonstrated to be useful in detecting pneumothorax in injured adults and has been suggested as an appropriate strategy for injured children, but without clear guidelines. This study evaluated one methodology of incorporating lung ultrasound, a single-point exam anteriorly positioned as an extension of the focused assessment with sonography for trauma exam, for the detection of pneumothoraces. Our findings suggest that the sensitivity of lung US, via this methodology, is not as reliable in the pediatric trauma population as it is in adults. Future research should examine effectiveness of differing methodologies of lung US for the detection of pneumothoraces in the injured pediatric population.
References
Hamilton BE, Hoyert DL, Martin JA et al (2013) Annual summary of vital statistics: 2010–2011. Pediatrics 131:548–555
Tovar JA, Vazquez JJ (2013) Management of chest trauma in children. Paediatr Respir Rev 14:86–91
Lee LK, Rogers AJ, Ehrlich PF et al (2014) Occult pneumothoraces in children with blunt torso trauma. Acad Emerg Med 21:440–448
Subcommittee ATLS (2013) Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg 74:1363–1366
Blaivas M, Lyon M, Brannam L et al (2005) Feasibility of FAST examination performance with ultrasound contrast. J Emerg Med 29:307–311
Ball CG, Kirkpatrick AW, Laupland KB et al (2005) Factors related to the failure of radiographic recognition of occult posttraumatic pneumothoraces. Am J Surg 189:541–546
Alrajhi K, Woo MY, Vaillancourt C (2012) Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 141:703–708
Kirkpatrick AW, Sirois M, Laupland KB et al (2004) Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the extended focused assessment with sonography for trauma (EFAST). J Trauma Acute Care Surg 57:288–295
Azad A, Juma S, Bhatti J, Dankoff J (2015) Validity of ultrasonography to diagnosing pneumothorax: a critical appraisal of two meta-analyses. CJEM 17:199–201
Wernecke K, Galanski M, Peters PE, Hansen J (1987) Pneumothorax: evaluation by ultrasound — preliminary results. J Thorac Imaging 2:76 –78
Goodman TR, Traill ZC, Phillips AJ et al (1999) Ultrasound detection of pneumothorax. Clin Radiol 54:736–739
Lichtenstein DA (2007) Ultrasound in the management of thoracic disease. Crit Care Med 35:S250–S261
Dulchavsky SA, Schwarz KL, Kirkpatrick AW et al (2001) Prospective evaluation of thoracic ultrasound in the detection of pneumothorax. J Trauma Acute Care Surg 50:201–205
Nandipati KC, Allamaneni S, Kakarla R et al (2011) Extended focused assessment with sonography for trauma (EFAST) in the diagnosis of pneumothorax: experience at a community based level I trauma center. Injury 42:511–514
Marin JR, Abo AM, Arroyo AC et al (2016) Pediatric emergency medicine point-of-care ultrasound: summary of the evidence. Crit Ultrasound J 8:16
Brook OR, Beck-Razi N, Abadi S et al (2009) Sonographic detection of pneumothorax by radiology residents as part of extended focused assessment with sonography for trauma. J Ultrasound Med 28:749–755
Knudtson JL, Dort JM, Helmer SD, Smith RS (2004) Surgeon-performed ultrasound for pneumothorax in the trauma suite. J Trauma Acute Care 56:527–530
Riccabona M (2008) Ultrasound of the chest in children (mediastinum excluded). Eur Radiol 18:390–399
Kircher LT, Swartzel RL (1954) Spontaneous pneumothorax and its treatment. JAMA 155:24–29
Collins CD, Lopez A, Mathie A et al (1995) Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis measurements from helical CT. AJR Am J Roentgenol 165:1127–1130
Di Bartolomeo S, Sanson G, Nardi G et al (2001) A population-based study on pneumothorax in severely traumatized patients. J Trauma Acute Care Surg 51:677–682
Williams K, Thomson D, Seto I et al (2012) Standard 6: age groups for pediatric trials. Pediatrics 129:S153–S160
Rowan KR, Kirkpatrick AW, Liu D et al (2002) Traumatic pneumothorax detection with thoracic US: correlation with chest radiography and CT — initial experience. Radiology 225:210–214
Volpicelli G, Elbarbary M, Blaivas M et al (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38:577–591
Staub LJ, Biscaro RRM, Kaszubowski E, Maurici R (2018) Chest ultrasonography for the emergency diagnosis of traumatic pneumothorax and haemothorax: a systematic review and meta-analysis. Injury 49:457–466
Soldati G, Testa A, Pignataro G et al (2006) The ultrasonographic deep sulcus sign in traumatic pneumothorax. Ultrasound Med Biol 32:1157–1163
Srinivasan S, Cornell TT (2011) Bedside ultrasound in pediatric critical care: a review. Pediatr Crit Care Med 12:667–674
Raimondi F, Rodriguez Fanjul J, Aversa S et al (2016) Lung ultrasound for diagnosing pneumothorax in the critically ill neonate. J Pediatr 175:74–78
Cattarossi L, Copetti R, Brusa G, Pintaldi S (2016) Lung ultrasound diagnostic accuracy in neonatal pneumothorax. Can Respir J 2016:6515069
American College of Emergency Physicians (2017) Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med 69: e27-354
Lichtenstein DA Meziere G Lascols N et al (2005) Ultrasound diagnosis of the occult pneumothorax. Crit Care Med 33:1231-1238
Volpicelli G (2017) Lung ultrasound in pneumothorax: the continuing need for appropriate use and correct interpretation. J Emerg Med 53:e25–e26
Ball CG, Kirkpatrick AW, Feliciano DV (2009) The occult pneumothorax: what have we learned? Can J Surg 52:E173–E179
Lichtenstein DA (2014) Lung ultrasound in the critically ill. Ann Intensive Care 4:1–12
Gillman LM, Ball CG, Panebianco N et al (2009) Clinician performed resuscitative ultrasonography for the initial evaluation and resuscitation of trauma. Scand J Trauma Resusc Emerg Med 17:34
Zhang M, Liu ZH, Yang JX et al (2006) Rapid detection of pneumothorax by ultrasonography in patients with multiple trauma. Crit Care 10:R112
Kristensen MS, Teoh WH, Graumann O, Laursen CB (2014) Ultrasonography for clinical decision-making and intervention in airway management: from the mouth to the lungs and pleurae. Insights Imaging 5:253–279
Bai W, Golmirzaie K, Burke C et al (2016) Evaluation of emergency pediatric tracheal intubation by pediatric anesthesiologists on inpatient units and the emergency department. Paediatr Anaesth 26:384–391
Hipolito RB, Milstein JM, Sherman J, Sherman MP (2015) Neonatal endotracheal tubes and prevention of bronchial intubation. J Pediatr Congen Disord 1:1–5
Williams SR, Perera P, Gharahbaghian L (2014) The FAST and E-FAST in 2013: trauma ultrasonography: overview, practical techniques, controversies, and new frontiers. Crit Care Clin 30:119–150
Alrajab S, Youssef AM, Akkus NI, Caldito G (2013) Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care 17:R208
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Normal ultrasound in a 15-year-old boy. Because absence of sliding pleura is highly suggestive but not diagnostic of pneumothorax [12], findings are generally reported in our institution as presence or absence of sliding pleura (AVI 2421 kb)
ESM 2
Pneumothorax in a 15-year-old boy. This cine demonstrates the loss of lung slide. The motion is contributed to heart beat and diaphragmatic motion (AVI 3637 kb)
ESM 3
Left apical pneumothorax in a 16-year-old girl. Lung ultrasound (false negative) shows a sliding pleural line (AVI 3807 kb)
Rights and permissions
About this article
Cite this article
Vasquez, D.G., Berg, G.M., Srour, S.G. et al. Lung ultrasound for detecting pneumothorax in injured children: preliminary experience at a community-based Level II pediatric trauma center. Pediatr Radiol 50, 329–337 (2020). https://doi.org/10.1007/s00247-019-04509-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04509-y