Abstract
It has been reported that more than half of the ejected blood from the left ventricle is stored in the aorta during systole and expelled during diastole. One important organ that receives blood flow mainly during diastole is the heart. It is also reported that the cardiac blood supply–workload balance in small children is disadvantageous to the heart. Therefore, we measured the aortic reservoir function and examined the relationship between the aortic reservoir function and the cardiac blood supply–workload balance. The percent diastolic runoff, which is the percentage of the diastolic blood flow of the total cardiac output, was measured as the index of the aortic reservoir function. The subendocardial viability ratio—the ratio of the diastolic pressure time index (the blood supply to the heart) to the tension time index (implying the myocardial oxygen demand)—was investigated as an index of the cardiac blood supply–workload balance in children. The percent diastolic runoff was 51.7 ± 4.5%, smaller than that in adult. It had a significant positive relationship to age (r2 = 0.32, p = 0.0052). The subendocardial viability ratio was 100.8 ± 19.6% and had a strong relationship to the percent diastolic runoff (r2 = 0.92, p < 0.0001). The percent diastolic runoff had a positive relationship with age during childhood. The value had a strong impact on the cardiac blood supply–workload balance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that more than half of the ejected blood from the left ventricle runs off during diastole [1]. That means that the more than half of the ejected blood is temporarily stored in the aorta during systole; the capacity to store ejected blood in an aorta is called the aortic reservoir function. One of the most important organs that receives blood flow during diastole is the heart, that is, the coronary circulation mainly flows during diastole [2]. There are several important parameters that can influence the aortic reservoir function: aortic diameter, distensibility, and pressure wave reflection. The aortic diameter and distensibility contribute to determining the amount of blood that can be stored in the aorta, and a late return of the reflected pressure wave (during diastole) pushes the stored blood in the aorta to the heart.
We previously reported that the cardiac blood supply–workload balance in small children is disadvantageous to the heart and one of the reasons for that is an enhanced pressure wave reflection in children [3]. It is possible that the aortic reservoir function plays an important role in maintaining the cardiac blood supply–workload balance. However, little evidence is available regarding the aortic reservoir function in children. The purpose of this study was to evaluate the aortic reservoir function in children. Moreover, we aimed to investigate the relationship between the aortic reservoir function and cardiac blood supply–workload balance in children.
Methods
Study Subjects
This study enrolled 43 patients who had undergone a cardiac catheterization for small left-to-right shunt disorders (pulmonary-systemic flow ratio < 2.0) below 15 years of age. No patients had any significant leakage at the aortic level (e.g., patent ductus arteriosus, aortic regurgitation, etc.). All patients were in good health, had no symptoms of cardiovascular dysfunction, and were not taking any medications. These patients were adopted as patients with a “normal” aortic circulation. To eliminate the influence of obesity, the patients whose body mass index exceeded the age-gender-specific 95th percentile were excluded. The patients demonstrated neither systemic nor pulmonary hypertension. Because the diastolic pressure waveforms should be approximated to an exponential curve to calculate the diastolic runoff, meaning the aortic reservoir function (mentioned below), the patients whose diastolic pressure waveforms could not be approximated to the exponential curve were excluded. Finally, 23 patients were selected for analysis (Table 1).
Data Acquisition
Cardiac catheterization was performed by a femoral arterial puncture. The ascending aortic pressure was measured using a pressure sensor-mounted catheter (model SPC-454D, Millar Instrument, Inc., Houston, Texas) before the injection of any contrast material. The ascending aortic pressure waveform was recorded at one vertebral body thickness higher than the aortic valve. During the cardiac catheterization, patients were sedated by a venous injection of midazolam. The waveform was recorded on a hard disk through an analog–digital converter with a sample rate of 1000 Hz (1000 samples per second), and it was also simultaneously recorded with an electrocardiogram.
Aortic Pressure Waveform Analysis
Because the diastolic pressure waveforms were approximated to an exponential curve to calculate the diastolic runoff, which means the aortic reservoir function, the diastolic pressure waveform was approximated as an exponential curve. Therefore, the patients whose diastolic pressure waveforms did not adequately correlate to the approximated exponential curve (coefficient of determination r2 < 0.95) were excluded from this study. From the recorded pressure waveforms, the systolic blood pressure, diastolic blood pressure, and pulse pressure were measured in each patient, and the percent diastolic runoff and subendocardial viability ratio were calculated.
Percent Diastolic Runoff
The diastolic runoff is defined as the part of the stroke volume that is stored in large arteries during systole and flows into the small peripheral arteries during diastole by means of the elastic properties of the arterial walls. When expressed as a percentage of the stroke volume, it constitutes an index of the reservoir role of the large arteries. We measured the percentage of the diastolic runoff in our patients according to the method described by Levenson and colleagues [4].
Because the pressure–volume relationship can be approximated as linear between the timing of the aortic valve closure (dicrotic notch) and end-diastole, the diastolic runoff was calculated as follows:
where SAC is the systemic arterial compliance, DR is the diastolic runoff, Pd is the diastolic pressure, and the Ps’ is the pressure at the dicrotic notch, which is the pressure at the closure of the aortic valve.
The SAC was calculated as follows:
where Tau is the diastolic decay time constant and TPR is the total peripheral resistance, which is calculated as the mean arterial pressure divided by the cardiac output.
Because the pressure–time curve during diastole is an exponential curve, the time constant of the diastolic pressure decay Tau can be calculated as follows:
where Ln is the natural logarithm.
Subendocardial Viability Ratio
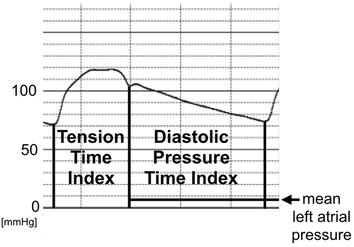
The subendocardium is thought to be more sensitive to a shortage of blood supply than the subepicardium. Buckberg and colleagues demonstrated that the ratio of the diastolic phase area (diastolic pressure time index) to the area of the systolic phase (tension time index) in the central aortic pressure profile has a close correlation to the blood supply to the subendocardium [5,6,7]. The ratio was designated as the subendocardial viability ratio:
Therefore, they coined this index as a measure of the hemodynamic capacity for the supply divided by the myocardial oxygen demand. The tension time index was obtained by measuring the area under the aortic systolic pressure curve and it equaled the mean aortic systolic pressure multiplied by the duration of systole. The diastolic pressure time index was obtained by measuring the area under the diastolic aortic pressure curve and subtracting the mean left atrial pressure (assumed to be equal to the left ventricular diastolic pressure) multiplied by the diastolic time from it. When the left atrial pressure was not recorded, we used the pulmonary capillary wedge pressure instead.
Statistical Analysis
All data were presented as the mean value ± standard deviation. Pearson’s correlation analysis was used to determine the correlation between two parameters. PRISM software version 4.0c (Graph Pad Software, Inc. La Jolla, CA, USA) was used for all statistical analyses. A p value less than 0.05 was considered to be statistically significant.
All subjects or their parents gave their written informed consent, and the Ethics Committees of Chiba Children’s Hospital and Hokkaido University Hospital approved the study protocol.
Results
The Table shows the demographic data. The patients were affected with a ventricular septal defect (9) or atrial septal defect (14), and their pulmonary to systemic flow ratio was 1.4 ± 0.3. The aortic systolic pressure was 91.5 ± 8.5 mmHg, diastolic pressure 58.2 ± 7.1 mmHg, and pulse pressure 33.2 ± 5.4 mmHg. When blood pressure waveforms during diastole were approximated to an exponential curve, the coefficient of the determination r2 between the real blood pressure waveform and approximated waveform was 0.992 ± 0.005.
The percent diastolic runoff, which was the percentage of the aortic blood flow during diastole to the stroke volume, was 51.7 ± 4.5%. Figure 1 demonstrates the relationship between age and percent diastolic runoff. The percent diastolic runoff had a significant positive correlation to age (r = 0.57, p = 0.0052).
The subendocardial viability ratio, which meant the cardiac blood supply–workload balance, was 100.8 ± 19.6% and had a significant correlation to age (r = 0.60, p = 0.0024) (Fig. 2). The subendocardial viability ratio had a strong correlation with the percent diastolic runoff (r = 0.96, p < 0.0001) (Fig. 3).
Discussion
The present study demonstrated that the aortic reservoir function had a positive correlation with age in children. Moreover, the aortic reservoir function had a strong impact on the cardiac blood supply–workload balance.
In the present data, the percent diastolic runoff (the aortic reservoir function) in children with a normal aorta was 51.7 ± 4.5%. This value was lower than that in adults. Levenson and colleagues reported that the diastolic runoff in adults without hypertension is 55% of the stroke volume [4]. The positive correlation between the percent diastolic runoff and the patient’s age in the present study also supported the hypothesis that the aortic reservoir function in small children is low. This is feasible, because the aortic pressure wave reflection is enhanced in childhood. We previously reported an elevation in the aortic pressure wave reflection in childhood, and the value was negatively correlated with the body height [8]. Because the late return (during diastole) of the reflected pressure wave expels the stored blood in aorta, the early return of it in small children due to their short height could be a disadvantage to the aortic reservoir function even if their aorta has adequate elasticity.
Another possible cause of the low percent diastolic runoff in small children is their high heart rate. Generally speaking, diastolic time becomes short in high heart rate. Practically, the heart rate negatively correlated with age (r = −0.58, p = 0.0034), and there was a positive correlation between diastolic time/RR interval and age (r = 0.58, p = 0.035) in this study. Therefore, it is possible that the percent diastolic runoff correlated with age through the interval of diastolic time.
The percent diastolic runoff had a significant relationship to the subendocardial viability ratio (Fig. 3), which meant the cardiac blood supply–workload balance. This strong correlation implied that the aortic reservoir function was important for maintaining the cardiac blood supply–demand balance. Buckberg and colleagues demonstrated that the subendocardial muscle is underperfused relative to the subepicardial muscle when the subendocardial viability ratio is below 70%, and when the value falls below 50%, the ratio of subendocardial to subepicardial flow falls precipitously, indicating subendocardial ischemia [2]. In the present study, no patients had a subendocardial viability ratio under 50%, which meant subendocardial ischemia. However, it can be imagined that even mild coronary obstruction in a youth could result in serious myocardial ischemia. Fortunately, such a condition is uncommon, because atherosclerotic lesions are unusual and ischemic heart disease is rare in childhood.
One of the reasons why the percent diastolic runoff had a significant relationship to the subendocardial viability ratio is the influence of the diastolic time. Judging from the formulas, both indices could be strongly affected by the diastolic time. Therefore, slowing heart rate is probably advantageous to management of heart failure, although cardiac output in children is said to be dependent on heart rate. Practically, it has been recognized that lowering heart rate by beta-blockers and ivabradine [9] is effective for chronic heart failure in adults [10]. Further studies are needed in order to prove the mechanism of the lowering heart rate in patients with heart failure.
Our study had several strengths. Especially, it was important that the pressure waveforms were recorded using a pressure sensor-mounted catheter. The analysis in this study would have been impossible without that catheter.
Our study had some limitations. One of the most important problems was that the patients had a left-to-right shunt. Therefore, the cardiac workload could have been slightly overestimated. Judging from the product of their flow ratio and pulmonary artery pressure, the increment of the cardiac workload was 10% at most. However, it could influence the cardiac blood supply–workload balance. Small sample size is another important limitation. It is difficult to collect much data about aortic pressure waveform in patients with normal aortic arch using a pressure sensor-mounted catheter. Because we recorded the pressure waveforms in children using an invasive method, it was necessary to administer sedative drugs to the patients. Therefore, the level of sedation could have influenced the cardiac blood supply–workload balance. Although there were such limitations, the relationship between the parameters should be reliable for each condition.
In conclusion, the aortic reservoir function was low in small children and improved with their growth. The aortic reservoir function had a strong impact on the cardiac blood supply–workload balance. Further studies are needed to clarify the relationship between the cardiac blood supply–workload balance and growth in children.
References
London GM, Guerin AP (1999) Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J 138:220–224
The coronary circulation. In: Nichols WW, O’Rourke MF, Vlachopoulos C (eds) (2011) McDonald’s blood flow in arteries, 6th edn. Hodder Arnold, London, pp 375–396
Murakami T, Takeda A, Takei K, Tateno S, Kawasoe Y, Niwa K (2014) The cardiac blood supply–workload balance in children. Heart Vessels 30:626–631
Levenson JA, Safar ME, Simon AC, Kheder AI, Daou JN, Levy BI (1981) Systemic arterial compliance and diastolic runoff in essential hypertension. Angiology 32:402–413
Buckberg GD, Fixler DE, Archie JP, Hoffman JI (1972) Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 30:67–81
Buckberg GD, Olinger GN, Mulder DG, Maloney JV (1975) Depressed postoperative cardiac performance. Prevention by adequate myocardial protection during cardiopulmonary bypass. J Thorac Cardiovasc Surg 70:974–994
Hoffman JIE, Buckberg GD (2014) The myocardial oxygen supply: demand index revisited. J Am Heart Assoc 3:e000285
Murakami T, Takeda A, Takei K, Ueno M, Yakuwa S, Yamazawa H, Frukawa T (2010) Aortic pressure wave reflection in children. Hypertens Res 33:225–228
Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, SHIFT Investigators (2010) Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376(9744):875–885
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18:891–975
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest.
Rights and permissions
About this article
Cite this article
Murakami, T., Takeda, A. Aortic Reservoir Function has a Strong Impact on the Cardiac Blood Supply–Workload Balance in Children. Pediatr Cardiol 39, 660–664 (2018). https://doi.org/10.1007/s00246-017-1803-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1803-6