Abstract
Objective
To identify if placental thickness measured from MRI images correlates with placenta accreta spectrum (PAS) disorders.
Methods
Placental thickness of 245 patients was retrospectively measured from October 2016 to March 2020. The measurement was made at the thickest portion of the placenta on the mid-sagittal plane of the placenta from MRI by two independent radiologists. Surgical report and pathology of the delivered placenta were used as a reference standard. Association between clinical features, placental thickness, and PAS disorders was evaluated with univariate and multivariate analyses. The inter-reader and intra-reader reproducibility of the measurements and receiver operating characteristic curve analysis were also performed.
Results
Placental thickness was significantly higher in patients with PAS disorders (3.45 cm) than that in patients without PAS disorders (2.90 cm) (p < 0.05). Multivariate analyses revealed that prior cesarean section, placenta previa, and placental thickness > 4 cm were independent risk factors for PAS disorders. The inter-reader and intra-reader reproducibility of placental thickness measurement were 0.979 (95% CI 0.960–0.989) and 0.981 (95% CI 0.9640–0.990), respectively.
Conclusion
The reproducibility of the measurement made from MRI images was high between two radiologists. Patients with PAS disorders had increased placental thickness. Placental thickness > 4 cm correlated with PAS disorders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Placenta accreta spectrum (PAS) disorder is a series of diseases, including abnormally adherent and invasive placentas. The adherent placenta is defined as placental villi simply adhering to the myometrium, while invasive placenta refers to a condition in which the villi invade or even penetrate the myometrium and includes placenta increta and placenta percreta [1]. The annual incidence of invasive placentation is projected to be over 9000/year and is estimated to be 1/300 pregnancies by 2020 [2, 3]. The increase of PAS disorders is a consequence of cesarean section (CS) and modern obstetric and reproductive medical processes, including operative hysterectomy, suction curettage, surgical termination, endometrial ablation, and any other procedures causing damage to the uterine wall [4].
The main complications of PAS disorders include massive hemorrhage, peripartum hysterectomy, organ injuries, and even death [5,6,7]. Patients with PAS disorders are also at higher risks for transfusion, anemia, bladder surgery, and ICU admission [8].
Since the first ultrasound (US) image of PAS disorders in the early 1980s, US imaging remains the primary method for diagnosing PAS disorders [9]. However, US imaging is operator-dependent because the diagnosis relies on individual subjective interpretations of visual sonographic findings on the gray scale and color Doppler imaging [10]. Although not routinely recommended by FIGO (The International Federation of Gynecology and Obstetrics), MRI is increasingly adopted in tertiary centers for prenatal diagnosis and birth plans. The Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) also released a consensus statement for MRI features in evaluating PAS disorders [11].
Li et al. reported that increased thickness of placenta in women with placenta previa in the lower uterine segment correlated with PAS disorders [10]. However, the reproducibility of the measurement of placental thickness from US imaging remains controversial because different operators may have different perceptions of the true sagittal plane [12]. It is relatively more objective to orient the true sagittal plane of the placenta, not just the sagittal plane of the mother from MRI, and the use of placental thickness has not been validated by MRI. Therefore, this study aims to determine whether measurement of placental thickness by MRI is reliable and, secondly, whether increased placental thickness correlates with PAS disorders.
Materials and methods
Patient selection
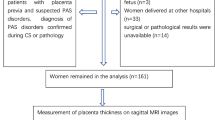
This retrospective study investigated consecutive patients who underwent placental MRI in our hospital between October 2016 and March 2020. The study protocol was approved by the Institutional Review Board. The inclusion criteria were as follows: ① patients with high risks of PAS disorders, ② patients with suspected PAS disorders detected by US imaging and MRI, ③ patients with a singleton pregnancy. The exclusion criteria included the following: ① patients with severe motion artifact from the fetus (n = 2), ② patients delivered at other hospitals (n = 37), ③ surgical or pathological results were unavailable (n = 12). Flowchart of the study design was demonstrated in Fig. 1.
Clinical characteristic analysis
The demographic information of patients was evaluated by consulting the clinical records of patients in the study, including maternal age, gravidity, parity, number of previous CS, and number of abortions. Gestational age at examination and gestational age at delivery were also recorded.
MRI protocols
All patients underwent placental MRI using a 1.5-T MR scanner (Aera, Siemens Healthineers, Erlangen, Germany). The image protocols were (1) axial, coronal, and sagittal half-Fourier acquisition single-shot turbo spin-echo (HASTE) of the placenta: field of view (FOV) 420 × 80 mm, 5-mm-thick section, 20% gap, matrix 272 × 320, TR 1 300 ms, TE 93 ms, and a scan duration of 50 s; (2) axial, coronal, and sagittal true fast imaging with steady-state precession (True-FISP) of the placenta: FOV of 420 × 80 mm, 5-mm-thick section, 30% gap, matrix 234 × 384, TR 4.11 ms, TE 1.63 ms, and a scan duration of 48 s; (3) 3D-volumetric interpolated breath-hold examination (3D-VIBE) of the placenta: FOV 400 mm, 5-mm-thick section, 20% gap, matrix 180 × 320, and a scan duration of 8 s.
Imaging analysis
Placental thickness was independently measured by 2 experienced radiologists with expertise in obstetric imaging with 3 and 13 years of experience, respectively. The radiologists were blinded to the clinical and pathological results. The thickest portion of the placenta at the mid-sagittal plane from HASTE was measured in all patients (see Fig. 2). The measurement was performed twice for each reader, and the average of the measurements was recorded as the final placental thickness.
Reference standard
The diagnosis of PAS disorders was made intraoperatively. Placenta percreta was diagnosed when the placental tissue invaded the uterine serosa and surrounding organs, including the broad ligament, vaginal wall, and bladder visually. Despite active management in the 3rd stage of labor, placenta increta was diagnosed when the placenta did not separate after 20 min, resulting in the difficulty in manual removal of the placenta piecemeal and heavy continuous bleeding from the implantation site. Placenta accreta was diagnosed when the placenta firmly adhered to the endometrium with non-self-controlled bleeding at the time of detachment. Pathological examination was made from the uterine specimen in hysterectomy cases or placental tissue in the invasive site of the removed placenta to support surgical diagnosis.
Statistical analysis
Continuous variables with a normal or non-normal distribution were expressed as mean ± standard deviation (SD) or median (range), respectively, and categorical variables were expressed as numbers (proportions, %). Student’s t test, Mann–Whitney U test, and χ2 test were used to compare the clinical features between patients with and without PAS disorders. A multivariable logistic regression analysis was used to determine the most significant risk factors for predicting PAS disorders. The following cutoff values of the risk factors were used: maternal age < 35 years or ≥ 35 years and placental thickness ≤ 4 cm or > 4 cm. Receiver operating characteristic curve analysis was used to determine the predictive value. The inter-reader and intra-reader reproducibility for placental thickness measurements were evaluated using the intraclass correlation coefficient (ICC) with 95% CI. An ICC of less than 0.20 indicated slight reproducibility, between 0.21 and 0.40 indicated fair reproducibility, between 0.41 and 0.60 indicated moderate reproducibility, between 0.61 and 0.80 indicated substantial reproducibility, and between 0.81 and 1.00 indicated perfect reproducibility. p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 21.0 (IBM Inc).
Results
A total of 245 patients were included in the study. The mean maternal age was 30 years, ranging between 19 and 45 years. The mean gestational age at examination was 32 weeks, ranging between 19 and 39 weeks. The clinical characteristics of the 2 groups are shown in Table 1. Of the 245 patients, 120 patients (47.24%) were diagnosed as PAS disorders, including 26 patients (10.61%) of placenta accreta, 81 patients (33.06%) of placenta increta, and 13 (5.31%) patients of placenta percreta. A total of 129 patients (52.65%) with placenta previa had CS, and 116 patients (47.35%) without placenta previa had a vaginal delivery.
Patients with PAS disorders were older than patients without PAS disorders, and more patients with PAS disorders were older than 35 years (p < 0.05). More patients with PAS disorders had prior CS, dilation and curettage of the uterus and placenta previa (p < 0.05). The number of prior CS, gravidity, and parity were all higher in patients with PAS disorders (p < 0.05). Placental thickness was 3.45 (1.98) cm in patients with PAS disorders and was significantly higher than that in patients without the disease at 2.90 (1.35) cm (p < 0.05) (Fig. 3). The placental thickness of patients with PAS disorders had increased (> 4 cm) (p < 0.05).
The inter-reader and intra-reader reproducibility of placental thickness measurement were 0.979 (95% CI 0.960–0.989) and 0.981 (95% CI 0.9640–0.990), respectively.
Multiple logistic regression analysis showed that prior CS, placenta previa, and placental thickness > 4 cm remained statistically significant (all p < 0.05) (Table 2). Table3 presents the accuracy, sensitivity, specificity, PPV (positve predictive value) and NPV(negative predictive value) of single risk factor and the combination with 3 risk factors together for predicting PAS disorders. For predicting PAS disorders, placenta previa demonstrated the highest AUC of 0.80 (95% CI 0.74–0.86), followed by prior CS and placental thickness of 0.71 (95% CI 0.64–0.77) and 0.61 (95% CI 0.54–0.68), respectively. A combination of all 3 risk factors demonstrated an AUC of 0.87 (95% CI 0.83–0.92) (Fig. 4).
Discussion
Our study showed that placental thickness > 4 cm, placenta previa, and prior CS were independent risk factors for PAS disorders. The specificity and PPV of placental thickness for predicting PAS disorders were 87.20% and 73.22%, respectively. Increased placental thickness can be used as a parameter for interpreting MRI and help stratify patients into those at high risk for PAS disorders and those at low risk for it.
The consensus statement released by SAR and ESUR recommended a series of MRI features to suggest PAS disorders. However, the diagnostic value of MRI features significantly depends on observers’ experience from a previous report [13]. Thickening of the placenta did not reach enough consensus and thus was not recommended by the consensus, probably due to a lack of standard of the placental thickness to differentiate PAS disorders.
Li et al. reported that increased placental thickness (> 4.5 cm) correlated with PAS disorders using US imaging [10]. Bhide et al. reported that placental thickness of the lower uterine segment was 5.03 cm in patients with PAS disorders, significantly higher than women with normal placentas (3.09 cm). Their study also showed that prior CS and placental thickness measured on US imaging were independent risk factors for PAS disorders [14]. Other studies showed that placental thickness correlated with blood loss in PAS disorders or antepartum hemorrhage [15, 16]. However, measurement of placental thickness using MRI has rarely been reported. Chen et al. identified the placental thickness on the uterine scar area > 3.8 cm using MRI as one of the independent risk factors for massive intraoperative hemorrhage in patients with placenta previa and accreta [17].
Our study showed placental thickness was 3.45 (1.98) cm in patients with PAS disorders using MRI, which was significantly higher than that in patients without the disease 2.90 (1.35) cm. Normally, the placenta is discoid with a thickness between 2 and 4 cm. Therefore, we adopted a cutoff of 4 cm in our study to determine abnormal thickening of the placenta. Our study showed that placental thickness > 4 cm was an independent risk factor for PAS disorders and was very specific to predict PAS disorders. Normally, the placenta spreads over the uterine mucosa like a pancake with uniform thickness. In PAS disorders, the placenta is abnormally implanted in the lower uterine segment, cervix, or the uterine scar, where had poor vascularization and subsequently limited placenta migration, causing abnormal thickening of the placenta [18].
The previous measurement of placental thickness has some limitations in using US imaging. Since previous studies were retrospective, the thickness of the placenta was measured on stored 2-D images, which may not have been representative of the maximal placental thickness [14]. The reproducibility of the measurement was also hard to evaluate. Discrepancies of identifying a true sagittal plane between different operators also raised the concern of reproducibility. With the ability of multiplanar imaging, it is relatively easy to orient the true sagittal plane of the placenta from MRI so that the reproducibility of the measurement can be evaluated between different radiologists. The high reproducibility confirmed the accuracy of our study. However, the sensitivity of placental thickness in predicting PAS disorders is only 35%, this is probably due to a lot of overlap of placental thickness between patients with and without PAS disorders. Therefore, it is imprudent to use placental thickness alone to diagnose PAS disorders. A combination of MRI features and placental thickness may help diagnose the disease accurately, especially for less experienced radiologists of placental imaging.
Our study still had some limitations. Firstly, this was a retrospective study with a small sample size. Selection bias was inevitable since we mainly included patients with equivocal US findings and patients with high risk factors for PAS disorders. Secondly, previous studies mainly measured the placenta thickness in the lower uterine segment in patients with placenta previa. As nonprevia placenta accreta was not uncommon in clinical practice, we also included patients without placenta previa. Our measurement was made at the thickest portion of the placenta, which varied in the location of the uterus. Thirdly, we did not evaluate the diagnostic value of different MRI features as they were more subjective than the measurement of placental thickness. However, the presence of accepted MRI features can greatly promote the confidence of accurate diagnosis.
In conclusion, measurement of placental thickness from MRI is objective and easy to stratify patients even for less experienced radiologists. Placental thickness > 4 cm is correlated with PAS disorders. A more accurate cutoff of placental thickness in diagnosing PAS disorders is needed in future studies.
References
Jauniaux E, Ayres-de-Campos D. FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel.FIGO consensus guidelines on placenta accreta spectrum disorders: introduction .Int J Gynaecol Obstet, 2018 ,140: 261-264
Solheim KN, Esakoff TF, Little SE,et al. The effect of cesarean delivery rates on the future incidence of placenta previa, placenta accreta, and maternal mortality. J Matern Fetal Neonatal Med,2011, 24:1341–1346
Chen T, Xu XQ, Shi HB, et al. Conventional MRI features for predicting the clinical outcome of patients with invasive placenta. Diagn Interv Radiol.2017, 23:173–179
Jauniaux E, Kingdom JC,Silver RM. A comparison of recent guidelines in the diagnosis and management of placenta accreta spectrum disorders.Best Practice & Research Clinical Obstetrics and Gynaecology,2021,72:102-116
Bodelon C, Bernabe-Ortiz A, Schiff MA, Reed SD.Factors associated with peripartum hysterectomy. Obstet Gynecol.2009, 114:115–123
Jin R, Guo Y, Chen Y. Risk factors associated with emergency peripartum hysterectomy. Chin Med J (Engl),2014, 127:900–904
Gielchinsky Y, Rojansky N, Fasouliotis SJ, Ezra Y. Placenta accreta—summary of 10 years: a survey of 310 cases. Placenta,2002 23:210–214
Upson K, Silver RM, Greene R, Lutomski J, Holt VL. Placenta accreta and maternal morbidity in the Republic of Ireland,2005-2010. J Matern-Fetal Neonat Med: Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2014;27(1):24–29.
Fox KA and Lee W.Prenatal Diagnosis and Evaluation of Abnormal Placentation.CLINICAL OBSTETRICS AND GYNECOLOGY,2017,60(3):596-607
Li Y,Choi HH, Goldstein R, et al.Placental thickness correlates with placenta accreta spectrum (PAS) disorder in women with placenta previa.Abdominal Radiology,2021,46(6):2722-2728
Jha P,Pōder L,Bourgioti C,et al.Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders.European Radiology .2020, 30:2604-2615
Pijnenborg R, Brosens I, Romero R (2010) Placental bed disorders:basic science and its translation to obstetrics. Cambridge University Press
Alamo L, Anaye A, Rey J, et al. Detection of suspected placen tal invasion by MRI: do the results depend on observer' experience? . Eur J Radiol,2013,82:e51-e57
Bhide A,Laoreti A, Agten AK, et al.Lower uterine segment placental thickness in women with abnormally invasive placenta.Acta Obstetricia et Gynecologica Scandinavica,2018, 98(1):95-100
Zaitoun MM, El Behery MM, Abd El Hameed AA, et al, Does cervical length and the lower placental edge thickness measurement correlates with clinical outcome in cases of complete placenta previa? Arch Gynecol Obstet ,2011,284:867-873
Liu Z, Wei Y, Dai Q,et al; Antenatal Sonographic Diagnosis and Clinical Signifcance of Placenta Previa Accreta After Cesarean Section. Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 2017,30;39(5):693–698
Chen D, Xu J, Ye P, et al. Risk scoring system with MRI for intraoperative massive hemorrhage in placenta previa and accreta. JMRI,2020,51:947-958
Craven CM, Zhao L, Ward K. Lateral placental growth occurs by trophoblast cell invasion of decidual veins. Placenta. 2000;21(2-3):160-169.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, T., Wang, Y., Guo, A. et al. Correlation of placental thickness and PAS disorders: findings from MRI. Abdom Radiol 47, 1150–1156 (2022). https://doi.org/10.1007/s00261-022-03420-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03420-9