Abstract
Background
MR enterography is increasingly utilized for noninvasive evaluation of disease activity in young patients with Crohn disease and has great impact on clinical management. Diffusion-weighted imaging (DWI) is a rapid MR imaging technique that measures molecular diffusion of water and is sensitive to the inflammatory process; however, its value to MR enterography has not been rigorously evaluated.
Objective
To determine whether the addition of DWI to MR enterography is helpful in evaluating Crohn disease activity in young patients when compared to a histological reference.
Materials and methods
In this single-institution retrospective study, we searched an imaging database for the period January 2010 to December 2012 to identify patients age 19 years and younger who had MR enterography with diffusion-weighted imaging (DWI). We used an electronic medical record search to identify those who had MR enterography and colonoscopy performed within 28 days of each other. All MR enterography scans were performed on a 1.5-T or 3-T clinical MR scanner with phased-array torso coil configuration using standard pulse sequences as well as axial DWI with b values of 50, 400 and 800. Bowel segments were evaluated for disease activity based on standard MR enterography sequences; in addition, segmental apparent diffusion coefficient (ADC) values were calculated based on DWI. Histological reference for disease activity was based on assessment for mucosal inflammatory changes on endoscopic biopsy. MR enterography and DWI evaluation were performed in a blinded fashion with respect to histological results.
Results
We included imaging of 78 bowel segments from 27 patients (mean age 14.5 ± 3.02 years) with known Crohn disease in the study. The mean ADC for bowel segments with active disease was 1.56 ± 0.7 × 103 mm2/s compared with 2.58 ± 1.4 × 103 mm2/s for segments without active disease, a difference that was statistically significant (P < 0.01, Student’s t-test). Using a threshold value of 2.0 × 103 mm2/s, DWI demonstrated lower accuracy (64.1%) but higher sensitivity (78.8%) for detecting active disease compared with standard MR enterography (69.2% and 54.6%, respectively). Combining DWI with MR enterography, using DWI as the initial screen and MR enterography afterward to reduce false negativity, led to a significant increase in accuracy (76.9%; P = 0.03, McNemar’s test) compared with either imaging technique alone.
Conclusion
Although DWI does not perform as well as standard MR enterography for detection of active Crohn disease, the combination of DWI and MR enterography increases imaging accuracy for determining disease activity compared with either technique alone. These results indicate that DWI adds value to MR enterography and supports the incorporation of DWI into MR enterography protocols for evaluation of Crohn disease in young patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance enterography is increasingly utilized for imaging evaluation of disease activity in young patients with Crohn disease [1–4]. Besides the advantages of lack of ionizing radiation [5] and the ability to assess for both intestinal and extra-intestinal disease activity [6], MR enterography helps in the detection of active disease and the differentiation of active disease from fibrosis, which have important implications for disease management [7].
Because Crohn disease has a chronic inflammatory nature, patients often must undergo multiple imaging exams over time to evaluate symptom recurrence. MR enterography has become the primary imaging modality for Crohn disease in many pediatric centers because of its lack of ionizing radiation exposure and established accuracy for detecting active disease [8, 9]. Diffusion-weighted imaging (DWI) is a rapid MR imaging technique that measures molecular diffusion of water and is sensitive to microscopic changes accompanying the inflammatory process [10]. Recent studies have established a role for DWI in detecting active bowel inflammation in Crohn disease [10–13], although its performance compared with standard MR enterography sequences has not been established. DWI is an attractive option for imaging children and young adults because it does not require the use of intravenous contrast material and sometimes precludes the need for intravenous access in young patients. In addition, the quantitative nature of DWI and its resultant apparent diffusion coefficient maps might be less subjective than some of the more qualitative MR enterography sequences. Because DWI is used in MR enterography as an additional sequence rather than a replacement for standard sequences, the purpose of our study was to determine whether there is a benefit to adding DWI to standard MR enterography for evaluating disease activity in children with Crohn disease. This is particularly important to demonstrate, given the trend toward shortening MRI scan times and eliminating unnecessary sequences, especially for young children being scanned awake. Therefore we studied children with established Crohn disease who had MR enterography with DWI to evaluate disease activity. We compared the performance of standard MR enterography sequences, DWI, and MR enterography plus DWI for detecting active disease in individual bowel segments with histological reference from endoscopic biopsies.
Materials and methods
Patient selection
This single-institution retrospective study was approved by the institutional review board and complies with the Health Insurance Portability and Accountability Act (HIPAA). We searched the electronic medical record database for the period of 2010–2012 to identify all patients 19 years of age and younger with established Crohn disease who had MR enterography (including DWI) and colonoscopy within 28 days of each other. We excluded patients with technically poor-quality studies caused by inadequate oral contrast agent intake or poor-quality DWI images.
MR enterography protocol
All MR enterography studies were performed on a 1.5-tesla scanner (HD Excite; GE Healthcare, Milwaukee, WI) or a 3-T scanner (Magnetom Trio; Siemens Healthcare, Malvern, PA) using a multichannel phased-array body coil configuration. Patients consumed an oral contrast mixture over a period of 45 min prior to MR enterography consisting of 900 ml of dilute barium and sorbitol (VoLumen; Bracco Diagnostics, Monroe, NJ) combined with 450 ml of a flavored barium sulfate suspension (Readi-Cat; Bracco Diagnostics). The total volume consumed was based on patient weight, with patients weighing less than 50 kg drinking a total of 900 ml and those weighing more than 50 kg drinking a total of 1,350 ml. The patients were encouraged to drink as much contrast agent as possible from the volumes offered. Intravenous contrast agent was also administered: gadopentetate dimeglumine (Magnevist; Bayer Imaging, Whippany, NJ) was administered at a dose of 0.1 mmol/kg injected (maximum of 20 ml) at 2 ml/s using a power injector followed by a 10-ml saline flush at the same rate.
Standard MR enterography sequences included the following: (1) tri-plane localizers; (2) coronal and axial T2 half-Fourier acquisition single-shot fast spin-echo (half-fourier acquired single-shot turbo spin echo [HASTE]/single-shot fast spin echo [SSFSE]) free-breathing images through the entire abdomen and pelvis (repetition time [TR]/echo time [TE] 1,200/90 ms, 5-mm thickness/0-mm gap, 320 × 256 matrix); (3) coronal 2-D balanced steady-state free precession (true fast imaging with steady-state precession [TrueFISP]/fast imaging employing steady state acquisition [FIESTA]) breath-hold images through the abdomen and pelvis (TR/TE 3.8/1.9 ms, 5-mm thickness/2.5-mm gap, 320 × 256 matrix); (4) axial T2 forced recovery fast spin-echo (RESTORE/fast recovery fast spin echo [FRFSE]) fat-suppressed free-breathing images through the pelvis from bottom of the pelvis to top of pelvic brim (TR/TE 2,100/100 ms, 5-mm thickness/5-mm gap, echo-train length 15, 320 × 290 matrix, free-breathing with respiratory triggering); (5) coronal dynamic T1 3-D volumetric gradient echo volume interpolated breathhold examination (VIBE)/liver acquisition with volume accleration (LAVA) fat-suppressed breath-hold images through the abdomen and pelvis with acquisitions at 45 s, 70 s and 180 s post-injection (TR/TE 4.5/2.2 ms, 4-mm thickness/0-mm gap, 320 × 290 matrix, multiple breath-hold acquisitions); (6) axial T1 3-D volumetric gradient echo (VIBE/LAVA) fat-suppressed breath-hold high-resolution delayed images through the abdomen and pelvis post-contrast (TR/TE 4.5/2.2 ms, 4-mm thickness/4-mm gap, 512 × 320 matrix, multiple breath-hold acquisitions).
Diffusion-weighted imaging (DWI) was performed using an axial breath-hold echoplanar imaging fat-suppressed sequence (TR/TE 4,400/50 ms, field of view [FOV] 30 cm, slick thickness/gap 5/5 mm, matrix 128 × 192) with three directions and b values of 50, 400 and 800 centered on the terminal ileum. The total axial coverage was 10 cm. The bowel segments imaged included the terminal ileum, cecum, adjacent ascending colon and a portion of the descending colon. Apparent diffusion coefficient (ADC) maps were generated automatically on the scanner and transmitted to a picture archiving and communication workstation (Agfa HealthCare, Greenville, SC) for analysis.
MRE image interpretation
All bowel segments known to have a corresponding histological reference for disease activity were reviewed on MR enterography for the presence or absence of active inflammation by a single board-certified radiologist with fellowship training in pediatric and abdominal imaging and 9 years of experience interpreting MR enterography studies. The radiologist was blinded to the histological results and the original clinical MR enterography interpretation. Standard MR enterography and diffusion-weighted images were interpreted in separate sessions by the same radiologist at an interval of 28 days. Standard MR enterography features of active inflammation include mural T2 hyperintensity relative to muscle, mural hyperenhancement on post-contrast images, bowel wall thickening (>3 mm for small bowel, >4 mm for large bowel [14, 15]) and mesenteric hypervascularity. A segment was scored as positive for active disease on standard MR enterography if at least two of these features were present. For DWI interpretation, bowel segments were identified anatomically based on b = 0 and the axial T2 fat-suppressed MR enterography images. Three regions of interest (ROI) were then placed over the bowel wall on the corresponding apparent diffusion coefficient (ADC) map. Each ROI spanned the entire bowel wall thickness from mucosal to serosal surfaces, excluding the lumen, at the location of maximum bowel wall thickness for each segment. A mean ADC value +/- the standard deviation was then calculated and recorded for each bowel segment based on these regions of interest. The ADC values of all 78 bowel segments were calculated in this manner.
Combined MR enterography–diffusion-weighted image interpretation
Two algorithms were used to determine value of DWI to MR enterography in determining disease activity. In the first, DWI was used as the initial screen, with all DWI non-restricted (ADC ≥ 2.0 × 10−3) segments considered negative, followed by standard MR enterography evaluation of DWI restricted segments. DWI restricted segments were considered positive if any two MR enterography features of active inflammation (mural T2 hyperintensity, wall thickening, hyperenhancement, or mesenteric hypervascularity) were present, and negative if no MR enterography features of active disease were seen. In the second algorithm, standard MR enterography was the initial screen, with all enterography-positive segments considered positive, followed by DWI of enterography-negative segments. Enterography-negative/DWI-restricted (ADC < 2.0 × 10−3) segments were considered positive and enterography-negative/DWI-non-restricted segments were considered negative.
Histological assessment of bowel inflammatory activity
The clinical pathology reports were reviewed for all endoscopic biopsies obtained from corresponding colonoscopy exams by a single pathologist. A bowel segment was considered positive for active inflammation by histology based on the presence of mucosal inflammatory changes (neutrophilic infiltration of mucosal glands or mucosal ulceration). In bowel segments with an indeterminate report or conflicting findings, a secondary review of slides was conducted. Both the original pathology report and the secondary review were conducted in a blinded fashion with respect to MR enterography interpretation.
Statistical analysis
McNemar’s test was used to evaluate for differences in test performance for detecting active inflammation between MR imaging parameters. Two-tailed Student’s t-test was used to evaluate for differences in mean ADC values. Ninety-five percent confidence intervals for test sensitivity and specificity were calculated using the Wilson score method (GraphPad, LaJolla, CA).
Results
A database query of radiology exams performed at our institution yielded 27 patients in whom MR enterography with DWI and colonoscopy were performed within 28 days of each other. Table 1 details the patient cohort. A total of 78 bowel segments had enterography–DWI–histology correlation, including 27 segments from the terminal ileum, 25 from the ascending colon and 26 from the descending colon. The mean time between MR enterography and endoscopy was 9.6 ± 8.5 days.
Performance of standard MR enterography for detecting active inflammation compared with histology
Out of the 78 bowel segments evaluated, 33 (42.3%) had histological evidence of active inflammation (Table 2). An imaging evaluation of disease activity in these segments using either standard MR enterography sequences or DWI was then performed in a blinded fashion with respect to the histology. Standard MR enterography was found to have an accuracy of 69.2% (54/78 segments) for detection of active inflammation, associated with a specificity of 80.0% (36/45 segments) and a positive predictive value of 66.7% (18/27 segments).
Performance of DWI for detecting active inflammation compared with histology
Diffusion-weighted imaging was evaluated in all of the individual bowel segments and was correlated with the presence or absence of active inflammation (Figs. 1 and 2). The ADC values for bowel segments with active disease overall were lower (Fig. 3), with a mean ADC for bowel segments with active disease of 1.56 ± 0.7 × 10−3 mm2/s compared with 2.58 ± 1.4 × 10−3 mm2/s for segments without active disease. This difference was found to be statistically significant; P < 0.01, Student’s t-test). Based on receiver operating characteristic analysis of the data using different ADC cutoffs for activity, an ADC threshold value <2.0 × 10−3 mm2/s was found to be the best DWI discriminator of active disease, with an accuracy of 64.1% (50/78 segments) that was similar to but lower than that of standard MR enterography. The sensitivity of DWI was 78.8% (26/33 segments), specificity 53.3% (24/45 segments) and positive predictive value 55.3% (26/47 segments).
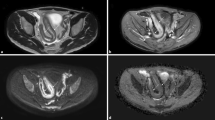
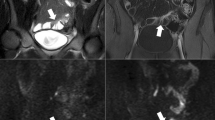
Examples of diffusion-weighted imaging (DWI) evaluation of nonactive (arrows, top row) and active (arrows, bottom row) bowel segments in an 18-year-old girl (a-d) and a 10-year-old boy (e-h). a-d Terminal ileum identified on b = 0 echoplanar images (EPI) (a) exhibits low signal on DWI (b) and high average apparent diffusion coefficient (ADC) (2.69 × 10−3) (c) with histology (d) showing normal mucosal crypt architecture (hematoxylin and eosin, 100x). e-h Descending colon identified on b = 0 EPI image (e) exhibits high signal on DWI (f) and low average ADC (1.35 × 10−3) (g) with histological evidence (h) of mucosal fissuring and neutrophil infiltration (hematoxlin and eosin 20x)
Diffusion-weighted imaging (DWI) discrimination between noninflamed and inflamed bowel segments compared with histological reference. a Dot plot depicts all of the ADC values for the 45 nonactive bowel segments (blue diamonds) and the 33 segments with active inflammation (red squares) by histology (blue). Dashed lines indicate median ADC values for each group. b Summary of overall mean ADC values between active (white) and nonactive (gray) groups. Error bars indicate standard error of the mean. Asterisk indicates statistical significance (P = 0.0007, Student’s t-test). ADC apparent diffusion coefficient
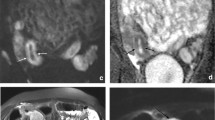
Three imaging examples show added value of diffusion-weighted imaging (DWI) to standard MR enterography (MRE) for assessing active disease. a–c The terminal ileum (arrows) in a 10-year-old boy demonstrates high signal on DWI (a) and restricted diffusion (average ADC of 1.29 × 10−3) with T2 hyperintensity and wall thickening on standard MR enterography (b), consistent with active disease as confirmed by histological findings (c). d–f The ascending colon (arrows) in a 17-year-old girl also demonstrates restricted diffusion on DWI (ADC 1.35 × 10−3)(d); however, standard MR enterography (e) was normal and histological findings (f) showed no active disease. g–i The terminal ileum in (arrows) a 16-year-old boy shows no restricted diffusion on DWI (ADC 2.43 × 10−3)(g) despite wall thickening and T2 hyperintensity on standard MR enterography (h), with histological findings showing no active disease and confirming the DWI findings (i). These examples show the benefit of DWI as an initial screen for Crohn disease activity, with standard MR enterography serving as confirmation for segments exhibiting low ADC. ADC apparent diffusion coefficient, DWI diffusion-weighted imaging
Value of combining DWI with MR enterography for assessing disease activity
Our results indicated that DWI had lower accuracy compared with standard MR enterography for detecting active Crohn disease inflammation. However, the high sensitivity of DWI suggested it could add value for detecting subtle areas of inflammation that standard MR enterography might miss. To test this, we reanalyzed our dataset using a combined enterography–DWI approach. Two algorithms were applied. Algorithm 1 (DWI as initial screen) had an accuracy of 76.9%, which was significantly higher than either MR enterography or DWI alone (P = 0.031 and P = 0.032 respectively, McNemar’s test) as well as increased specificity (88.9%). The improved accuracy compared with standard MR enterography alone is attributable to two positive cases in which DWI + MR enterography was true positive because of low ADC and the presence of MR enterography features of inflammation that were initially interpreted on standard MR enterography alone as negative, and four negative cases in which DWI+ enterography showed true negatives because of high ADC while standard MR enterography showed false positives. Algorithm 2 (MR enterography as initial screen) had an accuracy of 60.3%, which was lower than either MR enterography or DWI alone, and primarily because of low specificity (44.4%).
Discussion
Various studies have demonstrated the high accuracy of MR enterography in detection of Crohn disease in young patients [3, 13, 16, 17]. Standard MR enterography assessment for active inflammation primarily involves evaluation on T2-weighted and T1-weighted fat-suppressed multiphase post-contrast images [3, 14–16, 18, 19]. DWI is a quantitative rapid imaging technique that assesses changes in diffusivity of water protons that has been used to image numerous abdominal disease processes [20]. It has been hypothesized that DWI is more sensitive than standard MR enterography pulse sequences for detection of Crohn disease activity because of its potential to assess microscopic immune cell infiltration of bowel [11]. An additional benefit of DWI is its ability to assess inflammation without the need for intravenous contrast agent, avoiding the need for intravenous access and the potential risk of adverse contrast agent reaction. DWI has been shown in several recent studies to be an accurate noninvasive method to detect Crohn disease inflammation in adults [19, 21, 22] and children [13, 22–24]. In these studies, the presence of active bowel inflammation has been associated with restricted diffusion manifest as a reduction in ADC. DWI also adds value in detection of extraluminal complications of Crohn disease such as abdominopelvic abscesses and fistulae [25–27]. Presence of sacroiliitis can be detected [28].
Our study goal was to establish whether there is value in incorporating DWI into standard MR enterography protocols. Previous studies establishing the accuracy of DWI for evaluating Crohn disease activity have studied the performance of DWI alone rather than in combination with standard MR enterography. However in clinical practice DWI is typically added to standard MR enterography pulse sequences rather than replacing them. Given the desire to reduce overall MRI scan times, particularly in young patients, the additional time associated with DWI must be justified. An additional strength of this study is the use of a histological reference for inflammation from endoscopic biopsy that is generally considered a more reliable standard [3, 29] for assessing inflammatory activity on a bowel-segment basis compared with imaging or clinical-based criteria used in other studies.
Our results show that bowel segments with histological evidence of mucosal inflammation demonstrated lower ADC values (Fig. 3 and Table 2) on DWI (mean ADC 1.56 ± 0.71 × 10−3 mm2/s) compared with non-inflamed bowel segments (mean ADC 2.58 ± 1.4 × 10−3 mm2/s), a difference that was statistically significant. The calculated ADC values for both groups in our study are very similar to those observed in other recent studies examining DWI in inflammatory bowel disease [22, 31]. We then compared the performance of DWI to that of standard MR enterography pulse sequences (T2-weighted, balanced steady-state free precession, and dynamic T1-weighted fat-suppressed post-contrast images) for detecting active disease, using an ADC threshold of 2.0 × 10−3 mm2/s. Our results indicate that DWI has higher sensitivity but lower specificity and overall accuracy for assessing Crohn disease activity compared with standard MR enterography. The higher sensitivity and lower specificity of DWI for active disease that we observed are not surprising and can be attributed to DWI’s high sensitivity for active inflammatory changes (e.g., neutrophilic infiltration and mural edema) at the microscopic level, as well as the presence of chronic inflammatory changes (e.g., lymphocytic infiltration and fibrosis) in some nonactive bowel segments that would lead to reduction in ADC [20].
Given these test performance characteristics of DWI and standard MR enterography, we hypothesized that DWI could add value in detecting some areas of active inflammation that standard MR enterography might miss. To test this, we applied two algorithms for combined MR enterography–DWI evaluation of activity: one used DWI as the initial screen followed by standard MR enterography assessment of DWI-positive segments, and the other used standard MR enterography as the initial screen followed by DWI assessment of MR enterography-negative segments. The accuracy of the DWI followed by MR enterography algorithm (Table 3) was 76.9%, which was statistically higher than that of either technique alone. In contrast, the accuracy and specificity of the MR enterography followed by DWI algorithm were both lower than those of either technique alone. Our results validate the incorporation of DWI into MR enterography protocols and suggest a way to utilize DWI for image interpretation within the context of standard MR enterography pulse sequences. DWI appears to perform best when it is used as the initial screen for active disease, followed by standard MR enterography confirmation of evidence of active disease in segments showing ADC restriction (Fig. 4). In this way, DWI can serve a similar role to that of [F-18]2-fluoro-2-deoxyglucose PET/CT exams, where positron emission tomography is often used as the primary screen for hypermetabolic foci followed by CT confirmation of anatomical localization.
Flowchart depicts combined DWI–MR enterography (MRE) algorithm. Diffusion-weighted imaging (DWI) is used as the initial screen for active disease. Bowel segments with high apparent diffusion coefficient (ADC) values are considered negative, while those with low ADC values are then screened by standard MR enterography. Segments with MR enterography features of active disease are considered positive, while those with no active features are considered negative
Our study has limitations in terms of the total number of bowel segments included, as well as its retrospective nature. Future studies are needed to determine whether this combined MR enterography–DWI approach is generalizable for evaluation of Crohn disease activity on MR enterography. In particular, a prospective study would be helpful to evaluate children with Crohn disease pre- and post-treatment to assess its accuracy for detecting treatment response. With the increasing number of novel biological agents entering clinical trials for inflammatory bowel disease, noninvasive biomarkers of response are crucial for evaluation of these agents in the pediatric population. Finally, the accuracy values for DWI reported in our study are lower than those observed in some other studies [22, 30]. It is unclear what accounts for this difference, although our study used a histological reference versus an imaging-based reference for inflammatory activity, which might explain this difference. Other factors related to the specific MR vendor and echoplanar imaging (EPI) DWI sequence used might also be contributory.
Conclusion
Although DWI alone does not perform as well as standard MR enterography for detection of active Crohn disease, a combined DWI–MR enterography algorithm utilizing DWI as the initial screen for disease activity followed by standard MR enterography evaluation of DWI-restricted segments increases the accuracy in determining Crohn disease activity compared with either technique alone. These results indicate that DWI adds value to MR enterography and support the incorporation of DWI into MR enterography protocols for evaluation of Crohn disease in young patients. Our study also suggests a method to utilize DWI for clinical image interpretation within the context of standard MR enterography pulse sequences.
References
Silverstein J, Grand D, Kawatu D et al (2012) Feasibility of using MR enterography for the assessment of terminal ileitis and inflammatory activity in children with Crohn disease. J Pediatr Gastroenterol Nutr 55:173–177
Darge K, Anupindi SA, Jaramillo D (2008) MR imaging of the bowel: pediatric applications. Magn Reson Imaging Clin N Am 16:467–478
Gee MS, Nimkin K, Hsu M et al (2011) Prospective evaluation of MR enterography as the primary imaging modality for pediatric Crohn disease assessment. AJR Am J Roentgenol 197:224–231
Mollard BJ, Smith EA, Dillman JR (2015) Pediatric MR enterography: technique and approach to interpretation — how we do it. Radiology 274:29–43
Brenner DJ (2008) Should computed tomography be the modality of choice for imaging Crohn’s disease in children? The radiation risk perspective. Gut 57:1489–1490
Smith EA, Dillman JR, Adler J et al (2012) MR enterography of extraluminal manifestations of inflammatory bowel disease in children and adolescents: moving beyond the bowel wall. AJR Am J Roentgenol 198:W38–W45
Leyendecker JR, Bloomfeld RS, DiSantis DJ et al (2009) MR enterography in the management of patients with Crohn disease. Radiographics 29:1827–1846
Feng S-T, Law MW-M, Huang B et al (2010) Radiation dose and cancer risk from pediatric CT examinations on 64-slice CT: a phantom study. Eur J Radiol 76:e19–e23
Goske MJ, Applegate KE, Boylan J et al (2008) The ‘Image Gently’ campaign: increasing CT radiation dose awareness through a national education and awareness program. Pediatr Radiol 38:265–269
Qayyum A (2009) Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics 29:1797–1810
Oto A, Kayhan A, Williams JTB et al (2011) Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 33:615–624
Oto A, Zhu F, Kulkarni K et al (2009) Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol 16:597–603
Ream JM, Dillman JR, Adler J et al (2013) MRI diffusion-weighted imaging (DWI) in pediatric small bowel Crohn disease: correlation with MRI findings of active bowel wall inflammation. Pediatr Radiol 43:1077–1085
Sempere GAJ, Martinez Sanjuan V, Medina Chulia E et al (2005) MRI evaluation of inflammatory activity in Crohn’s disease. AJR Am J Roentgenol 184:1829–1835
Florie J, Wasser MNJM, Arts-Cieslik K et al (2006) Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. AJR Am J Roentgenol 186:1384–1392
Darbari A, Sena L, Argani P et al (2004) Gadolinium-enhanced magnetic resonance imaging: a useful radiological tool in diagnosing pediatric IBD. Inflamm Bowel Dis 10:67–72
Alexopoulou E, Roma E, Loggitsi D et al (2009) Magnetic resonance imaging of the small bowel in children with idiopathic inflammatory bowel disease: evaluation of disease activity. Pediatr Radiol 39:791–797
Tolan DJM, Greenhalgh R, Zealley IA et al (2010) MR enterographic manifestations of small bowel Crohn disease. Radiographics 30:367–384
Oto A, Fan X, Mustafi D et al (2009) Quantitative analysis of dynamic contrast enhanced MRI for assessment of bowel inflammation in Crohn’s disease. Acad Radiol 16:1223–1230
Moore WA, Khatri G, Madhuranthakam AJ et al (2014) Added value of diffusion-weighted acquisitions in MRI of the abdomen and pelvis. AJR Am J Roentgenol 202:995–1006
Kiryu S, Dodanuki K, Takao H et al (2009) Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging 29:880–886
Freiman M, Perez-Rossello JM, Callahan MJ et al (2013) Characterization of fast and slow diffusion from diffusion-weighted MRI of pediatric Crohn’s disease. J Magn Reson Imaging 37:156–163
Neubauer H, Pabst T, Dick A et al (2013) Small-bowel MRI in children and young adults with Crohn disease: retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol 43:103–114
Sohn B, Kim M-J, Koh H et al (2014) Intestinal lesions in pediatric Crohn disease: comparative detectability among pulse sequences at MR enterography. Pediatr Radiol 44:821–830
Oto A, Schmid-Tannwald C, Agrawal G et al (2011) Diffusion-weighted MR imaging of abdominopelvic abscesses. Emerg Radiol 18:515–524
Schmid-Tannwald C, Agrawal G, Dahi F et al (2012) Diffusion-weighted MRI: role in detecting abdominopelvic internal fistulas and sinus tracts. J Magn Reson Imaging 35:125–131
Yoshizako T, Wada A, Takahara T et al (2012) Diffusion-weighted MRI for evaluating perianal fistula activity: feasibility study. Eur J Radiol 81:2049–2053
Bozgeyik Z, Ozgocmen S, Kocakoc E (2008) Role of diffusion-weighted MRI in the detection of early active sacroiliitis. AJR Am J Roentgenol 191:980–986
Fefferman DS, Farrell RJ (2005) Endoscopy in inflammatory bowel disease: indications, surveillance, and use in clinical practice. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 3:11–24
Hordonneau C, Buisson A, Scanzi J et al (2014) Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 109:89–98
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shenoy-Bhangle, A.S., Nimkin, K., Aranson, T. et al. Value of diffusion-weighted imaging when added to magnetic resonance enterographic evaluation of Crohn disease in children. Pediatr Radiol 46, 34–42 (2016). https://doi.org/10.1007/s00247-015-3438-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-015-3438-1