Abstract
Background
Abnormalities of the placenta affect 5–7% of pregnancies. Because disturbances in fetal growth are often preceded by dysfunction of the placenta or attenuation of its normal expansion, placental health warrants careful surveillance. There are limited normative data available for placental volume by MRI.
Objective
To determine normative ranges of placental volume by MRI throughout gestation.
Materials and methods
In this cross-sectional retrospective analysis, we reviewed MRI examinations of pregnant females obtained between 2002 and 2017 at a single institution. We performed semi-automated segmentation of the placenta in images obtained in patients with no radiologic evidence of maternal or fetal pathology, using the Philips Intellispace Tumor Tracking Tool.
Results
Placental segmentation was performed in 112 women and had a high degree of interrater reliability (single-measure intraclass correlation coefficient =0.978 with 95% confidence interval [CI] 0.956, 0.989; P<0.001). Normative data on placental volume by MRI increased nonlinearly from 6 weeks to 39 weeks of gestation, with wider variability of placental volume at higher gestational age (GA). We fit placental volumetric data to a polynomial curve of third order described as placental volume = –0.02*GA3 + 1.6*GA2 – 13.3*GA + 8.3. Placental volume showed positive correlation with estimated fetal weight (P=0.03) and birth weight (P=0.05).
Conclusion
This study provides normative placental volume by MRI from early first trimester to term gestation. Deviations in placental volume from normal might prove to be an imaging biomarker of adverse fetal health and neonatal outcome, and further studies are needed to more fully understand this metric. Assessment of placental volume should be considered in all routine fetal MRI examinations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abnormalities of the placenta affect 5–7% of pregnancies [1, 2]. Evidence is accumulating that the placenta is directly responsible for both the immediate and long-term health of the fetus [3, 4]. Fetal growth is the primary indicator of overall fetal health, and birth weight is strongly linked to infant survival [5]. Because disturbances in fetal growth are often preceded by dysfunction of the placenta or attenuation of its normal expansion [6], placental growth and development warrant careful surveillance. Normal placental imaging demonstrates increasing size and heterogeneity as gestation progresses (Fig. 1), but this progression does not always occur. Imaging studies that can identify predictors of impending fetal growth disruption should be a major focus of efforts to improve fetal and neonatal health outcomes. Abnormal placental volume might prove to be an imaging biomarker of an adverse fetal environment and provide an opportunity for intervention before fetal health is compromised.
Increasing heterogeneity and complexity of the placenta (*) across gestation in T2-weighted single-shot fast spin-echo MR images in the coronal plane in a 35-year-old woman at 10 weeks 3 days of gestation (a), in the axial plane in a 33-year-old woman at 23 weeks 5 days of gestation (b) and in the axial plane in a 25-year-old woman at 37 weeks 6 days of gestation (c)
Evaluation of the placenta is part of routine antenatal ultrasound (US), but the lack of soft-tissue contrast and narrow field of view can limit this technique. Furthermore, depending on placental location within the uterus, US might be limited by its ability to penetrate tissues. When fetal growth becomes compromised, umbilical artery Doppler US studies are frequently used to assess fetal blood supply and provide an indirect assessment of the placenta. Non-contrast MR imaging has become more accessible and useful for more detailed evaluation in the setting of fetal anomalies, but little to no quantitative information about the placenta is obtained from these studies. One contributor to our limited understanding of abnormal placental growth is the lack of established normative data, particularly normal ranges for placental volume by MRI. In 2001, Duncan et al. [7] published the first large-scale study of fetal organ volume and placental volume throughout gestation using echoplanar MRI at 0.5 tesla (T). In 2016, those ranges were updated in a study of placental growth by MRI at 1.5 T in the second and third trimesters in a longitudinal cohort of 20 healthy pregnant women [8]. However little is known about placental volume in the first trimester or whether this small sample size accurately represents population norms because no larger studies have replicated these findings to date.
The objective of our study was to determine placental volume by MRI from early first trimester to term gestation. We used semi-automated segmentation of the placenta to create normative ranges of placental volume by MRI throughout gestation in a radiologically normal cohort of pregnant women.
Materials and methods
Study participants
The institutional review board of our academic health care system approved this retrospective cross-sectional imaging study with waiver of participant consent. The institutional radiology database was queried for MR imaging of pregnant females between 2002 and 2017 and images were categorized by diagnosis. Imaging was performed for a variety of clinical indications including concern for fetal pathology, suspected invasive placenta, and concern for maternal intra-abdominal inflammatory processes. We excluded from analysis imaging studies with any radiologic evidence of maternal or fetal pathology, as determined by the clinical radiology report and chart review. Any immediate maternal or fetal postnatal abnormality documented in the chart was assessed for its impact on the placental size; studies with intrapartum documentation of placenta accreta were excluded from analysis. We excluded from the analysis imaging studies with multiple gestation, as well as imaging studies on fetuses found to have pathology later in pregnancy or at birth. Finally, we excluded studies from pregnancies resulting in a neonate with birthweight below the 3rd percentile or above the 97th percentile for gestational age.
We obtained maternal clinical and demographic information by retrospective chart review, including age, height, weight, body mass index (BMI), gestational age, parity, race, health conditions (specifically tobacco use, hypertension and diabetes) and medications used during pregnancy. Gestational age was determined by either first-trimester US or last menstrual period. In fetuses for whom gestational age was not available in the maternal medical record, we compared MRI measures of the fetal biparietal diameter, anteroposterior cerebral dimension, anteroposterior pons, transverse cerebellar dimension, overall brain maturation, and femur length against published normative data to determine gestational age at time of MRI [9]. We obtained clinical information about the fetus by chart review and included fetal gender and estimated fetal weight percentile by US obtained within 30 days of MRI. For those infants later born within our medical system, we obtained gestational age at delivery and growth parameters at birth including weight, length and occipito-frontal circumference.
Magnetic resonance imaging studies
Imaging studies were obtained on a 1.5-T scanner between 2002 and 2013 (Magnetom Avanto-Fit; Siemens, Erlangen, Germany) and on a 3.0-T scanner between 2014 and 2017 (Magnetom Skyra; Siemens, Erlangen, Germany). Exact pulse sequences differed depending on the indication for MRI but placental segmentation was performed using either the half-Fourier acquisition single-shot fast spin-echo (SSFSE) sequence or the balanced steady-state free precession (SSFP) gradient-echo sequence. All scans utilized for placental segmentation included three planes of imaging.
Image analysis
We reviewed magnetic resonance studies prior to segmentation to ensure adequate quality of placental imaging, and we excluded from analysis those with inadequate visualization of the placenta (partially outside field of view, low resolution or motion artifact; n=43). We performed semi-automated placental segmentation on images in the maternal axial plane, which frequently (but not uniformly) corresponded to the placental axial plane. We found that this plane allowed for clearest demarcation of placental margins (Fig. 2). Segmentation was performed by a single observer (R.L.L., physician in fellowship) using Philips Intellispace Tumor Tracking Tool (Koninklijke Philips N.V., Amsterdam, the Netherlands). Although propagation of the region of interest (ROI) with edge detection occurred automatically through the image series, we manually adjusted the ROI in each slice of the selected sequence to ensure accuracy. The software calculated volume of the ROI based on slice thickness and recorded it for analysis. A subset of images of the study population was measured by a second observer (B.P.B., pediatric radiologist with 5 years’ post-fellowship experience) to assess interrater reliability.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 24 (IBM, Armonk, NY). Placental volume data were fit to a polynomial curve of third order and differences from expected placental volume based on the equation of the best-fit curve were calculated for each patient measurement. Correlation of the nonparametric placental volume data with continuous, nominal or ordinal dependent variables was determined by the Spearman rho, Mann–Whitney U or Kruskal–Wallis test, respectively. Intraclass correlation coefficient (ICC) using a two-way random model to measure absolute agreement was calculated to determine interrater reliability on a subset of the total population analyzed [10]. For all analyses, the level of significance was set at P<0.05.
Results
Study population
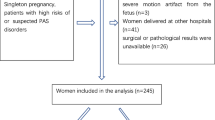
A total of 1,010 abdominal or fetal MR imaging studies of pregnant women were performed at our institution between 2002 and 2017 for concern for maternal or fetal pathology. A total of 848 studies were excluded from analysis because of positive finding of radiologic abnormality in mother or fetus, leaving 162 studies (16%) with no maternal or fetal radiologic abnormality. A total of 43 studies were excluded for inadequate placental imaging, an additional 4 studies were excluded for multiple gestation, and 3 studies were excluded for neonatal birthweight <3rd percentile or >97th percentile (Fig. 3). Placental segmentation was performed on a total of 112 MRIs.
Gestational age at time of MRI ranged from 6 weeks to 39 weeks. Gestational age was unavailable in seven MRI studies and was determined by fetal biometry. Maternal age ranged from 16 years to 45 years, with an average age of 26 years. Of the included women, 25% were primiparous, 42% were multiparous, 18% were grand-multiparous (gravida 5 or more) and 15% had no available information on parity. The clinical indication for MRI was divided nearly equally between maternal (48%) and fetal concerns (46%). An additional 6% of MR imaging examinations came from healthy volunteers recruited in a prior investigation (Table 1). The majority of maternal indications for MRI were right lower quadrant pain with no abnormality on imaging and the majority of the fetal indications were concern for absent cavum septum pellucidum ultimately found to have normal anatomy on MRI. Neonatal characteristics were available for 60% of the study population. Neonates were delivered on average at 38 2/7 weeks of gestation and weighed an average of 3,181 g at birth.
Placental volume
Between 6 weeks and 9 weeks of gestation the mean placental volume was 10.1 mL, which increased to a mean of 1,039 mL by term (Table 2). The equation that best described the placental volume increase throughout gestation in this population (R2=0.75) is reported below, where gestational age (GA) is expressed in weeks (Fig. 4):
Placental volume by gestational age in a radiologically normal cohort of 112 pregnant women demonstrates nonlinear distribution of placental volume across gestation and increasing variability in placental volume at higher gestational ages, with best-fit curve described by the third-order polynomial equation
We found a high degree of interrater reliability between the subset of placental volume measurements representing 30% of the total population studied, with an average measure ICC of 0.978 (95% CI=0.956, 0.989; P<0.001). No subjective difference in placental volume or ability to discern placental margins was noted between imaging studies at 1.5 T versus 3.0 T or based on sequence used to perform segmentation (SSFP versus SSFSE). Placental location within the uterus was recorded, with most placentae located anteriorly (47%), followed by 38% in posterior position, and 5% located laterally. Inferiorly located placentae in this cohort were exclusively observed in women in the first-trimester of pregnancy and any imaging with the finding of placenta previa beyond the first trimester was deemed abnormal and excluded from analysis.
The Spearman rho test for this nonparametric data set demonstrated no correlation between placental volume and maternal pre-pregnancy weight, height, BMI or age (Table 3). There was a positive correlation between estimated fetal weight and placental volume (ρ=0.378, n=32, P=0.03). Birth weight percentile had a similar positive association with placental volume (ρ=0.249, n=61, P=0.05). In the few cases of maternal hypertension (n=12) in our data set, no statistically significant differences in placental volume were measured (P=0.90). Similarly, for those with maternal diabetes mellitus (n=8), no correlation with placental volume was found (P=0.90). Information on maternal medications and level of control of these conditions was not available. Tobacco use was documented in the medical chart of 27 women in the study, but no information on amount or duration was recorded, and there was no significant association with placental volume (P=0.11).
Discussion
The present study provides normative placental volume by MRI measured at as early as 6 weeks of gestation and as late as 39 weeks of gestation in 112 women whose imaging studies showed no fetal or maternal radiologic abnormalities. We found a nonlinear relationship between placental volume and gestational age, with increasing variability in placental volume at higher gestational ages. A large portion of our sample data represent the time when most women would be referred for fetal MRI in response to concerns on US in the mid-second trimester. Our results add to the placental volume data reported in recent MRI placental segmentation studies with smaller cohorts [8, 11, 12].
In a prospective study by Langhoff et al. [8], the authors provided longitudinal placental volume in seven repeated MRI scans from second trimester to term gestation in a cohort of 20 healthy primiparous women using no medications and with body mass index of 18 to 30. This data set has the advantage of excluding women with complicating conditions that likely affect placental volume. Langhoff et al. [8] also performed placental segmentation seven times on each participant, providing information on interval placental growth. Our placental volume data are significantly higher, particularly at higher gestational ages compared to Langhoff et al.’s [8], are slightly lower than those reported by Duncan et al. [7], and are very close to those described by Andescavage et al. [11]. For example, placental volume at term gestation is approximately 1,250 mL in the report by Duncan et al. [7], compared to our volume of 1,039 mL between 37 weeks and 40 weeks, Andescavage et al.’s [11] approximately 1,000 mL at term and Langhoff et al.’s [8] 787 mL between 37 weeks and 39 weeks. These variations can likely be attributed to differences in populations studied, imaging equipment, segmentation tools and technical experience. Both our patient population and the one described by Duncan et al. [7] included a large number of multiparous women, which has been shown in previous studies to be associated with larger volume of the delivered placenta [13]. In addition, Duncan et al. [7] obtained images with 0.5-T MRI, which likely affected resolution and complicated determination of the placental plane at the basal plate [7]. At both 1.5-T and 3.0-T magnetic strengths, we found that placental contrast with amniotic fluid at the chorionic plate was easily visualized in all sequences, but the demarcation of the basal plate was slightly more difficult to discern in our earlier studies at lower magnetic field. We observed that these tissue planes are equally discernible on both SSFSE and SSFP sequences.
There were also differences in segmentation software used in each of these studies. Andescavage et al. [11] utilized ITK-SNAP, while Langhoff et al. [8] measured placental volume with a segmentation tool by Circle Cardiovascular Imaging. Our use of the Philips Intellispace Tumor Tracking Tool for segmentation analysis allows our results to be comparable to those obtainable by most radiologists who evaluate fetal MR imaging. This tool allows users to reproducibly measure the placenta in multiple planes of MR imaging sequences. Although the Tumor Tracking Tool was created for the purposes of repeated measures of tumors to determine response to chemotherapy, this segmentation tool has demonstrated excellent precision, with only 0.1–0.6-cm3 discrepancy in tumor volume by imaging compared with excised hepatic tumor size in rabbits [14]. In addition, our interrater reliability statistics demonstrated a high degree of reproducibility of placental volume measurements by this method.
Alternative methods of placental volumetric analysis have been reported, with US imaging comprising the majority of these studies. New US technologies have been developed to render three-dimensional organ reconstructions and have proved useful in assessing placental shape but have limitations in volumetric analysis. Namely, the low soft-tissue contrast limits the ability to clearly discern the tissue plane that creates the interface between uterus and basal plate of the placenta. Likewise, the narrow field-of-view of US limits full visualization of the placenta, forcing software and technician to piecemeal together imaging of the complete organ. Placental volumetric analysis by US, therefore, has had varying degrees of success [15,16,17,18,19,20,21,22,23,24], with some reports showing low levels of intra- and interrater reliability [17, 24, 25]. Volumetric analysis of the placenta by US is most concordant with MRI measurements in the first trimester [16, 18, 23] because later US measurements significantly underestimate placental volume. Placental volumes from term pregnancies reported in some studies are significantly less than the delivered, partially exsanguinated placenta [15, 19], underscoring the fact that US is not the ideal imaging technique for placental volume beyond the first trimester. Compared to one of the largest US placental volumetric analyses [15], our data correlate well in the first half of pregnancy but show increasingly larger discrepancies beyond the second trimester. Although this US study has the benefit of including 423 patient measurements, it is limited by the fact that most placental volume measures were obtained at 12 weeks and 20 weeks of gestation, thus relying heavily on extrapolation to determine the remainder of the placental growth curve.
With US readily available in most obstetric practices and significantly more cost-effective than MRI, it is well-suited as a screening examination for abnormalities of the placenta. In our retrospective cohort, only 16% of the women referred for fetal MRI because of concerns for maternal or fetal abnormalities during the 15-plus years of this study were found to be radiologically normal, demonstrating the high specificity of prenatal US in identifying fetal anomalies. Increasing evidence suggests that MRI provides reliable additional data that are useful for prognostication, treatment planning and even guidance for intrauterine intervention, for those select cases where it is indicated. Its utility has been particularly well established for evaluation of fetal intracranial anomalies. Accordingly, the number of referrals for fetal MRI are steadily increasing at our institution.
Despite the advantages of MRI to study the intrauterine environment, there are limitations to studies, such as the one presented here. Our data are limited by their observational nature. Each woman in our study was referred for MRI for a specific maternal or fetal concern, demonstrating a potential selection bias in our population, although only imaging without radiologic abnormalities was included in our analysis. In addition, our study population reflects the demographics of our location, with few non-Caucasian women and a high number of overweight and obese women in this cohort, although no correlation was found between placental volume and weight or BMI. As with all retrospective studies, we cannot draw conclusions on the causative relationships between placental volume and clinical factors analyzed here. Information on maternal hypertension, diabetes and tobacco use was collected by medical chart review but was only available for 82% of subjects, and no data were collected on whether medical management of these conditions was successful. Likewise, fetal outcome and growth parameters at birth were unknown for a large portion of our study participants because many women who underwent MRI received the remainder of their obstetric care outside our hospital system.
These limitations highlight the need for future investigations of placental volume in larger prospective cohorts in a more diverse study population. Future studies should not, however, be limited to primiparous women or only those with normal BMI because this does not accurately reflect population norms. Future investigations might also examine changes in placental signal intensity compared to an internal control, such as muscle, to determine whether this is predictive of placental abnormalities. In addition, studies of placental volume in people with maternal and fetal pathology are necessary to clarify how this metric can best be used to identify fetuses at risk of impaired placental growth.
A key question raised by this study is the physiological relevance of placental volume; specifically, how placental volume relates to placental function in vivo. We do not know whether a larger placenta uniformly enhances blood flow to the fetus, or whether in some cases placental growth might be deleterious to fetal health. The case of the morbidly adherent placenta poses a particularly uncertain clinical scenario. With Cesarean section rates (the greatest risk factor for morbidly adherent placenta) climbing [26], studies suggest that we should expect increasing incidence of morbidly adherent placenta. Understanding the hemodynamic effects of the invasive placenta is paramount to the obstetric management of these women. Advanced MRI analysis techniques evaluating placental function, such as intravoxel incoherent motion (IVIM) of diffusion-weighted imaging, have promising application to placental imaging research, specifically in elucidating the hemodynamic consequences of abnormal placental volume [27,28,29,30].
Alterations of placental structure and function in cases of fetal pathology are also poorly understood. In fetuses with congenital heart disease, placental growth as compared to birthweight percentile is larger than in healthy fetuses [11]. This might be interpreted as placental compensation for the structurally abnormal heart and subsequent disruption of normal blood flow patterns resulting in decreased oxygen delivery to target organs. In fetal gastroschisis, we know that placental microstructure is altered, with evidence of vascular hyperplasia or chorangiosis reported within the terminal chorionic villi of delivered placentae from these women [31]. Chorangiosis is thought to arise in states of chronic low-level hypoxemia, as encountered with gestation at high altitudes [32]. Coupled with the high rate of intrauterine growth restriction in cases of gastroschisis, the conclusion arises that this placental remodeling is likely associated with an attempted compensation of the organ. How this structural change at the microscopic level translates into a functional compensation of the placenta in vivo remains unknown. Abnormalities of the placenta in other forms of fetal pathology are less well defined. Because the placenta is almost entirely of fetal origin, there is good reason to postulate that aberrations in placental structure or function exist concomitant with other forms of fetal pathology.
In addition, many studies have established the effects of maternal exposures on placental growth and subsequent effects on the fetus. Perhaps the best studied of these is maternal smoking, which has well-known deleterious effects on placental and fetal growth [33] and imparts a significantly elevated risk of both fetal and early neonatal mortality [34]. Pathological characteristics of the placenta exposed to maternal smoking include decreased placental vascularization, thickening of the villous and trophoblast membranes and higher rates of syncytiotrophoblastic necrosis [35]. Using MR imaging, Anblagan et al. [33] demonstrated that maternal smoking is associated with smaller fetal organ size, including reduced brain and placenta volume. Contrast-enhanced MR imaging studies in non-human primates have shown that nicotine exposure alone also adversely affects placental hemodynamics [36]. Similar studies demonstrating in vivo functional effects of maternal smoking on the placenta in humans have not been reported. Maternal diabetes is also a well-defined fetal exposure with adverse effects. It leads most often to increased placental volume and surface area at birth but with villous immaturity [37]. As in maternal smoking, the in vivo characterization of dysglycemia on the placenta is uncertain. The use of advanced MR imaging techniques might be the key to understanding the pathophysiology of these and other specific exposures on placental structure and function.
Conclusion
This study provides normative placental volume ranges by MRI from early first trimester to term gestation. Future studies are indicated to determine normative placental volume in larger and more diverse populations in order to further refine this metric, which might prove to be an imaging biomarker of fetal and neonatal health outcomes. Assessment of placental volume should be considered in all routine fetal MRI examinations.
References
Silver RM (2015) Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol 126:654–668
Hladunewich M, Karumanchi SA, Lafayette R (2007) Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol 2:543–549
Gluckman PD, Hanson MA, Cooper C et al (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359:61–73
Godfrey KM (2002) The role of the placenta in fetal programming — a review. Placenta 23:S20–S27
McIntire DD, Bloom SL, Casey BM et al (1999) Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 340:1234–1238
Maulik D, Frances Evans J, Ragolia L (2006) Fetal growth restriction: pathogenic mechanisms. Clin Obstet Gynecol 49:219–227
Duncan KR, Sahota DS, Gowland PA et al (2001) Multilevel modeling of fetal and placental growth using echo-planar magnetic resonance imaging. J Soc Gynecol Investig 8:285–290
Langhoff L, Gronbeck L, von Huth S et al (2016) Placental growth during normal pregnancy — a magnetic resonance imaging study. Gynecol Obstet Investig 82:462–467
Kline-Fath B, Bahado-Singh R, Bulas D (2014) Fundamental and advanced fetal imaging: ultrasound and MRI. Lippincott Williams & Wilkins, Philadelphia
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Andescavage N, Yarish A, Donofrio M et al (2015) 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta 36:1024–1030
Derwig IE, Akolekar R, Zelaya FO et al (2011) Association of placental volume measured by MRI and birth weight percentile. J Magn Reson Imaging 34:1125–1130
Wallace JM, Bhattacharya S, Horgan GW (2013) Gestational age, gender and parity specific centile charts for placental weight for singleton deliveries in Aberdeen, UK. Placenta 34:269–274
Pellerin O, Lin M, Bhagat N et al (2013) Comparison of semi-automatic volumetric VX2 hepatic tumor segmentation from cone beam CT and multi-detector CT with histology in rabbit models. Acad Radiol 20:115–121
Arleo EK, Troiano RN, da Silva R et al (2014) Utilizing two-dimensional ultrasound to develop normative curves for estimated placental volume. Am J Perinatol 31:683–688
Aye CY, Stevenson GN, Impey L et al (2015) Comparison of 2-D and 3-D estimates of placental volume in early pregnancy. Ultrasound Med Biol 41:734–740
Cheong KB, Leung KY, Li TK et al (2010) Comparison of inter- and intraobserver agreement and reliability between three different types of placental volume measurement technique (XI VOCAL, VOCAL and multiplanar) and validity in the in-vitro setting. Ultrasound Obstet Gynecol 36:210–217
Collins SL, Stevenson GN, Noble JA et al (2013) Rapid calculation of standardized placental volume at 11 to 13 weeks and the prediction of small for gestational age babies. Ultrasound Med Biol 39:253–260
de Paula CF, Ruano R, Campos JA et al (2008) Placental volumes measured by 3-dimensional ultrasonography in normal pregnancies from 12 to 40 weeks' gestation. J Ultrasound Med 27:1583–1590
Pala HG, Artunc-Ulkumen B, Koyuncu FM et al (2016) Three-dimensional ultrasonographic placental volume in gestational diabetes mellitus. J Matern Fetal Neonatal Med 29:610–614
Simcox LE, Higgins LE, Myers JE, Johnstone ED (2017) Intraexaminer and Interexaminer variability in 3D fetal volume measurements during the second and third trimesters of pregnancy. J Ultrasound Med 36:1415–1429
Titapant V, Cherdchoogieat P (2014) Nomogram of placental thickness, placental volume and placental vascular indices in healthy pregnant women between 12 and 20 weeks of gestation. J Med Assoc Thail 97:267–273
Meengeonthong D, Luewan S, Sirichotiyakul S, Tongsong T (2017) Reference ranges of placental volume measured by virtual organ computer-aided analysis between 10 and 14 weeks of gestation. J Clin Ultrasound 45:185–191
Jones NW, Raine-Fenning NJ, Mousa HA et al (2011) Evaluating the intra- and interobserver reliability of three-dimensional ultrasound and power Doppler angiography (3D-PDA) for assessment of placental volume and vascularity in the second trimester of pregnancy. Ultrasound Med Biol 37:376–385
Florido J, Ocon O, de Dios Luna del Castillo J et al (2014) Analysis of measurement process of placental volume in early pregnancy: an interobserver reliability study. J Perinat Med 42:559–564
Zhang J, Troendle J, Reddy UM et al (2010) Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol 203:326.e1–326.e10
Alison M, Chalouhi GE, Autret G et al (2013) Use of intravoxel incoherent motion MR imaging to assess placental perfusion in a murine model of placental insufficiency. Investig Radiol 48:17–23
Moore RJ, Strachan BK, Tyler DJ et al (2000) In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Placenta 21:726–732
Sohlberg S, Mulic-Lutvica A, Lindgren P et al (2014) Placental perfusion in normal pregnancy and early and late preeclampsia: a magnetic resonance imaging study. Placenta 35:202–206
Siauve N, Chalouhi GE, Deloison B et al (2015) Functional imaging of the human placenta with magnetic resonance. Am J Obstet Gynecol 213:S103–S114
Payne NR, Simonton SC, Olsen S et al (2011) Growth restriction in gastroschisis: quantification of its severity and exploration of a placental cause. BMC Pediatr 11:90
Tissot van Patot M, Grilli A, Chapman P et al (2003) Remodelling of uteroplacental arteries is decreased in high altitude placentae. Placenta 24:326–335
Anblagan D, Jones NW, Costigan C et al (2013) Maternal smoking during pregnancy and fetal organ growth: a magnetic resonance imaging study. PLoS One 8:e67223
Cnattingius S, Haglund B, Meirik O (1988) Cigarette smoking as risk factor for late fetal and early neonatal death. BMJ 297:258–261
Jauniaux E, Burton GJ (2007) Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev 83:699–706
Lo JO, Schabel MC, Roberts VH et al (2015) Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am J Obstet Gynecol 212:370.e371–370.e378
Huynh J, Dawson D, Roberts D et al (2015) A systematic review of placental pathology in maternal diabetes mellitus. Placenta 36:101–114
Acknowledgments
This publication was made possible in part with support from the Indiana Clinical and Translational Sciences Institute, funded in part by award number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Additional support came from the Joyce Victoria McRobbie Pediatric Fellowship grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
León, R.L., Li, K.T. & Brown, B.P. A retrospective segmentation analysis of placental volume by magnetic resonance imaging from first trimester to term gestation. Pediatr Radiol 48, 1936–1944 (2018). https://doi.org/10.1007/s00247-018-4213-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4213-x