Abstract
One of the most important problems in patients with aortic coarctation after aortic arch repair is future cardiovascular disease. We have previously reported that the enhancement of aortic pressure wave reflection in patients could be one of the causes of future cardiovascular diseases, because it results in an increase of the left ventricular workload and is disadvantageous for coronary circulation. Seventeen patients who had undergone aortic arch repair without pressure gradient in their aortic arch were enrolled. An ascending aortic pressure waveform was recorded by a pressure-sensor-mounted catheter, and a subendocardial viability ratio, which measures cardiac blood supply–workload balance, was calculated. The values were compared with those in age-matched controls. The patients’ mean age was 6.8 ± 2.8 years. The mean ascending aortic systolic pressure was higher (100.4 ± 12.9 vs. 90.2 ± 8.9 mmHg, p = 0.0011) and the pulse pressure was wider (38.1 ± 7.1 vs. 32.5 ± 5.4 mmHg, p = 0.0072) in patients than in control subjects. There was no difference in the mean subendocardial viability ratio (1.01 ± 0.25 vs. 1.01 ± 0.24, ns), while the mean tension time index (27.4 ± 5.6 vs. 23.0 ± 3.3, p = 0.0001) and diastolic pressure time index (28.4 ± 11.1 vs. 23.6 ± 8.0, p = 0.0082) were higher in patients than in controls. The cardiac blood supply–workload balance was preserved in patients after aortic arch repair, despite an increase in their cardiac workload.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular complications are encountered following surgical repair of aortic coarctation, despite apparently successful procedures [1,2,3,4,5,6]. Although many of the complications are related to anatomical problems such as restenosis and aneurysm formation, there are some complications that are not directly related to the morphological problems of the reconstructed aorta. One of the most important complications is the early onset of cardiovascular diseases, such as hypertension, myocardial infarction, cardiac failure, stroke, and sudden death [7,8,9]. Complications have been reported even in cases where aortic arch repair is completely successful. Cohen and colleagues have reported that the most important predictor of long-term survival and hypertension in patients was the age of patients at the time of the initial repair [4]. The report suggested that preoperative cardiovascular damage influences the postoperative condition. However, O’Sullivan and colleagues reported that patients suffered from hypertension after an early and successful aortic coarctation repair [9]. Many hypotheses have been proposed to explain these observations.
We have previously reported that the repaired site generates an extra pressure wave reflection, which might cause future cardiovascular disease [10]. The enhancement of the pressure wave reflection leads to an increase in left ventricular workload and is disadvantageous for coronary circulation. The damaged cardiac blood supply–workload balance could induce heart failure, arrhythmia, and sudden death. Therefore, we analyzed cardiac blood supply–workload balance in patients after aortic arch repair.
Methods
Patients (Table 1)
We enrolled 17 patients who had undergone aortic arch repair for a coarctation of the aorta or an interruption of the aortic arch without an aortic recoarctation. Aortic recoarctation was defined as an aorta with a structural stenosis or an existence of a systolic blood pressure (SBP) difference at the repaired site. No patients had any significant leakage at the aortic level, including patent ductus arteriosus and aortic regurgitation. The mean age of the patients was 6.8 ± 2.8 years (range 1–13 years). The patients underwent an aortic arch repair at 28.9 ± 29.0 days (range 3–103 days). The aortic arch repair was performed by an extended end-to-end anastomosis in 12 patients (70.6%), subclavian flap in 4 patients (23.5%), and a Blalock–Park operation in one patient (5.9%). Thirteen patients (76.5%) had a ventricular septal defect and underwent a closure of the defect at the mean age of 0.76 ± 0.49 years (range 0.17–2.0 years). The postoperative course was uneventful. Seven patients (41.2%) were diagnosed with a bicuspid aortic valve without aortic stenosis or aortic regurgitation. All patients were fully active and asymptomatic. None of the patients were on cardiovascular medications.
Control 1-to-1, age-matched patients had small left-to-right shunt disorders (12 patients with ventricular septal defects and five with atrial septal defects) [11], because it has been reported that the value of a subendocardial viability ratio in children, which we used to evaluate cardiac blood supply–workload balance, depends on the patient’s age [12]. Their mean pulmonary-to-systemic flow ratio was 1.2 ± 0.2 (range 1.0–1.5). They were also asymptomatic and were given no medications. There were no differences in height (117.1 ± 21.3 cm), weight (25.2 ± 15.9 kg), or body surface area (0.88 ± 0.34 m2) compared with the patient test group (Table 1). There were no differences in cardiac index (4.1 ± 0.7 l/min m2) and heart rate (94.0 ± 17.0 bpm) between the control and test groups.
Parents of all subjects gave their informed consent. The study protocol was approved by the ethics committee of Chiba Children’s Hospital and Hokkaido University Hospital.
Data Acquisition
Ascending aortic pressure waveforms were recorded using a catheter-mounted pressure sensor (Millar, SPC-454D; Millar Instruments, Inc. Houston, TX) during cardiac catheterization for evaluation of postoperative hemodynamics. The ascending aortic pressure waveform was recorded at one vertebral body thickness above the aortic valve. Patients were sedated by a venous injection of midazolam during cardiac catheterization. The waveform was recorded on a hard disk through an analog–digital converter with a sampling rate of 1000 Hz (1000 samples per second). It was also simultaneously recorded with an electrocardiogram.
Data Analysis
From the recorded pressure waveforms, we measured the SBP, diastolic blood pressure (DBP), mean blood pressure (MBP), and pulse pressure (PP) in each patient. Moreover, we calculated a subendocardial viability ratio according to the following equation:
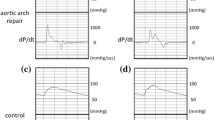
The subendocardium is thought to be more sensitive to shortage of the blood supply than the subepicardium. Buckberg and colleagues demonstrated that the ratio of the diastolic phase area (diastolic pressure time index) to the area of the systolic phase (tension time index) in the central aortic pressure profile has a close correlation to the blood supply to the subendocardium. That ratio was designated as the subendocardial viability ratio [13,14,15] (Fig. 1).
Therefore, they coined this index as a measure of the hemodynamic capacity for the supply divided by the myocardial oxygen demand. The tension time index was obtained by measuring the area under the aortic pressure curve in systole; it equaled the mean aortic pressure in systole multiplied by the duration of systole. The diastolic pressure time index was obtained by measuring the area under the aortic pressure curve in diastole and subtracting the mean left atrial pressure (assumed to be equal to the left ventricular diastolic pressure) multiplied by the diastolic time from it. When the left atrial pressure was not recorded, we used the pulmonary capillary wedge pressure instead.
Statistical Analysis
All values are reported as mean ± SD where applicable. We compared the difference in the hemodynamic data and subendocardial viability ratio values in patients after the aortic arch repair with those in the age-matched control subjects by a paired t test.
Results
Cardiac catheterization data are shown in Table 2. Blood pressure values were measured in the ascending aorta. The SBP was significantly higher (100.4 ± 12.9 vs. 90.2 ± 8.9 mmHg, p = 0.0011) and PP was significantly wider (38.1 ± 7.1 vs. 32.5 ± 5.4 mmHg, p = 0.0072) in patients after aortic arch repair compared with age-matched controls. Moreover, DBP (62.3 ± 9.8 vs. 57.7 ± 6.7 mmHg, p = 0.019) and MAP (80.2 ± 13.5 vs. 74.4 ± 7.3 mmHg, p = 0.049) were significantly higher in patients compared with the control subjects. Table 3 summarizes the subendocardial viability ratio data. Although there was no difference in the subendocardial viability ratio between the patients and control subjects (1.01 ± 0.25 vs. 1.01 ± 0.24, ns), the tension time index (27.4 ± 5.6 vs. 23.0 ± 3.3, p = 0.0001), which reflects the workload of the left ventricle, and the diastolic pressure time index (28.4 ± 11.1 vs. 23.6 ± 8.0, p = 0.0082), which reflects the blood supply to the subendocardium, were significantly higher in patients after aortic arch repair.
Discussion
The current study demonstrates that the subendocardial viability ratio, which is a measure of cardiac blood supply–workload balance, was not significantly affected following successful aortic arch repair. However, tension time index, which measures cardiac workload, and diastolic pressure time index, which measures coronary blood supply, both increased. Because the subendocardial viability ratio is a ratio of diastolic pressure time index to tension time index, the value was not significantly affected even though the two parameters increased.
We have previously reported on subendocardial viability ratio in patients after an arterial switch operation [16]. The results were almost identical to those described here, i.e., no significant difference in subendocardial viability ratio despite an increase in tension time index of the patients. Maintaining a cardiac blood supply–workload balance is essential for survival. Hence, it is important to maintain the subendocardial viability ratio and many reports have demonstrated the relationship between low subendocardial viability ratio and poor outcome [17,18,19,20,21].
Our results demonstrate that blood pressure parameters were higher in patients after aortic arch repair compared with controls. Previous studies have reported decreased distensibility of the proximal aorta [22,23,24,25,26] and hence left ventricular hypertrophy [24, 27, 28] in patients after aortic coarctation. We have previously reported that the degree of left ventricular hypertrophy is associated with the enhancement of pressure wave reflection in patients after aortic arch repair, and that the hypertrophy may lead to abnormal pathogenesis associated with myocardial fibrosis [28, 29].
Czernin and colleagues have previously reported that a gradual decline of myocardial blood flow reserve with aging correlates with an age-related increase of baseline myocardial work and blood flow [30]. Systolic blood pressure in aged subjects was higher than that in young subjects and may increase cardiac workload. This scenario resembles the one we describe in the current study where increased coronary blood flow is associated with augmented cardiac workload. Hauser and colleagues have reported decreased coronary flow reserve with the increase of resting basal coronary blood flow in patients after an arterial switch procedure [31]. This finding is in agreement with the study by Czernin and colleagues regarding coronary circulation and the aging process [30]. Therefore, cardiac blood supply–workload balance in young patients after aortic surgery may be similar to that in aged individuals, i.e., aortic function may be damaged in a way that is disproportionate with chronological age. Further studies are needed to elucidate the mechanism of cardiac blood supply–workload balance in patients after aortic arch repair.
One of the most important limitations of the current study is that the control subjects had a left-to-right shunt. Therefore, cardiac workload could be slightly overestimated. Judging from the product of their flow ratio and pulmonary artery pressure, the increment of the cardiac workload was under 5%. However, it could influence cardiac blood supply–workload balance. Because we recorded pressure waveforms in children by an invasive method, it was necessary to administer sedative drugs, which could influence cardiac blood supply–workload balance. Despite these limitations, the relationship between the parameters should be reliable in each condition.
Conclusion
Cardiac blood supply–workload balance is preserved in patients after aortic arch repair, despite an increase in cardiac workload.
References
Presbitero P, Demarie D, Villani M, Perinetto EA, Riva G, Orzan F, Bobbio M, Morea M, Brusca A (1987) Long term results (15-30 years) of surgical repair of aortic coarctation. Br Heart J 57:462–467
Stewart AB, Ahmed R, Travill CM, Newman CG (1993) Coarctation of the aorta life and health 20-44 years after surgical repair. Br Heart J 69:65–70
Jenkins NP, Ward C (1999) Coarctation of the aorta: natural history and outcome after surgical treatment. QJM 92:365–371
Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC (1989) Coarctation of the aorta. Long-term follow-up and prediction of outcome after surgical correction. Circulation 80:840–845
Daniels SR (2001) Repair of coarctation of the aorta and hypertension: does age matter. Lancet 358:89
Lee MGY, Allen SL, Kawasaki R, Kotevski A, Koleff J, Kowalski R, Cheung MM, Konstantinov IE, Brizard CP, d’Udekem Y (2015) High prevalence of hypertension and end-organ damage late after coarctation repair in normal arches. Ann Thorac Surg 100:647–653
Celermajer DS, Greaves K (2002) Survivors of coarctation repair: fixed but not cured. Heart 88:113–114
Toro-Salazar OH, Steinberger J, Thomas W, Rocchini AP, Carpenter B, Moller JH (2002) Long-term follow-up of patients after coarctation of the aorta repair. Am J Cardiol 89:541–547
O’Sullivan JJ, Derrick G, Darnell R (2002) Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart 88:163–166
Murakami T, Takeda A (2005) Enhanced aortic pressure wave reflection in patients after repair of aortic coarctation. Ann Thorac Surg 80:995–999
Murakami T, Takeda A, Takei K, Ueno M, Yakuwa S, Yamazawa H, Furukawa T (2010) Aortic pressure wave reflection in children. Hypertens Res 33:225–228
Murakami T, Takeda A, Takei K, Tateno S, Kawasoe Y, Niwa K (2015) The cardiac blood supply-workload balance in children. Heart Vessels 30:626–631
Buckberg GD, Fixler DE, Archie JP, Hoffman JI (1972) Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 30:67–81
Buckberg GD, Olinger GN, Mulder DG, Maloney JV (1975) Depressed postoperative cardiac performance. Prevention by adequate myocardial protection during cardiopulmonary bypass. J Thorac Cardiovasc Surg 70:974–994
Hoffman JIE, Buckberg GD (2014) The myocardial oxygen supply: demand index revisited. J AM Heart Assoc 3:e000285
Murakami T, Takei K, Ueno M, Takeda A, Satoshi Y, Nakazawa M (2008) Aortic reservoir function after arterial switch operation in elementary school-aged children. Circ J 72:1291–1295
Di Micco L, Salvi P, Bellasi A, Sirico ML, Di Iorio B (2013) Subendocardial viability ratio predicts cardiovascular mortality in chronic kidney disease patients. Blood Purif 36:26–28
Vizinho RS, Santos C, Lucas C, Adragao T, Barata JD (2014) Effect of the arteriovenous access for hemodialysis on subendocardial viability ratio, pulse pressure and hospitalizations. J Nephrol 27:563–570
Theilade S, Hansen TW, Rossing P (2014) Central hemodynamics are associated with cardiovascular disease and albuminuria in type 1 diabetes. Am J Hypertens 27:1152–1159
Saiki H, Kuwata S, Kurishima C, Masutani S, Senzaki H (2016) Vulnerability of coronary circulation after Norwood operation. Ann Thorac Surg 101:1544–1551
Laugesen E, Hoyem P, Fleischer J, Kumarathas I, Knudsen ST, Hansen KW, Christiansen JS, Hansen TK, Poulsen PL (2016) Reduced subendocardial viability ratio is associated with unfavorable cardiovascular risk profile in women with short duration of type 2 diabetes. Am J Hypertens 29:1165–1172
Lombardi KC, Northrup V, McNamara RL, Sugeng L, Weismann CG (2013) Aortic stiffness and left ventricular diastolic function in children following early repair of aortic coarctation. Am J Cardiol 112:1828–1833
Kuhn A, Baumgartner D, Baumgartner C, Horer J, Schreiber C, Hess J, Vogt M (2009) Impaired elastic properties of the ascending aorta persist within the first 3 years after neonatal coarctation repair. Pediatr Cardiol 30:46–51
Ou P, Celermajer DS, Jolivet O, Buyens F, Herment A, Sidi D, Bonnet D, Mousseaux E (2008) Increased central aortic stiffness and left ventricular mass in normotensive young subjects after successful coarctation repair. Am Heart J 155:187–193
Vitarelli A, Conde Y, Cimino E, D’Orazio S, Stellato S, Battaglia D, Padella V, Caranci F, Continanza G, Dettori O, Capotosto L (2008) Assessment of ascending aorta distensibility after successful coarctation repair by strain Doppler echocardiography. J Am Soc Echocardiogr 21:729–736
Voges I, Kees J, Jerosch-Herold M, Gottschalk H, Trentmann J, Hart C, Gabbert DD, Pardun E, Pham M, Andrade AC, Wegner P, Kristo I, Jansen O, Kramer HH, Rickers C (2016) Aortic stiffening and its impact on left atrial volumes and function in patients after successful coarctation repair: a multiparametric cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 18:56
Rinnstrom D, Dellborg M, Thilen U, Sorensson P, Nielsen N-E, Christersson C, Johansson B (2016) Left ventricular hypertrophy in adults with previous repair of coarctation of the aorta; association with systolic blood pressure in the high normal range. Int J Cardiol 218:59–64
Murakami T, Takeda A, Yamazawa H, Tateno S, Kawasoe Y, Niwa K (2013) Aortic pressure wave reflection in patients after successful aortic arch repair in early infancy. Hypertens Res 36:603–607
Yamazawa H, Murakami T, Takeda A, Takei K, Furukawa T, Nakajima H (2015) Serum concentration of procollagen type III amino-terminal peptide is increased in patients with successfully repaired coarctation of the aorta with left ventricular hypertrophy. Pediatr Cardiol 36:555–560
Czernin J, Müller P, Chan S, Brunken RC, Porenta G, Krivokapich J, Chen K, Chan A, Phelps ME, Schelbert HR (1993) Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 88:62–69
Hauser M, Bengel FM, Kühn A, Sauer U, Zylla S, Braun SL, Nekolla SG, Oberhoffer R, Lange R, Schwaiger M, Hess J (2001) Myocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and Ross operation. Circulation 103:1875–1880
Acknowledgement
We thank Ashraf Malhas, PhD, from Edanz Group for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Murakami, T., Takeda, A. Preserved Cardiac Blood Supply–Workload Balance in Pediatric Patients After Aortic Arch Repair. Pediatr Cardiol 39, 294–298 (2018). https://doi.org/10.1007/s00246-017-1754-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1754-y