Abstract
Ejection fraction (EF) and fractional shortening (FS) are standard methods of quantifying left ventricular (LV) systolic function. 2D global longitudinal strain (2D GLS) is a well-established, but underutilized method for LV function quantification. The aim of this study was to assess precision of GLS compared to EF & FS in pediatrics. Echocardiograms were prospectively analyzed by 2 blinded observers. FS, EF, and GLS were calculated following standard methods. Bland–Altman was applied to assess agreement. Intraclass correlation coefficient (ICC) was used to measure reliability. Coefficient of variation was used to demonstrate relative variability between methods. 103 pediatric echos were evaluated for inter-observer reproducibility, and 15 patients for intra-observer reproducibility. GLS had higher inter-observer agreement and reliability (bias 7%, 95% LOA − 3.4 to + 3.5, ICC 0.86 CI 0.80–0.90) compared to EF (bias 27%, 95% LOA − 18.9 to + 19.5; ICC 0.25 CI 0.07–0.43) and FS (bias 12%, 95% LOA − 11.9 to + 12.2; ICC 0.53 CI 0.38–0.66). GLS also had higher intra-observer agreement (bias 4%, 95% LOA − 3.6 to + 3.7; ICC 0.87 CI 0.66–0.96) compared to EF (bias 11%, 95% LOA − 14.9 to + 15.1; ICC 0.26 CI − 0.28–0.67) and FS (bias 12%, 95% LOA − 12.2 to + 12.5; ICC 0.38 CI − 0.15–0.74). GLS is a more precise method for quantifying LV function in pediatrics, with lower variability compared to EF and FS. GLS provides a more reliable evaluation of LV systolic function and should be utilized more widely in pediatrics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The assessment of left ventricular (LV) function is one of the most frequent indications for obtaining echocardiograms in the pediatric population. Measurement of fractional shortening (FS) and ejection fraction (EF) are long-standing conventional methods of assessing LV systolic function, however, both have important limitations [1,2,3]. Cardiac magnetic resonance imaging (MRI) is the clinical non-invasive gold standard for measuring ventricular function; however, due to availability, cost, and time requirements, echocardiography is more routinely used for screening and monitoring cardiac dysfunction in the clinical setting. Two-dimensional speckle-tracking strain echocardiography (2D STE) is a method of quantifying ventricular systolic function by tracking acoustic backscatter within the ventricular myocardium. 2D STE has been prevalent in the cardiac literature for the past two decades, and no longer considered a “novel” functional technique. The technique has been validated against in vitro and in vivo gold standards, with sonomicrometry and cardiac MRI. The normal values for LV strain have been defined in large populations in adults [4, 5] and pediatrics [6, 7]. Additionally, clinical advantages of GLS over EF and FS has been reported, including greater sensitivity for detecting pre-clinical cardiac dysfunction [8,9,10,11]. Recent studies in adult patients have reported lower variability in LV GLS measurements compared to EF, with GLS having superior prognostic value over LV EF for predicting major adverse cardiac events [12]. Despite these apparent advantages over EF and FS, strain continues to be an underutilized measurement in clinical echocardiography, especially in pediatrics. To our knowledge the precision of GLS compared to FS and EF has not been investigated in pediatrics. Thus, we aimed to assess the precision of LV GLS compared to LV EF and FS, with the hypothesis that LV GLS is a more precise LV functional parameter than EF or FS in pediatrics.
Methods
Study Design and Patient Population
Echocardiograms were obtained prospectively using standardized protocols between October 2013 and December 2015 at the St. Louis Children’s Hospital Heart Center at Washington University School of Medicine. All echocardiograms had been ordered for routine clinical evaluations. The study protocol was approved by the Institutional Review Board at Washington University School of Medicine. A total of 103 pediatric patients (< 22 years of age) echocardiograms meeting the following criteria were analyzed.
Inclusion and Exclusion Criteria
All patients above 1 month of age with a four-chambered heart and echocardiograms suitable for 2D strain, EF and FS analysis were included. Patients with poor echocardiographic windows, poor ECG tracings, or inadequate LV endomyocardial border visualization were excluded. Neonatal subjects (< 30 days old) were excluded due to the known ventricular remodeling that occurs during the neonatal period. Echos with significant wall motion abnormalities (dyskinesis or paradoxical motion), a known pitfall of M-mode analysis, were also excluded. Finally, patients with hemodynamically significant structural congenital heart disease, history of cardiac surgery, or arrhythmias were excluded.
Sample Size Determination
Post-hoc power analysis was performed based on results of the intraclass correlation coefficient (ICC) below. A sample size consisting of 52 subjects with two observations per subject was able to achieve 99% power to detect an ICC of 0.925 under the alternative hypothesis when the ICC under the null hypothesis is 0.692 using an F test with a significance level or α of 0.001. Based on this analysis, a study population of 103 patients was found to be more than adequate.
Data Acquisition
All echocardiograms were obtained prospectively by experienced pediatric cardiac sonographers following pediatric guidelines from the American Society of Echocardiography [13]. Conventional two-dimensional images were acquired on commercially available ultrasound machines (Vivid 7 or E9; GE Medical Systems, Milwaukee, WI) according to the standardized American Society of Echocardiography (ASE) protocol [13, 14]. The images were obtained using a phased-array transducer with central frequency ranging between 5 and 12 MHz. Cine image data were digitally stored for at least 3 cardiac cycles in native (uncompressed) frame rates for offline analysis on analysis software (EchoPAC version 110.1.2; or Digisonics version 3.8.4.2).
Two observers (M.D.P, C.M) were simultaneously trained in functional analysis by an expert pediatric echocardiographer (S.A). After a training period, the two observers analyzed 10 randomly selected echocardiograms and an analysis of variance was performed. Results were disclosed to the observers and techniques compared to narrow variations in analysis. These 10 initial echocardiograms were excluded from the final dataset. Following the training period, 2D global longitudinal strain, ejection fraction and fractional shortening were calculated for each study by the 2 observers blinded to each other’s analysis. The same echocardiographic cardiac cycle was utilized by both observers for each measurement. 15% of the studies had repeat assessments by each observer to evaluate intra-observer variability.
2D Strain Analysis
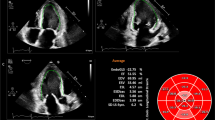
2D Lagrangian longitudinal strain (GLS) was obtained for each patient utilizing a standardized LV 2D strain protocol [15,16,17]. Offline 2D strain analysis of each study was performed for the entire cardiac cycle. Automated tracking of myocardial deformation was performed using EchoPAC v110.1.2 (GE Medical Systems, Milwaukee WI) for determination of six segment LV longitudinal strain for each of the 3 following echocardiographic views: Apical 4 chamber, Apical 2 chamber, and Apical 3 chamber views. Peak GLS from each of the views (determined by the nadir of the strain curve) was averaged to produce GLS. Tracking was visually inspected throughout the cardiac cycle and minimally manually adjusted as needed. Strain analysis on 15 randomly selected subjects was repeated by both observers (M.D.P, C.M) at least 4 weeks after the initial measurement to obtain data regarding inter-observer variability.
Ejection Fraction
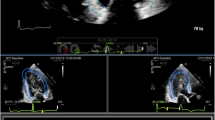
Echocardiographic 2D images were obtained in the apical 4 chamber LV focused view, and the apical 2 chamber LV view for each study. Only studies showing the entirety of the endocardial border of the left ventricle were included. Biplane method of disks (also known as biplane Simpson method) was utilized for absolute LV volume measurements for each study using Digisonics Digiview v3.8.4.2 (Digisonics Inc., Houston, TX). The same cardiac cycles and clips were measured by each observer in the both views, per ASE guidelines [14]. The observers manually traced the endocardial border at end-diastole and end-systole in each view. End-diastole was defined as the peak of the electrocardiographic R-wave or just prior to onset of mitral valve closure in 2-D imaging. End systole was defined as the frame prior to opening of the mitral valve. Volume measurements were then used to calculate ejection fraction with the following formula: [(EDV − ESV)/EDV] × 100 = EF (%).
Fractional Shortening
M-mode echocardiography was performed in the parasternal short axis view through center of the LV at the level of the papillary muscles, per standard ASE protocol [13]. Both observers calculated FS for each study and measurements were performed offline using Digisonics Digiview v3.8.4.2. Again, the same cardiac cycles and clips were analyzed by each observer. FS was calculated using the following formula: [(LVEDD − LVESD)/LVEDD] × 100 = FS (%).
Statistical Analysis
Agreement between the blinded measurements was evaluated with inter-observer and intra-observer variability analysis. The two observers (M.D.P and C.M) performed offline analysis using the same measurement protocol. Variability was evaluated via Bland–Altman analysis [percentage bias and 95% limits of agreement (LOA)]. Coefficients of variation (CV) were calculated to demonstrate relative variability between methods of measure, and the CVs for each method were compared using the Friedman test with the Bonferroni adjustment [18].
Intraclass correlation coefficient (ICC) was used to measure intra-observer and inter-observer reliability within measurements. ICC is a commonly used statistic to assess rater reliability of measurements and incorporates the magnitude of disagreement rather an all or nothing agreement. Higher ICC values indicate greater reliability between measurements, with an ICC of 1 indicating perfect agreement and 0 indicating only random agreement. Post-hoc power analysis was performed as above utilizing ICC results for each clinical method. The above statistical analysis was performed using SPSS version 22.0 (SPSS, Inc., Armonk, NY).
Results
Functional analysis was performed on 103 individual studies determined to have adequate imaging. Patient characteristics are listed in Table 1. Median age was 13.2 years with 58% males. The most common indication for echocardiogram in this population was for surveillance after receiving chemotherapy. The second most common was routine outpatient screening; more specifically this included screenings for chest pain, palpitations, murmur, syncope or a family history (cardiomyopathy, bicuspid aortic valve, predisposing genetic condition, etc.) requiring evaluation by echocardiogram. Another common indication was surveillance after orthotopic heart transplantation. Three patients with history of cardiomyopathy related to coronary abnormalities (n = 2) or myocarditis (n = 1) were also included. Mean heart rate (72 bpm, range 41–104) approximated the mean frame rate (70 f/s, range 53–94) for source images.
Agreement, Bland–Altman Analysis
Mean values for each functional analysis method are shown in Table 2. No significant differences were found between means for GLS and FS, however the means between individual users were significantly different for EF (p < 0.001). The mean differences for inter-observer and intra-observer measurements are shown in Table 3. GLS had higher precision, i.e. higher inter-observer agreement and lower variability compared to EF and FS (Fig. 1; Table 4). Inter-observer variability was significant for EF, with high bias, wide limits of agreement. FS showed lower bias but wide limits of agreement. GLS had the strongest agreement and lowest variability.
Intra-observer analysis of 15 cases (15% of subjects) showed similar results, with low bias, and narrow limits of agreement for GLS. FS again showed low bias, but wide limits of agreement. EF had highest variability (Fig. 2).
Reliability, Intra-class Correlation Analysis
A high degree of reliability was found between the two observers’ measurements for GLS, with an ICC of 0.86 (Table 4). A moderate degree of reliability was found between the two individual observer measurements for FS, with an ICC of 0.53. Poor reliability was found for inter-observer EF with an ICC of 0.25.
Similar results were found for intra-observer measurements of GLS, with an ICC of 0.87. Intra-observer reliability for FS was poor with an ICC of 0.38. Again, poor reliability was found for intra-observer measurements of EF, with an ICC of 0.26 (Table 4).
For the inter-observer measurements, the coefficient of variation was lowest for GLS, with a CV of 3.61. It was slightly higher for FS with a CV of 5.88, and highest for EF with a CV of 18.95, showing the highest variation between measurements in EF. Using the Friedman test with Bonferroni adjustment, the CV for GLS was found to be significantly different from the CVs for EF (p < 0.0001), and FS (p = 0.0006). The CVs for EF and FS were also found to be significantly different (p < 0.0001).
Similar results were found for intra-observer measurements with a CV of 0.55 for GLS, compared to 3.97 for FS, and 4.11 for EF. Variability was lowest for observer 1 repeated measurements of GLS, and highest for observer 1 repeated measurements of EF. Using the Friedman test with Bonferroni adjustment, the CV for GLS was significantly different from the CV for EF (p < 0.02), but not significantly different from the CV for FS (p = 0.071). The CVs for EF and FS were not found to be significantly different (p = 0.071).
Discussion
The results of this study support our hypothesis that GLS is a more precise LV functional parameter than EF or FS. For the more routinely used analysis methods, FS by M-mode was more reproducible than EF calculated by the biplane method of disks. This finding is noteworthy and may be partly explained by our exclusion criteria. Consistent with the clinical practice in many echocardiography labs, we excluded subjects with considerable wall motion abnormalities (significant dyskinesis or paradoxical motion), a known pitfall of M-mode-based measurements. This may have resulted in a selection bias resulting in lower variability in FS measurements. In addition, the manual calculation of biplane EF from two separate images (versus one image for FS) may create opportunity for greater variability in EF compared to FS.
Limitations in EF and FS have been well established in the literature, though to-date they remain the most commonly used LV function quantification methods. Lee et al. showed inter-acquisition variability led to the near doubling of intra-reader variability when measuring LV dimensions and FS [19]. EF and FS are subject to limitations in acquisition, reader variability and differences in measurement techniques. Some examples include apical dropout, single beat versus 3 beat averaging, and operator variability. Margossian et al. compared multiple EF analysis methods and found significant differences between the various algorithms in pediatric subjects [20]. In this study, reproducibility of EF increased by using an average of three heart beats, though this may not be feasible in routine clinical practice due to time constraints. In potential pediatric cardiac transplant donors, Chen et al. found significant variability between local donor hospital measurements of FS and a central laboratory FS analysis of the same study, enough so to alter donor candidacy [21]. This raises considerable concern regarding the reliance on FS and EF for functional assessment in pediatrics. On the other hand, 2D LV GLS has been validated against in vitro and in vivo gold standards [22, 23], and normal values have been established from multiple large studies in adult [4, 5, 17] and pediatric populations [6, 7]. While 2D strain analysis is subject to some of the same limitations as EF (endocardial border visualization, apical dropout, etc.), strain analysis has key differentiators that may contribute to higher precision. These include the inherent algorithm of tracking acoustic backscatter that is relatively stable through the cardiac cycle, integration of key landmarks (mitral annulus, apex, endocardial border) into the analysis, and angle independence. This “semi-automated” method of functional analysis may reduce reader variability [24,25,26]. Strain also provides regional myocardial deformation patterns and is less dependent on ventricular loading conditions, both of which are well known limitations of EF and FS.
Clinical Implications
Clinical decisions are regularly based on the echocardiographic assessment of LV systolic function. The ability to follow changes over time and maintaining reliable measurements between readers is paramount. In addition to higher precision, strain offers several clinical advantages over EF and FS. Superiority of GLS over LVEF in predicting outcomes has been reported in adults [27], including a recent large meta-analysis by Kalam et al. showing superior prognostic value of GLS over LV EF for predicting major adverse cardiac events [12]. Several studies have shown the capability of 2D GLS in early detection of cardiac dysfunction. This has been shown extensively in adult patients following cardiotoxic chemotherapy [9], as well as in pediatrics [9, 28, 29], allowing earlier treatment of heart disease and alterations in treatment plans [30, 31] Similarly, strain has shown promise in recipients of cardiac transplants for detection of rejection [10, 32]. The potential clinical applications for pediatric patients continue to expand [33,34,35]. Given higher reproducibility and prognostic ability, the data from our study and the studies referenced argue for the inclusion of LV GLS as a routinely reported measurement of LV systolic function in clinical pediatric echo reports.
Historically, a major limitation in 2D strain analysis has been variability in measurements across different echo platforms and strain analysis software, limiting widespread clinical utilization. Prior literature has shown these differences, while significant, are similar across vendors [36]. To address this important limitation there has been a coordinated effort among the medical and scientific communities and commercial imaging vendors to standardize deformation imaging, resulting in recommendations summarized in a consensus document in 2015 [37]. Follow-up studies have subsequently shown reduction in inter-vendor variability for GLS [18, 38]. Of note, important variability in regional (segmental) longitudinal strain measurements remains an active issue [39, 40]. At this time, experience over the past two decades has proven LV GLS to be a robust and reliable measure of LV systolic function, with distinct advantages over EF and FS. Findings from this paper suggest the same may be said of LV GLS in pediatrics, albeit from a single-center experience with limitations as below.
Limitations
We note that there are limitations in this study. We utilized Simpson’s biplane method of disks for measurement of EF, though in pediatrics the 5/6 area length method with a 3-beat average has shown greater reproducibility [20]. The ASE currently recommends Simpson’s biplane method of disks for EF calculation [13], and this continues to be the method of EF calculation for most clinical echocardiography labs. Thus, to produce the most generalizable results, our group chose the biplane method of disks methods for EF calculation. The population we investigated was largely healthy. As a follow-up, the same analysis needs to be conducted in a larger cohort, preferably multi-center, and include patients with LV pathology. Also, we did not assess test–retest variability. Additionally, we compared the precision (reproducibility) of LV functional parameters though accuracy (comparison against a gold standard) was not addressed in this study. Prospective study comparing the above methods with cardiac MRI measurements would be of benefit in order to access accuracy. Lastly, our variability analysis was limited to a single vendor software and version. As discussed in the preceding section, inter-vendor variability in strain measurements has historically been a significant issue. Though standardization initiatives [37] have led to decrease in variability in GLS [18, 38], validation of this study’s findings with other ultrasound platforms and strain analysis software is warranted.
Conclusion
LV GLS is a more precise measure of LV systolic function compared to LV EF and LV FS in pediatric subjects with four-chambered hearts. FS in this cohort was more reproducible than LV EF. Given these findings and recent data showing improvements in inter-vendor variability, we propose LV GLS be included as a routinely reported functional parameter in pediatric (> 1 month old) echo reports, in addition to LV FS and EF. Follow-up of our study’s findings could include a multi-center trial in a heterogeneous pediatric population with a spectrum of LV disease using multi-vendor systems, and assessment of accuracy against cardiac MRI.
Abbreviations
- GLS:
-
Global longitudinal Lagrangian strain
- ICC:
-
Intraclass correlation coefficient
- CV:
-
Coefficient of variation
- 2D STE:
-
Two-dimensional speckle tracking echocardiography
- LV:
-
Left ventricular
- Obs:
-
Observer
References
Wood PW, Choy JB, Nanda NC, Becher H (2014) Left ventricular ejection fraction and volumes: it depends on the imaging method. Echocardiography 31:87–100
Picard MH, Popp RL, Weyman AE (2008) Assessment of left ventricular function by echocardiography: a technique in evolution. J Am Soc Echocardiogr 21:14–21
Cameli M, Mondillo S, Solari M et al (2016) Echocardiographic assessment of left ventricular systolic function: from ejection fraction to torsion. Heart Fail Rev 21:77–94
Sugimoto T, Dulgheru R, Bernard A et al (2017) Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 18:833–840
Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH (2013) Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 26:185–191
Jashari H, Rydberg A, Ibrahimi P et al (2015) Normal ranges of left ventricular strain in children: a meta-analysis. Cardiovasc Ultrasound 13:37
Levy PT, Machefsky A, Sanchez AA et al (2016) Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr 29(209–225):e6
Ari ME, Cetin II, Kocabas A, Ekici F, Ceylan O, Surucu M (2016) Decreased deformation in asymptomatic children with isolated left ventricular non-compaction and normal ejection fraction. Pediatr Cardiol 37:201–207
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63:2751–2768
Dandel M, Hetzer R (2017) Post-transplant surveillance for acute rejection and allograft vasculopathy by echocardiography: usefulness of myocardial velocity and deformation imaging. J Heart Lung Transplant 36:117–131
Gunasekaran P, Panaich S, Briasoulis A, Cardozo S, Afonso L (2017) Incremental value of two dimensional speckle tracking echocardiography in the functional assessment and characterization of subclinical left ventricular dysfunction. Curr Cardiol Rev 13:32–40
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100:1673–1680
Lopez L, Colan SD, Frommelt PC et al (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23:465–495
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1–39):e14
Systems GM (2008). In: Systems GM (ed) EchoPAC PC user manuel, 1st edn. General Electric Co., Boston, pp. 1–627.
Lorch SM, Ludomirsky A, Singh GK (2008) Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J Am Soc Echocardiogr 21:1207–1215
Takigiku K, Takeuchi M, Izumi C et al (2012) Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J 76:2623–2632
Yang H, Marwick TH, Fukuda N et al (2015) Improvement in strain concordance between two major vendors after the strain standardization initiative. J Am Soc Echocardiogr 28(642–8):e7
Lee CK, Margossian R, Sleeper LA et al (2014) Variability of M-mode versus two-dimensional echocardiography measurements in children with dilated cardiomyopathy. Pediatr Cardiol 35:658–667
Margossian R, Chen S, Sleeper LA et al (2015) The reproducibility and absolute values of echocardiographic measurements of left ventricular size and function in children are algorithm dependent. J Am Soc Echocardiogr 28(549–558):e1
Chen S, Selamet Tierney ES, Khush KK et al (2015) Reliability of echocardiographic measurements of left ventricular systolic function in potential pediatric heart transplant donors. J Heart Lung Transplant 34:100–106
Korinek J, Wang J, Sengupta PP et al (2005) Two-dimensional strain—a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr 18:1247–1253
Amundsen BH, Helle-Valle T, Edvardsen T et al (2006) Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 47:789–793
Geyer H, Caracciolo G, Abe H et al (2010) Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 23:351–369
Risum N, Ali S, Olsen NT et al (2012) Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr 25:1195–1203
Takahashi M, Harada N, Isozaki Y et al (2013) Efficiency of quantitative longitudinal peak systolic strain values using automated function imaging on transthoracic echocardiogram for evaluating left ventricular wall motion: new diagnostic criteria and agreement with naked eye evaluation by experienced cardiologist. Int J Cardiol 167:1625–1631
Stanton T, Leano R, Marwick TH (2009) Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2:356–364
Pignatelli RH, Ghazi P, Reddy SC et al (2015) Abnormal myocardial strain indices in children receiving anthracycline chemotherapy. Pediatr Cardiol 36:1610–1616
Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW (2012) Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr 25:733–740
Florescu M, Magda LS, Enescu OA, Jinga D, Vinereanu D (2014) Early detection of epirubicin-induced cardiotoxicity in patients with breast cancer. J Am Soc Echocardiogr 27:83–92
Negishi K, Negishi T, Haluska BA, Hare JL, Plana JC, Marwick TH (2014) Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 15:324–331
Sarvari SI, Gjesdal O, Gude E et al (2012) Early postoperative left ventricular function by echocardiographic strain is a predictor of 1-year mortality in heart transplant recipients. J Am Soc Echocardiogr 25:1007–1014
Ruotsalainen H, Bellsham-Revell H, Bell A, Pihkala J, Ojala T, Simpson J (2016) Right ventricular systolic function in hypoplastic left heart syndrome: a comparison of velocity vector imaging and magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 17:687–692
Ghelani SJ, Harrild DM, Gauvreau K, Geva T, Rathod RH (2016) Echocardiography and magnetic resonance imaging based strain analysis of functional single ventricles: a study of intra- and inter-modality reproducibility. Int J Cardiovasc Imaging 32:1113–1120
Schlangen J, Petko C, Hansen JH et al (2014) Two-dimensional global longitudinal strain rate is a preload independent index of systemic right ventricular contractility in hypoplastic left heart syndrome patients after Fontan operation. Circ Cardiovasc Imaging 7:880–886
Anwar S, Negishi K, Borowszki A et al (2017) Comparison of two-dimensional strain analysis using vendor-independent and vendor-specific software in adult and pediatric patients. JRSM Cardiovasc Dis 6:2048004017712862
Voigt JU, Pedrizzetti G, Lysyansky P et al (2015) Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 28:183–193
Shiino K, Yamada A, Ischenko M et al (2017) Intervendor consistency and reproducibility of left ventricular 2D global and regional strain with two different high-end ultrasound systems. Eur Heart J Cardiovasc Imaging 18:707–716
Nagata Y, Takeuchi M, Mizukoshi K et al (2015) Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr 28:630–641
Mirea O, Pagourelias ED, Duchenne J et al (2017) Variability and Reproducibility of segmental longitudinal strain measurement: a report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging 11(1):15–24
Acknowledgements
The authors wish to thank the sonographers and staff at the Heart Station, St. Louis Children’s Hospital for their contributions to echocardiographic acquisitions related to this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors. This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, M.D., Myers, C., Negishi, K. et al. Two-Dimensional Strain is more Precise than Conventional Measures of Left Ventricular Systolic Function in Pediatric Patients. Pediatr Cardiol 41, 186–193 (2020). https://doi.org/10.1007/s00246-019-02243-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-019-02243-8