Abstract
In this study, we focused on evaluating the responses of the cockle, Cerastoderma glaucum to in situ exposures to metals at three sites in the Gulf of Gabes in the coastal zone of Tunisia differing in levels of metal contamination. Firstly, we examined the general physiological state of the organisms. Secondly, we evaluated the bioaccumulation of several metals (Cd, Cu, Zn, Ni) in the cockles. Thirdly, we focused on evaluating histologically changes in gametogenesis and sexual maturity of the organisms. Finally, we determined the expression of seven key genes encoding enzymes or proteins involved in responses to different types of environmental stressors. Results showed a decrease in the general physiological status of the cockles, including a reduced condition index, sex ratios skewed to females (70% and 80% females in the intermediate and the contaminated site, respectively) and greater mortalities in tests under anoxic conditions (i.e., stress on stress test) in cockles collected from the most contaminated site (LT50 = 2.88 days) compared to the cockles from the intermediate site (LT50 = 5 days) and the less contaminated site (LT50 = 6 days). Results for metal bioaccumulation showed that the levels of Cd, Cu, Zn and Ni in cockles were consistent with the contaminant gradient, with the highest levels in cockles from the most contaminated site (1.04; 4.92; 52.76 and 13.81 µg/g dw, respectively), followed by those from the intermediate site (0.34; 2.94; 36.94; 17.40 µg/g dw, respectively) and then the less contaminated site (0.065; 1.27; 21.62 and 5.40 µg/g dw, respectively). Results from the gametogenesis and maturity index showed few differences in the reproductive cycle of cockles collected from the three study sites. There were different patterns of gene expression that were divided into three groups in terms of responses: (1) expression of genes involved in metal detoxification, ATP Binding Cassette Subfamily B Member 1 (ABCB1) and metallothionein MT) and genes for superoxide dismutases (i.e., Mn SOD and CuZn SOD), which did not show any difference in their levels of expression; (2) heat shock protein 70 (HSP70) gene expression, which decreased in cockles according to the pollution gradient, and (3) expression of catalase (CAT) and cytochrome oxidase subunit 1 (COI) genes was threefold and 1000-fold higher in cockles from intermediate and most contaminated sites compared to the less contaminated site. Therefore, changes in overall physiological condition, sex ratios and expression of HSP70, CAT and COI genes may be appropriate biomarkers for in situ studies of the impacts of metals in cockles. However, these biomarkers should be coupled to proteomics studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Coastal ecosystems are complex environments with high productivity. They provide habitat, protection and food for native and migratory species. However, they are also very sensitive as they are increasingly affected by anthropogenic activities (e.g. pollution, tourism, over-fishing, sediment discharge, shipping, industrial and urban development) (Barbier et al. 2011; Day et al. 2012; Krishnakumar et al. 2018). These anthropogenic pressures have been reported to degrade marine habitats, decline of marine resources leading to serious economic problems (Liu et al. 2011; Ali et al. 2012) and human health risks (Liu et al. 2011; Kerambrun et al. 2012). Anthropogenic composites, such as trace metals are introduced into the coastal waters through natural process and anthropogenic activities through rivers, effluents, runoff, and from the atmosphere (Cobelo-Garcia et al. 2004). Trace metals occur naturally in the marine environment, many of which are essential to marine life at low concentrations. However, when their concentrations exceed natural levels, they pose a serious threat to marine life (Krishnakumar et al. 2018). Due to their toxicity, non-biodegradability and accumulation behaviour, heavy metals are considered as hazardous environmental pollutants and need to be carefully monitored in ecotoxicology studies (Naifar et al. 2018; Zouch et al. 2018; Altwaijry et al. 2022). Therefore, there is an urgent need to conduct regular monitoring and assessment programs in coastal waters to plan and implement mitigation measures as well as to control trace metal pollution. The "Mussel Watch" is an example of an international program that has been applied worldwide to monitor pollution levels in coastal areas (Goldberg et al. 1978; Goldberg and Bertine 2000). Indeed, bio monitoring of environment contamination by metals requires data on the biological responses which, in turn, should be supported by appropriate tools such as metal bioaccumulation. In this context, since 1960, tissue concentrations of pollutants in marine organisms, especially mussels, has been used for assessing coastal water quality, rather than water or sediment samples (Rainbow 1990). Moreover, utilisation of biomarkers as an early warning of pollution or degradation in ecosystems has increased over the past 20 years (kerbakmun et al. 2011). Using a single biomarker does not adequately reflect the impairment of the health of an organism. However, the use of a multi-biomarker approach is required for a more realistic evaluation of the biological impact of chemicals and to elucidate the mechanisms underlying the inception of such alterations (Zuykov et al. 2013). Biomarkers occur at different levels of organization, from subcellular to whole organisms and ecosystems. Responses that occur at individual, population and ecosystem level are generally accepted to have ecological relevance and tend to be less reversible and more detrimental than effects at lower levels. However, molecular and cellular biomarker responses of organisms are usually used to predict higher-level consequences of toxicant exposure (Van der Oost et al. 2003; Tankoua et al. 2013; Hamza-Chaffai 2014).
Bivalve mollusks have been adopted in the aquatic metallic biomonitoring programs mainly mussel, oyster and clam, (Zuykov et al. 2013; Krishnakumar et al. 2018). However less attention has been paid to the cockle, nevertheless, the cockle C. glaucum satisfies the criteria for an excellent bio-indicator of pollution firstly, for its wide geographical distribution, in the Mediterranean sea and southern Europe. C. glaucum has been recorded from the coasts of Egypt, Tunisia, Turkey, Sardinia, Italy, Greece, Portugal, Spain, France (Atlantic and Mediterranean coasts), The Netherlands, the British Isles, Denmark, Finland, Norway and in the Wadden Sea, Adriatic Sea, Red Sea, Aegean Sea and Caspian Sea (Brock and Wolowicz 1994; Malham et al. 2012; Kandeel et al. 2017). Secondly, C. glaucum lives in close proximity to sediment, which serves as a final sink for many organic and inorganic pollutants released into the environment as a result of human activity. Moreover, C. glaucum is a sedentary, filter-feeding marine bivalve that meets the criteria for a suitable bioindicator of pollution, according to studies of Machreki-Ajmi and Hamza-Chaffai (2006), Hamza-Chaffai (2014) and Karray et al. (2015a, b, c). Thirdly, this species plays a key ecological role, sharing in the food chain as some marine animals’ prey upon them. C. glaucum also plays an important role in nutrient cycles. It is eaten by humans and considered as a very cheap food resource due to their occurrence in high densities (Kandeel et al. 2017). For all these reasons, the cockle, C. glaucum deserves to be better studied, evaluated and integrated into national and international bio monitoring programs.
In the case of Tunisia, the cockle, C. glaucum represented one of the most dominant species in the Gulf of Gabes. This area is situated on Tunisia's southern coast. It offers unique physical and biological qualities (shallow waters, weak currents, high salinity and temperature) (El Zrelli et al. 2018; Fourati et al. 2018). Moreover, it is considered as a refuge for larvae and juveniles of animals (El Zrelli et al. 2018). This ecosystem is known to contribute to 42% of the halieutic Tunisian national production (DGPA 2015). At the same time, this area represents an ecosystem under heavy industrial pressure due to the rapid and uncontrolled industrialization (Sellami et al. 2022; Ajala et al. 2021; El Zrelli et al. 2018; Fourati et al. 2018). In fact, many industrial complexes have settled on southern coast of the Gulf of Gabès and discharge urban/industrial effluents into seawater, which has led to a high diversity of contaminants, including heavy metals (Zouch et al. 2018). The main operating industries, are phosphate treatment (SIAPE plant), soap manufacturing (SIOS-ZITEX), and secondary lead smelting (FP Sfax Sud) (Chifflet et al. 2019; Ajala et al. 2021; Sellami et al. 2022). According to Sellami et al. (2022) and Naifar et al. (2018), the SIAPE and the secondary lead smelting industry are the main sources of heavy metals. Indeed, untreated effluents from these manufactories contain high concentrations of metals mainly Cd, Cu, Zn and Ni that have been continuously discharged into the marine environment altering the marine environment and affecting its biodiversity (Ben Salem and Ayadi 2017; Mosbahi et al. 2019; Karray et al. 2015a, b, c; Zouch et al. 2018). Therefore, the Gulf of Gabes region is an ideal location for conducting ecotoxicological studies based on metal pollution and using the cockle, C. glaucum. Several studies conducted on C. glaucum in the Gulf of Gabes region focused on biotic variations linked to the reproductive cycle, size and sex (Derbali et al. 2009; Machreki-Ajmi et al. 2013; Karray et al. 2015a), the relationship between trace metal concentration in sediment and in cockle tissues in field condition (Machreki-Ajmi and Hamza Chaffai 2006; Machreki-Ajmi 2008; Machreki-Ajmi et al. 2009), response of some biomarkers to in vivo contamination by cadmium (Ladhar Chabbouni et al. 2009). Moreover, response of the cockle, C. glaucum to in vivo Cadmium and effluent contamination using biomarkers at different levels of biological organisation were studied (Karray et al. 2015b, c). To our knowledge, no field studies have applied a multi-level approach using physiological, biochemical and molecular biomarkers in the cockle, C. glaucum originated from the Gulf of Gabes. Indeed, biomarkers measured at molecular or cellular levels have been proposed as sensitive "early warning" tools for environmental quality assessment. These early warning biomarkers can be used in a predictive way before irreversible environmental damage of ecological consequence occurs. Therefore a set of molecular biomarkers were applied in this study. They are implicated in the response of different types of environmental stressors such as Metal and xenobiotic detoxification (metallothionein, MT; ATP Binding Cassette Subfamily B Member 1, ABCB1), oxidative stress protection (superoxide dismutases, MnSOD and CuZnSOD; catalase, CAT), mitochondrial alterations (cytochrome c oxidase1, CO1), and general stress (heat shock protein 70, HSP70).
The main goal of our study was to be able to select in C. glaucum the most relevant biomarkers associated with stress response in field conditions to be used in future in bio monitoring programs. To accomplish this objective, we conducted a multiple biomarker approach at different biological organisation levels. Therefore, we focused on the effect of metallic stressors on the cockle C. glaucum using biomarkers in three differently impacted sites in the Gulf of Gabes region through: (1) three parameters at the global physiological level (stress-on-stress, condition index and sex-ratio), (2) bioaccumulation of Cd, Cu, Zn and Ni metals in natural cockles, (3) The study of the reproductive cycle of cockles and (4) gene transcription and metals levels relationships in an attempt to highlight an mRNA expression pattern in natural cockles.
Materials and Methods
Studied Sites and Sampling
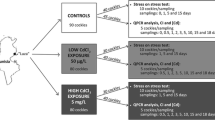
Cockles C. glaucum were collected at three sites differing by their contamination level along the Gulf of Gabès Ellouza (35°1’ N, 11°22’ E), Sidi Mansour(34°46’ N, 10°49’ E) and Gargour (33°31’ N, 10°42’ E) sites (Fig. 1). C. glaucum specimens were collected by hand from coastal silt (top 20 cm depth) at low tide, then transferred to the lab in cool boxes containing roughly 2 cm of seawater from the collection site. Specimens of C. glaucum (n = 20 for each sampling date, shell length ranging from 28 to32 mm) were collected from each site in mid-October, November, mid-November and December for reproductive and physiological parameters determination. Indeed, parameters in relation to the reproductive cycle and gametogenesis such as condition index, sexual maturity and reproductive stages were analysed according to temporal variations to distinguish active and rest sexual periods in the cockle, C. glaucum. However, in order to avoid certain confounding factors related to the biological cycle, analyzes such as metal accumulation and gene expression were performed when cockles were at the sexual rest. Water temperature was measured at each sampling date in the three studied sites. Values were 25 °C, 26 °C and 23 °C, respectively, in Ellouza, Sidi Mansour and Gargour site in mi October, 18 °C, 19 °C and 18 °C, respectively, in Ellouza, Sidi Mansour and Gargour sites in Novembre, 15 °C, 13 °C and 15 °C, respectively, in Ellouza, Sidi Mansour and Gargour sites in mi November and 12 °C, 10 °C and 11 °C, respectively, in Ellouza Sidi Mansour and Gargour in December. To study the responses at transcriptional levels and metal bioaccumulation, twenty cockles samples were collected from the three selected sites (sell length ranging from 28 to 32 mm).
Stress-on-Stress Test
Twenty animals were sampled from each site and subjected to anoxia in the laboratory by air exposure at 15 °C. Survival rate (%) under anoxia was followed daily during experiments. Death symptoms were considered to be open valves and absence of muscular activity. Dead animals were recorded until 100% mortality was reached (Viarengo et al. 1999). The median Lethal Time (time required for 50 percent of the animals to die (LT50)) was determined by Probit regression analysis (Hahn and Soyer 2008), using SPSS 13.0, and the results were expressed in days.
Condition Index and Histological Analysis
The reproductive cycle was studied using twenty cockles per site. Condition index (CI) was calculated individually on twenty cockles from each sampling site. Total and soft weights were obtained for each sample, and the CI was reported as a percentage of the ratio of fresh soft tissue weight to total weight according to Lobel et al. (1991).
To prepare tissue for histological analysis, foot was completely removed to avoid later difficulties in tissue sectioning. A 5-mm thick cross-section was then removed using a scalpel. The determination of reproductive stage is based on a histological evaluation of the maturation stage of cockle gonads located within/around the visceral mass. The tissue section is obtained such that the dorsal–ventral aspect passes through the digestive gland. Each cockle's gonads were then fixed for 48 h in aqueous Bouin's fixative for histological analysis. Tissues were then dehydrated by immersing them in increasingly higher alcohol concentrations (70°, 80°, 90°, and 95°) for one hour and thirty minutes at each concentration before being embedded in paraffin at 56 °C.
3 µm section were cut with a mechanical microtome (type HM315). Hematoxylin, Light green, and Eosin were employed for staining. The reproductive stages were determined using the parameters set out by Karray et al. (2015a, b, c). According to Karray et al. (2015a), the individual stages were coded with numbers ranging from 0 to 5. A maturity score was assigned to each stage (1, resting; 2, development; 3, gametogenesis; 4, maturity; 5, spawning; 2, spent), and a sexual maturity index (SMI) was generated using Siah et al. (2003) equation:
Metal Analysis
Metals analysis (Cd, Cu, Zn, Ni, Cr, Mn and Pb) was performed on sediments collected directly from the three sites. An inductively coupled plasma optical emission spectrometer (ICP-AOS, ThermoScientific iCAP 6300 DUO) was used to determine trace element concentrations in sediments. Characteristic parameters of each metal (Cd, Cr, Cu, Mn, Ni, Pb and Zn) including limit of detection (LOD) and quantification (LOQ) are presented in Table 1. The limit of detection (LOD) is the analyte concentration corresponding to the sample blank value plus three standard deviation and the limit of quantification (LOQ) is the analyte concentration corresponding to the sample blank value plus ten standard deviations. For cockles originating from the three sites (n = 20 from each site), metal concentrations were measured using flameless (Cd, Cu, and Ni) or flame (Zn) atomic absorption spectrophotometry with Zeeman correction in a graphite furnace, as previously described (Dedourge-Geffard et al. 2009; Geffard et al. 2010). (SpectrAA Zeeman 220).
Internal quality controls were based on the analysis of the metals of interest in standard reference material. One Standard was used in the study as certified reference material for tissues: TORT-2, Lobster Hepatopancreas, NRC–CNRC (TORT-2, NRC-CNRC, Table 2, n = 6). We also tested blanks representative of reagents and preparation technique. Blanks correspond to nitric acid at 65 °C.
RNA Extraction and Real Time Quantitative PCR
In each site, the same individuals were used for gene transcription and metal studies. Gill was used to assess the transcriptional responses of seven major genes encoding enzymes or proteins involved in environmental stressor responses. Table 3 lists the names of the genes, their accession numbers, annealing temperatures, and the individual primers used for cloning and characterisation of these various genes. Gills is a critical interface for metal absorption, storage, and excretion, as well as a high potential for protein synthesis (Al Kaddissi et al. 2012; Navarro et al. 2011). For each sample, the gills were dissected, preserved in RNA later (Sigma-Aldrich), and stored at 20 °C. Total RNA was extracted from 100 mg of gills using Tri-Reagent (Invitrogen) according to the manufacturer's instructions. The quality of the RNA was tested using the Experion system, and the concentration of RNA was measured using a spectrophotometric method at 260 nm (Bio-Rad).
In a final volume of 12.25 µl, three micrograms of total RNA were added to dT Race primer and nuclease free water. The mixture was first denatured at 70 °C for 5 min before being held on ice. First strand complementary DNAs (cDNAs) were then synthesized using dTRace primers (5-GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTTT-3), dNTPs, MMLV reverse transcriptase (Promega), RNAsin (Promega), and nuclease free water in a final volume of 25 µl for 90 min at 42 °C. An Applied Biosystems Step-One-plus apparatus was used for real-time PCR reactions, Fast SYBR Green Master Mix (Applied Biosystems), and specific primers (0.1 M) constructed on the basis of previously identified sequences, as detailed in Table 4 and in Karray et al. (2015b).
Correct PCR reactions without inhibitions were found for all genes according to efficiencies calculated (90 ≤ E ≤ 110) (Gašparič et al. 2008). Three (β-Actin, α-Tubulin, β-Tubulin) out of the 4 housekeeping genes studied were used to normalize the 7 target genes. A Bestkeeper index compiling these 3 housekeeping genes was calculated and used to normalize the target gene transcriptions (Pfaffl et al. 2004). Using an RNA sample from cockles from the Ellouza site as a calibrator, the relative expression was evaluated using the comparative Ct method (Livak and Schmittgen 2001).
Statistical Analysis
We employed the SPSS package program (version 13) for the statistical analysis. To ensure that the variances were equal, we used the Levene test. The normal distribution was checked using the P-Plot tool. To analyse differences between the means, a one-way ANOVA test was used. The effects of inter-site metallic pollution on gene transcription, metals bioaccumulation, sex ratio, and CI were determined using an independent ANOVA significance test after standardization of the data by log(x + 1) transformation and stabilization of their variances by square-root transformation. Principal component analysis (PCAs Biplot) were performed considering condition index, genes transcription and metals bioaccumulation variables. Correlations were statistically verified using Pearson's technique. Finally, to determine the crucial differences between the groups, a Tukey's HDS test was utilized. Significance was set at p < 0.05 in all cases.
Results
Metal Contamination of Sediments and Cockles
Metal analysis in sediments and cockles tissues included Cd, Cu, Zn and Ni. We chose these four metals because: (i) There are two types of metals: essential and non-essential. In fact, Cu and Zn are essential for bivalve metabolism; however, in cases when concentrations exceed normal levels in the environment, they become toxic (Krishnakumar et al. 2018). Non-essential metals such as Cd and Ni have no biological function and are toxic even at low concentrations (Ivanovic et al. 2016). (ii) These four metals are the main sources of metallic pollution in the Gulf of Gabes sediment (Feki et al. 2022; Sellami et al. 2022). (iii) Marine organisms that were collected from the Gulf of Gabes region contain high concentrations of Cd, Cu, Ni and Zn (Machreki-Ajmi and Hamza Chaffai 2009; Karray et al. 2015c; Ben Salem and Ayadi 2017). (iv) Cd, Cu, Ni and Zn have been shown to correlate with responses to the different biomarkers examined here (e.g. ABCB1; COI; CAT and SOD's) (Achard-Joris et al. 2006; Karray et al. 2015a, b, c) and are often used for marine contamination monitoring and assessment due to their dependence on anthropogenic activities (Naifar et al. 2018). Sediments were collected from the Ellouza, Sidi Mansour and Gargour sites and analyzed by ICP (Cd, Cu, Zn, Ni, Cr, Mn and Pb). Gargour sediment presented the highest values for different metals studied, followed by Sidi Mansour and Ellouza sites. Only Cd presented values above the threshold established by Long et al. (1995) (Table 1). All other metals values were below those thresholds.
Bioaccumulation of metals (Cd, Cu Zn and Ni) in cockles tissues from the three studied sites were presented in Fig. 2. Results show that Zn was present at the highest concentrations followed by Ni and Cu. The lowest values were for Cd. Inter-site variations for the different metals show that the highest values for all metals (p < 0.05) are registered in the Gargour site while the lowest ones (p < 0.05) were for the Ellouza site.
Global Physiological State of Cockles
Stress-on-Stress
For the cockles originating from the tree site, survival under anoxia was assessed (Fig. 3). Cockles from Gargour site presented the lowest values of lethal time (LT50 = 2.88 days) followed by cockles from Sidi Mansour site (LT50 = 5 days). Cockles from the Ellouza site presented the highest values of LT50 (LT50 = 6 days).
Condition Index
Condition index (CI) was assessed bi-monthly between October and December 2009 in cockles collected from the three studied sites (Fig. 4). Results show that variations in CI were synchronous in the three studied sites: CI decreased significantly between October and November and no variation was observed for the others sampling dates. This decline in CI values was more important in cockles from Ellouza (from 27.91 to 13.79%) and Sidi Mansour (from 27.45% to 12.13%) sites compared to Gargour (from 13.75% to 10.10%) site witch presented the lowest values.
Temporal variations (means ± SD) of condition index (CI) in C. glaucum collected in Ellouza (E), Sidi Mansour (SM) and Gargour (G) sites in the Gulf of Gabès. A, B, C and D represent the different sampling dates, i.e. mid-October, November, mid-November and December, respectively. Significant differences are indicated by an asterisk (p < 0.05, Tukey test)
Sex-Ratio
The percentage of males and females from the three studied sites was presented in Fig. 5. The sex ratio was highly unbalanced in favour of females in Gargour site. However, in the Ellouza site, percentages of males and females were equivalent.
Gametogenesis and Reproductive Cycle
Previous data dealing with the reproductive stages of C. glaucum demonstrated a trimodal sexual cycle with three periods of reproduction and five gametogenesis stages in males and females: development, gametogenesis, maturity, spawning, and spent stages (Karray et al. 2015a).
The distribution of each gonadal development stages and the seasonal sexual maturity index (SMI) cycle of C. glaucum from the three examined sites was presented in Fig. 6.
Sexual maturation with partitioning expressed in % between each observed development stages of gonads and sexual maturity index (SMI) from C. glaucum collected in Ellouza (A), Sidi Mansour (B) and Gargour (C) sites. SMI = ∑ (% of each sexual state*maturity factor). A, B, C and D represent the different sampling dates, i.e. mid-October, November, mid-November and December, respectively
We have not detected any differences in the reproductive stages between males and females at the three studied sites and the gametogenesis evolution was quite the same at all three sites. The majority of cockles were at the development stage in the first sampling date, at maturity for the second sampling, spawning in the third and finally spent in the fourth. However, two differences were detected: the first in the beginning of gametogenesis cycle in the contaminated site (Gargour) with a majority of individuals in gametogenesis stage (stage 3), while the majority of individuals were in development stage (stage 2) in the reference (Ellouza) and the intermediate (Sidi Mansour) sites. The second difference was observed in the last sampling date (December) in Sidi Mansour and Gargour sites with specimens in spent stages while specimens in Ellouza were in the spawning stage.
A quantitative evaluation of the successful reproductive cycle is determined by SMI. Evolution of this index (Fig. 6) showed a similar pattern in the three studied sites and at the three first sampling periods. An increase in this index was observed between October and November, corresponding to an evolution of gametogenesis that took place synchronously in the three studied sites. In the last sampling date, a decrease of SMI was observed in cockles from the intermediate(Sidi Mansour) and the most contaminated (Gargour) sites, however it was increased in cockles from the less contaminated site (Ellouza).
Inter-Sites Variation of Relative Gene Expression
Relative gene expression of seven-regulated genes was investigated in cockles from the three sites (Figs. 7, 8, 9 and 10). No difference was found in the expression of these genes between males and females and the results obtained for the two sexes have been therefore grouped. Results do not show inter-sites variations in the expressions of MT, ABCB1, MnSOD and CuZnSOD genes (Figs. 7, 8). HSP70 expression decreased when pollution increased. Indeed, the highest mRNA level of HSP70 was recorded in the Ellouza site while the lowest one was recorded in the Gargour site (Fig. 9). Catalase expression level was similar in the intermediate and the contaminated sites. It was significantly over expressed (threefold) in Sidi Mansour and Gargour sites compared to Ellouza site (Fig. 10). COI genes show the same expression pattern (Fig. 10). This gene was significantly over expressed (1000 fold) in the intermediate and the most contaminated sites with respect to the less contaminated site.
Principal Components Analysis
PCA (Fig. 11) was performed considering condition index, mRNA levels (ΔCt = Ct sample − CHKG) and bioaccumulation of metals (Cd, Cu, Zn and Ni) parameters. First and second principal components(PC) explained 32% and 18.83% of the total variance, respectively, with the first PC being related to metals (Cd, Cu, Ni and Zn) and gene expression of HSP70, CAT and COI whilst PC2 was related to the expression of CuZnSOD, MnSOD and MT. All pair-wise correlations were tested statistically (Pearson’s correlation test), and the results were presented in Fig. 12. Catalase and COI expression levels were significantly (p < 0.05) positively correlated. Moreover, Zn was significantly (p < 0.05) positively correlated to Cd, Cu, Zn, catalase and COI genes. Likewise, MnSOD and CuZnSOD were significantly positively correlated (p < 0.05). Individuals projections on the two main axes of the PCA indicate the separation of cockles from the three studied sites. Samples from Ellouza site were quite clustered on the left of the figure with the lowest metal level but the highest mRNA expression of MT, HSP70, MnSOD and CuZnSOD genes. Cockles from Gargour site were grouped on the right at the top of the figure with an important expression of COI and CAT genes, while samples from Sidi Mansour site are found on the right lower part of the figure with the highest metals bioaccumulation.
Discussion
Three sites (Gargour, Sidi Mansour and Ellouza) differing by their levels of contaminations were subject of this study. These sites were firstly selected for their richness in C. glaucum (Karray et al. 2015a, b, c; Machreki-Ajmi and Hamza Chaffai 2006; Machreki-Ajmi 2008; Machreki-Ajmi et al. 2009, 2013). Secondly, Gargour site located in the southern coast of the Gulf of Gabes was selected as representative of the contamination statute in the southern coast. On the other hand, Sidi Mansour site located in the north coast of the Gulf of Gabes was selected as representative of the state of contamination in the northern coast. Finally, Ellouza site was selected as a site so far from any source of urban or industrial contamination (Ben Salem and Ayadi 2016, 2017; Kessabi et al. 2009, 2014; Barhoumi et al. 2009). Gargour site, situated 15 km south of Sfax and 10 km south of the SIAPE plant was considered as the most polluted site. It was affected by industrial activity and effluents carried out by North–South streams which contain high concentrations of metals (Ajala et al. 2021; Ben Salem and Ayadi 2016; Karray et al. 2015c; Mosbahi et al. 2019). These authors reported high levels of metal mainly Cd, Cu, Zn and Pb. Moreover, the richness of this site in nutritive salts and especially in phosphate distributed by phosphogypsum discharges from SIAPE plant brought to a massive phytoplankton production characteristic of this region (Hamza-Chaffai et al. 2003). Sidi Mansour site located 12 km north of Sfax was considered as an intermediate contaminated site. It is an active fishing area but not very productive compared to the regions to the south in the Gulf of Gabes. This site has been impacted by diverse contaminants, including hydrocarbons, sulphates, trace metals (Cd, Cr and Pb) and microplastics directly related to existing Poudrière industrial zone (Fourati et al. 2018; Chouchene et al. 2019). The Ellouza site is located 45 km north of Sfax city and appeared to be unaffected by human activities and seemed to be so far from any source of urban or industrial contamination. Moreover, several studies interested in metal analyses (Cd, Cu,Ni and Zn) in this site showed lower levels compared to other sites in the same region. Ellouza site is usually considered as the less contaminated site according to the studies of Ben Salem and Ayadi (2016, 2017), Kessabi et al. (2009, 2014), Barhoumi et al. (2009). Furthermore, our results on metals analyses in sediments collected from these three sites were in accordance with studies presented above since Gargour sediment presented the highest values for almost all the different metals studied, followed by the Sidi Mansour and Ellouza sites.
Among studied metals, only Cd presented values above the threshold established by Long et al. (1995) (Table 1) This result was in accordance with several studies demonstrating that Cd is a major contaminant in the Gulf of Gabes region (Machreki-Ajmi et al. 2009; Karray et al. 2015b; Naifar et al. 2018; Feki et al. 2022). In general, contaminated sediment reflects a polluted site and a real risk to organisms living in contact with the sediment since it serves as nursery and breeding place for many benthic species including cockles (Machreki-Ajmi et al. 2009; Geffard et al. 2001).
Three parameters were used in this study to evaluate the global physiological state of cockles: stress-on-stress test, condition index and the sex ratio. The stress-on-stress test reveals the physiological capacity of cockles to survive under anoxia. Our data demonstrate that exposure to natural metallic pollution affects the LT50 of cockles, Indeed, the highest values of LT50 were recorded in cockles from the Ellouza site reflecting an important resistance to anoxia in cockles from this site compared to cockles from Gargour site that present the lowest values of LT50 reflecting metabolic perturbations and a general lower health status of cockles. Our results were in concordance with previous data performed on the same species demonstrating that cockles from a reference site are more resistant than cockles from a contaminated site (Machreki-Ajmi et al. 2009). Other studies carried out in controlled conditions on C. glaucum (Ladhar-Chaabouni et al. 2009; Karray et al. 2015b; c) and on others bivalves (Hamza-Chaffai et al. 1998; Viarengo et al. 1999) showed that, following an exposure to metallic contamination, a decrease in LT50 was observed with exposure time and increasing metals concentrations.
The CI is a general indicator of the physiological and nutritional state of animals in relation to environmental factors (Krishnakumar et al. 2018; Karray et al. 2015a). This index is routinely used in environmental monitoring studies to assess the health condition of bivalves (Filgueira et al. 2013; Peharda et al. 2007). In this study, CI was synchronous in the three studied sites. Moreover, the highest values of CI were observed in October in the three studied sites when cockles are in development/gametogenesis stages. This result demonstrated that cockles were in their best physiological and nutritional state which prepare them for spawning. However, a significant decrease in CI was observed then between October and November when cockles began their spawning period. This decrease may be attributed to the loss of body mass after spawning. to supply the reproductive effort as has been observed in the studies of Machreki-Ajmi et al. (2013) and Karray et al. (2015a, b, c) in the cockle C. glaucum originating from the Gulf of Gabes. Furthermore, CI values in Gargour site in October was significantly lower than those in Sidi Mansour and Ellouza site suggesting a perturbation in physiological and nutritional state in cockle from the most polluted site Gargour and an excellent physiological and nutritional state in cockles from Sidi Mansour and Ellouza sites. Our results are in accordance with previous studies in bivalves demonstrating a decrease in CI when increasing metallic pollution (Amiard and Berthet 1996; Mourgaud et al. 2002; Hummel et al. 1997). Furthermore, Kerambrun et al. (2011) demonstrated that for organisms collected at the same period of reproduction, CI index can highlight differences between control and contaminated sites. However, variations in CI could be also related to hydro-climatic conditions in studied sites (temperature, salinity, oxygen availability) and food availability (Sasikumar and Krishnakumar 2011; Kerambrun et al. 2011). Moreover CI could be also strongly related to seasonal variations and age of animals (Valentine et al. 2006). Therefore, this parameter must be considered carefully in ecotoxicological studies (Krishnakumar et al. 2018; Gilliers et al. 2004).
Our results on sex-ratio demonstrated that there was a significant imbalance in favour of females at the contaminated location Gargour. However, we have detected an equal percentage of males and females in the reference site Ellouza. These results may suggest that the unbalanced sex-ratio could be due to the gradient pollution factor, which can act in synergy with the other factors (temperature, trophic conditions, endocrine disruptors, "etc.").Our findings are consistent with research by Liu et al. (2013) that show a significant sex ratio imbalance in favour of males when metal exposure increased. Previous studies (Lango-Reynoso et al.1999; Ketata et al. 2007; Machreki-Ajmi et al. 2013) also reported an unbalanced sex-ratio that was often related to particular environmental conditions (temperature, trophic conditions, "etc.") as well as to reproductive stages. Moreover, in the contaminated site we have demonstrated that the predominance was for females. The same results was confirmed by the study of Derbali et al. (2009), demonstrating a predominance of females in cockles having a size above 24 mm in some sites in the gulf of Gabès region.
Results of gametogenesis and maturity index show few differences in the reproductive cycle of cockles collected from the three studied sites. However, two differences were detected: the first in the beginning of the gametogenesis cycle in cockles from the contaminated site (Gargour) with a majority of individuals in gametogenesis stage, while in cockles from the "less contaminated site" (Ellouza) and intermediate (Sidi Mansour) sites, the majority of specimens were in development stage (stage 2). The second difference was observed for the last sampling date (December) in Sidi Mansour and Gargour sites with animals in spent stages while cockles from the less contaminated site Ellouza were still in the spawning stage. These two differences were recorded between "less contaminated" and contaminated sites and were probably linked to pollution factors. Previous studies reported many effects on reproduction (delayed oogenesis or spermatogenesis, histological alterations, etc.) in several bivalves exposed to metallic or chemical contaminants such as Mya arenaria (Gautier-Clerc et al. 2002), Ruditapes deccussatus (Ketata et al. 2007; Smaoui-Damak et al. 2006), Mytilus galloprovincialis (Ruiz et al. 2011) and Donax trunculus (Tlili et al. 2011). Other factors may also alter gametogenesis of bivalves and spawning such as water temperature in C. glaucum (Machreki-Ajmi et al. 2013; Karray et al. (2015a) as well as in C. edule and R. dessucatus (Vázquez et al. 2021). These authors reported that water temperatures is considered as the main important factor that controls gametogenesis, and the timing and duration of spawning periods. However, in this study, values of water temperatures recorded in the three studied sites don't show important differences between sites in the four sampling periods. suggesting that differences in gametogenesis observed was not correlated to temperature factor. Moreover, food availability was also known to be important in regulating gametogenesis and spawning periods in bivalves (Stenyakina et al. 2010; Narváez et al. 2008; Machreki-Ajmi et al. 2013; Karray et al. 2015a). In this study, CI that reflects physiological and nutritional status presented the highest values in Ellouza and Sidi Mansour site when cockes were in development/gametogenesis suggesting a sufficient food availability in these sites which allow cockles to prepare spawning. In this regard, Machreki-Ajmi et al. (2013), reported that C. glaucum adjusted their life cycle to spawn at times when food was available in the water column, which helps to ensure larval survival. However, lowest values of CI recorded in Gargour site may be attributable to insufficient food availability. Salinity may also affect growth breeding activities of bivalves (Machreki-Ajmi et al. 2013). Furthermore, Karray et al. (2015a, b, c), demonstrated that C. glaucum were able to support high variations of salinity and even spawn under these conditions.
Metal accumulation analysis in cockles tissues is very important because metals can be accumulated at higher levels than those found in natural environments and can even reach toxic threshold levels (Veiga 2015; Varotto et al. 2013). Results in metal accumulation show that cockles from the most contaminated site, Gargour, present the highest values of Cd, Cu, Zn and Ni metals followed by those from the intermediate site, Sidi Mansour, and finally those from the less contaminated site, Ellouza. This result confirms that bioaccumulation in cockles follows levels of contamination in studied sites. The Ellouza site has been considered as a less contaminated site by many studies (Ben Salem and Ayadi 2016, 2017; Kessabi et al. 2009, 2014; Barhoumi et al. 2009) and our results on metal analysis supported this finding. The studies of Ajala et al. (2021), Ben Salem and Ayadi (2016), Karray et al. (2015c), Mosbahi et al. (2019), Fourati et al. (2018), Chouchene et al. (2019), Smaoui-Damak et al. (2011), Machreki-Ajmi (2008), and Hamza-Chaffai et al. (2003) also supported our findings for the highly contaminated site Gargour and the intermediate site Sidi Mansour. In general, investigations done in the Gulf of Gabès region have demonstrated that the concentration of heavy metals reduces the further one is away from the crude phosphate treatment (El Zreli et al. 2015; Rabaoui et al. 2013).
When exposed to contamination, an organism can modify the expression of its genes to acclimate to this new situation. This change is often considered as an early response from an organism under stress (Achard-Joris et al. 2006; Al Kaddissi et al. 2012; Ivanina et al. 2008a, b; Navarro et al. 2011). In this study, we succeeded to partially sequence certain genes in C. glaucum that were involved in response to different types of environmental stress. Different metabolic pathways were selected such as metal and xenobiotic detoxification (metallothionein, MT; ATP Binding Cassette transporters, ABCB1), oxidative stress defense (superoxide dismutases, MnSOD and CuZnSOD; catalase, CAT)) and stress in general (heat shock proteins, HSP70). Moreover, we chose cytochrome-c oxidase (COX) as an example of an energy metabolism gene. The expression of these genes was conducted on cockles from the three studied sites. Results show that we can divided expression profiles obtained into three groups. (1) Genes involved in metals detoxification (ABCB1 and MT) and superoxide dismutase (MnSOD and CuZnSOD) do not display any difference in their expressions levels that may be due to the interference of confounding factors such as biotic factors (age, size and sex) and abiotic factors (temperature, salinity turbidity and availability of food). However, the expression of these same genes has been induced following metal exposure in several bivalves (Fang et al. 2010; Balbi et al. 2014; Delong et al. 2020); (2) HSP70 gene expression decreased when environmental pollution increased. This gene presents the highest level in cockles from the most contaminated site, Gargour, and the lowest one in samples from the reference site, Ellouza. This fact could be explained by an inhibition of HSP70 synthesis in the contaminated sites following high metallic contamination. Similar results were reported for oysters demonstrating an inhibition of HSP70 synthesis in sites presenting high levels of metals (Boutet et al. 2003). (3) CAT and COI showed higher expression levels in both the intermediate and the most contaminated sites compared to the less contaminated site. Similar study (Giuliani et al. 2013) demonstrated that Mussels exposed to metallic pollution exhibited a dose-dependent induction of CAT gene transcription. Moreover, expression of COI gene was 1000 fold higher in cockles from the Gargour and Sidi Mansour sites compared to the reference site Ellouza. This result is similar to the study of Achard-Joris et al. (2006) and could be explained by the energy demand, which is particularly high when exposed to any environmental stress (David et al. 2005).
Conclusion
Our study presents an original work in using biomarkers at different levels of biological organization (global physiological state of organisms, reproductive cycle and molecular responses) for the assessment of the health status of the cockle C. glaucum exposed to multiple metallic pollution in situ. Results obtained show changes in the overall physiological state, sex ratios, and expression of the HSP70, CAT, and COI genes. These biomarkers may be suitable for in situ analyses of the effects of metals in cockles, and should be used as powerful tools for possibly incorporation of the cockle C. glaucum as bio indicator in national and international marine pollution biomonitoring programs. It would also be interesting in the future to complete these transcriptional studies with proteomic studies to have additional information on pollution effects mechanisms.
Data Availability
All data are mentioned in the body of manuscript, tables, and figure.
References
Achard-Joris M, Gonzalez P, Marie V, Baudrimont M, Bourdineaud JP (2006) Cytochrome c oxydase subunit I geneis up-regulated by cadmium in freshwater and marine bivalves. Biometals. Int J Role Met Ions Biol Biochem Med 19:237–244
Ajala M, Ben Ameur W, Annabi A (2021) First evidence of the utility of cephalopods for biomonitoring program in the field: case of Sepia officinalis south west of Mediterranean Sea (Gulf of Gabes, Tunisia). Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-17804-9
Al Kaddissi S, Legeay A, Elia AC, Gonzalez P, Floriani M, Cavalie I, Massabuau JC, Gilbin R, Simon O (2012) Mitochondrial gene expression, antioxidant responses, and histopathology after cadmium exposure. Environ Toxicol 8:893–907
Ali Z, Malik RN, Abdul Q (2012) Heavy metals distribution and risk assessment in soils affected by tannery effluents. Chem Ecol 29:676–692
Altwaijry N, Khan MS, Shaik GM, Tarique M, Javed M (2022) Redox status, immune alterations, histopathology, and micronuclei induction in Labeo rohita dwelling in polluted river water. Arch Environ Contam Toxicol. https://doi.org/10.1007/s00244-022-00976-x
Amiard JC, Berthet B (1996) Fluctuations of cadmium, copper, lead and zinc concentrations in field populations of the Pacifc oyster Crassostrea gigas in the Bay of Bourgneuf (Atlantic coast, France). Ann Inst Oceanogr 72:195–207
Balbi T, Smerilli A, Fabbri A, Ciacci C, Montagna M, Grasselli E, Brunelli A, Pojana G, Marcomini A, Gallo G, Canesi L (2014) Co-exposure to n-TiO2 and Cd2+ results in interactive effects on biomarker responses but not in increased toxicity in the marine bivalve M. galloprovincialis. Sci Total Environ 493:355–364
Barbier EB, Hacker SD, Kennedy CJ, Koch EW, Stier A, Silliman B (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193
Barhoumi S, Messaoudi I, Deli T, Said K, Kerkeni A (2009) Cadmium bioaccumulation in three benthic fish species, Salariabasilisca, Zosterisessor ophiocephalus and Solea vulgaris collected from the Gulf of Gabes in Tunisia. J Environ Sci China 21:980–984
Ben Salem Z, Ayadi H (2016) Heavy metal accumulation in Diplodus annularis, Liza aurata, and Solea vulgaris relevant to their concentration in water and sediment from the southwestern Mediterranean (coast of Sfax). Environ Sci Pollut Res 23:13895–13906
Ben Salem Z, Ayadi H (2017) Assessment of trace metals contamination in Diplodus annularis (Linnaeus, 1758) from the south coast of Sfax, Tunisia. Euro-Mediterr J Environ Integr 2:13
Boutet I, Tanguy A, Rousseau S, Auffret M, Moraga D (2003) Molecular identification and expression of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperones 8:76–85
Brock V, Wolowicz M (1994) Comparisons of European populations of the Cerastoderma glaucum C. lamarcki complex based on reproductive physiology and biochemistry. Oceanol Acta 17:97–103
Chifflet S, Tedetti M, Zouch H, Fourati R, Zaghden H, Elleuch B, Quéméneur M, Karray F, Sayadi S (2019) Dynamics of trace metals in a shallow coastal ecosystem: insights from the Gulf of Gabès (southern Mediterranean Sea). AIMS Environ Sci 6:277–297
Chouchene K, Costa JP, Wali A, Girão VA, Hentati O, Duarte AC, Rocha-Santos T, Ksibi M (2019) Microplastic pollution in the sediments of Sidi Mansour harbor in southeast Tunisia. Mar Pollut Bull 146:92–99
Cobelo-Garcia A, Prego R, Labandeira A (2004) Land inputs of trace metals, major elements, particulate organic carbon and suspended solids to an industrial coastal Bay of the NE Atlantic. Water Res 38:1753–1764
David E, Tanguy A, Pichavant K, Moraga D (2005) Response of the Pacific oyster Crassostrea gigas to hypoxia exposure under experimental conditions. FEBS J 272:5635–5652
Day JW, Yáñez-Arancibia A, Kemp WM (2012) Human impact and management of coastal and estuarine ecosystems. In: Day JW, Crump BC, Kemp WM, Yáñez-Arancibia A (eds) Estuarine ecology, 2nd ed. Wiley, Singapore, pp 483–495
Dedourge-Geffard O, Palais F, Biagianti-Risbourg S, Geffard O, Geffard A (2009) Effects of metals on feeding rate and digestive enzymes in Gammarus fossarum: an in situ experiment. Chemosphere 77:1569–1576
Derbali A, Jarboui O, Ghorbel M (2009) Reproductive biology of the cockle Cerastoderma glaucum (Mollusca: Bivalvia) from the north coast of Sfax (Gulf of Gabes, Tunisia). Cienc Mar 35:141–152
Direction Générale de la Pêche et de l'Aquaculture: DGPA (2015) Annuaire des statistiques des pèches en Tunisie
El Zrelli R, Rabaoui L, Ben Alaya M, Daghbouj N, Castet S, Besson P, Michel S, Bejaoui N, Courjault-Radé P (2018) Seawater quality assessment and identification of pollution sources along the central coastal area of Gabes Gulf (SE Tunisia): evidence of industrial impact and implications for marine environment protection. Mar Pollut Bull 127:445–452
Fang Y, Yang H, Wang T, Liu B, Zhao H, Chen M (2010) Metallothionein and superoxide dismutase responses to sublethal cadmium exposure in the clam Mactra veneriformis. Comp Biochem Physiol C 151:325–333
Feki N, Khannous L, Abdmouleh Keskes F, Ben Slama A, Levacher D (2022) Mobility of trace metals and microbiological pollution from dredged sediments to the Gulf of Gabes, Tunisia. Environ Monit Assess 194:815
Filgueira R, Comeau LA, Landry T, Grant J, Guyonde T, Mallet A (2013) Bivalve condition index as an indicator of aquaculture intensity: a meta-analysis. Ecol Ind 25:215–229
Fourati R, Tedetti M, Guigue C, Goutx G, Zaghden H, Sayadi S, Elleuch B (2018) Natural and anthropogenic particulate-bound aliphatic and polycyclic aromatic hydrocarbons in surface waters of the Gulf of Gabès (Tunisia, southern Mediterranean Sea). Environ Sci Pollut Res 25:2476–2494
Gašparič MB, Cankar K, Žel J, Gruden K (2008) Comparison of different real-time PCR chemistries and theirsuitability for detection and quantification of genetically modified organisms. BMC Biotechnol 8:26
Gautier-Clerc S, Pellerin J, Blaise C, Gagné F (2002) Delayed gametogenesis of Mya arenariain the Saguenay Fjord (Canada): a consequence of endocrine disruptors? Comp Biochem Physiol C 131:457–467
Geffard O, Budzinski H, Augagneur S, Seaman MNL, His E (2001) Assessment of sediment contamination by spermiotoxicity and embryotoxicity bioassays with sea urchins (Paracentrotus lividus) and oysters (Crassostrea gigas). Environ Toxicol Chem 16:2190–2199
Geffard A, Sartelet H, Garric J, Biagianti-Risbourg S, Delahaut L, Geffard O (2010) Subcellular compartmentalization of cadmium, nickel, and lead in Gammarus fossarum: Comparison of methods. Chemosphere 78:822–829
Gilliers C, Amara R, Bergeron JP, Le Pape O (2004) Comparison of growth and condition indices of flat fish juvenile (sole, dab and plaice) in different coastal nursery grounds. Environ Biol Fishes 71:189–198
Giuliani ME, Benedetti M, Arukwe A, Regoli F (2013) Transcriptional and catalytic responses of antioxidant and biotransformation pathways in mussels, Mytilus galloprovincialis, exposed to chemical mixtures. Aquat Toxicol 134:120–127
Goldberg ED, Bertine KK (2000) Beyond the mussel watch—new directions for monitoring marine pollution. Sci Total Environ 247:165–174
Goldberg ED, Bowen VT, Farrington JW, Harvey G, Martin JH, Parker PL, Risebrough RW, Robertson W, Schneider W, Gamble E (1978) The mussel watch. Environ Conserv 5:101–125
Hahn ED, Soyer R (2008) Probit and logit models: differences in a multivariate realm, vol 28, pp 1–14
Hamza-Chaffai A (2014) Usefulness of bioindicators and biomarkers in pollution biomonitoring. Int J Biotechnol Wellness Ind 3:19–26
Hamza-Chaffai A, Romeo M, Gnassia-Barelli M, El Abed A (1998) Effect of copper and lindane on some biomarkers measured in the clam Ruditapes decussatus. Bull Environ Contam Toxicol 61:397–404
Hamza-Chaffai A, Pellerin J, Amiard JC (2003) Health assessment of a marine bivalve Ruditapes decussatus from the Gulf of Gabes (Tunisia). Environ Int 28:609–617
Hummel H, Moderman R, Amiard-Triquet C, Rainglet F, Van Duijn Y, Herssevoor M, De Jong J, Bogaards R, Bachelet G, Desprez M, Marchand J, Sylvand B, Amiard JC, Rybarczyk H, De Wolf L (1997) A comparative study on the relation between copper and condition in marine bivalves and the relation with copper in the sediment. Aquat Toxicol 38:165–181
Ivanina AV, Cherkasov AS, Sokolova IM (2008a) Effects of cadmium on cellular protein and glutathione synthesis and expression of stress proteins in eastern oysters, Crassostrea virginica Gmelin. J Exp Biol 211:577–586
Ivanina AV, Habinck E, Sokolova IM (2008b) Differential sensitivity to cadmium of key mitochondrial enzymes in the eastern oyster, Crassostrea virginica Gmelin (Bivalvia:Ostreidae). Comp Biochem Physiol Part C Toxicol Pharmacol 148:72–79
Kandeel EK, Mohammed SZ, Mostafa AM, Abd-Alla ME (2017) Population dynamics of the cockle, Cerastoderma glaucum: a comparison between lake Qarun and lake Timsah, Egypt. Turk J Fish Aquat Sci 17:945–958
Karray S, Smaoui-Damak W, Rebai T, Hamza-Chaffai A (2015a) Reproductive cycle, condition index and glycogen reserves of the cockles Cerastoderma glaucum from the Gulf of Gabès (Tunisia). Environ Sci Pollut Res 22:17317–17323
Karray S, Marchand J, Moreau B, Tastard E, Thiriet-Rupert S, Geffard A, Delahaut L, Denis F, Hamza-Chaffai A, Chénais B (2015b) b) Transcriptional response of stress-regulated genes to cadmium exposure in the cockle Cerastoderma glaucum from the gulf of Gabès area (Tunisia). Environ Sci Pollut Res 22:17290–17302
Karray S, Tastard E, Moreau B, Delahaut L, Geffard A, Guillon E, Denis F, Hamza-Chaffai A, Chénais B, Marchand J (2015c) c) Transcriptional response of stress-regulated genes to industrial effluent exposure in the cockle Cerastoderma glaucum. Environ Sci Pollut Res 22:17303–18161
Kerambrun E, Sanchez W, Coise H, Amara F (2011) Are biochemical biomarker responses related to physiological performance of juvenile sea bass (Dicentrarchus Labrax) and turbot (Scophthalmus Maximus) caged in a polluted harbour? Comp Biochem Physiol C Toxicol Pharmacol 154:187–195
Kerambrun E, Henry F, Perrichon P, Courcot L, Meziane T, Spilmont N, Amara R (2012) Growth and condition indices of juvenile turbot, Scophthalmus maximus, exposed to contaminated sediments: effects of metallic and organic compounds. Aquat Toxicol 108:130–140
Kessabi K, Navarro A, Casado M, Said K, Messaoudi I, Pina B (2009) Evaluation of environmental impact on natural populations of the Mediterranean killifish Aphanius fasciatusby quantitative RNA biomarkers. Mar Environ Res 70:327–333
Kessabi K, Hwas Z, Sassi A, Said, K, Messaoudi I (2014) Heavy metal accumulation and histomorphological alterations in Aphanius fasciatus (Pisces, Cyprinodontidae) from the Gulf of Gabes (Tunisia). Environ Sci Pollut Res. https://doi.org/10.1007/s11356-014-3252-6
Ketata I, Smaoui-Damak W, Guermazi F, Rebai T, Hamza Chaffai A (2007) In situ endocrine disrupting effects of cadmium on the reproduction of Ruditapes decussatus. Comp Biochem Physiol C Toxicol Pharmacol 146:415–430
Krishnakumar PK, Qurban MA, Geetha Sasikumar G (2018) Biomonitoring of trace metals in the coastal waters using bivalve molluscs. In: Saleh HEM, El-Adham E (eds) Trace elements-human health and environment. Intech Open. https://doi.org/10.5772/intechopen.76938
Ladhar-Chaabouni R, Smaoui-Damak W, Hamza-Chaffai A (2009) In vivo variation of some biomarkers with time and cadmium concentration in the cockle, Cerastoderma glaucum. Mar Biol Res 5:487–495
Lango-Reynoso F, Devauchelle N, Le Pennec M, Hatt PJ (1999) Elements of reproductive strategy in oysters, Crassostrea gigas, from the “Rade de Brest”, France. Invertebr Reprod Dev 36:141–144
Liu S, Lou S, Kuang C, Huang W, Chen W, Zhang J, Zhong G (2011) Water quality assessment by pollution-index method in the coastal waters of Hebei Province in western Bohai Sea, China. Mar Pollut Bull 62:2220–2229
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Lobel PB, Bajdik CD, Belkhode SP, Jackson SE, Longerich HP (1991) Improved protocol for collecting mussel watch specimens taking into account sex, size, condition, shell shape, and chronological age. Arch Environ Contam Toxicol 21:409–414
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–87
Machreki-Ajmi M (2008) Hamza–Chaffai. Ecotoxicology 17:802–810
Machreki-Ajmi M, Hamza-Chaffai (2006) Accumulation of cadmium and lead in Cerastoderma glaucum originating from the Gulf of Gabes, Tunisia. Bull Environ Contam Toxicol 76:529–537
Machreki-Ajmi M, Ketata I, Ladhar-Chaabouni R, Hamza-Chaffai A (2009) The effect of in situ cadmium contamination on some biomarkers in Cerastoderma glaucum. Ecotoxicology 17:1–11
Machreki-Ajmi M, Rebai T, Hamza-Chaffai A (2013) Reproductive strategy in a littoral population of the cockle, Cerastoderma glaucum from the Gulf of Gabés area (southeastern Tunisia). J Shellfish Res 32:733–738
Malham SK, Hutchinson TH, Longshaw M (2012) A review of the biology of European cockle (Cerastoderma spp.). J Mar Biol Assoc UK 92:1563–1577
Mosbahi N, Serbaji M, Pezy JP, Neifar L, Dauvin JC (2019) Response of benthic macrofauna to multiple anthropogenic pressures in the shallow coastal zone south of Sfax (Tunisia, central Mediterranean Sea). Environ Pollut 253:474–487
Mourgaud Y, Martinez E, Geffard A, Andral B, Stansiere JY, Amiard JC (2002) Metallothionein concentration in the mussel Mytilus galloprovincialis as a biomarker of response to metal contamination: validation in the field. Biomarkers 7:479–490
Naifar I, Pereira F, Zmemla R, Bouaziz M, Elleuch B, Garcia D (2018) Spatial distribution and contamination assessment of heavy metals in marine sediments of the southern coast of Sfax, Gabes Gulf, Tunisia. Mar Pollut Bull 131:53–62
Narváez M, Freites L, Guevara M, Mendoza J, Guderley H, Lodeiros JC, Salazar G (2008) Food availability and reproduction affects lipid and fatty acid composition of the brown mussel, Perna perna, raised in suspension culture. Comp Biochem Physiol B 149:293–302
Navarro A, Faria M, Barata C, Pina B (2011) Transcriptional response of stress genes to metal exposure in zebra mussel larvae and adults. Environ Pollut 159:100–107
Peharda M, Zupan I, Bavcevick L, Frankick A, Klanjsflcek T (2007) Growth and condition index of mussel Mytilus galloprovincialis in experimental integrated aquaculture. Aquac Res 38:1714–1720
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: best Keeper-Excel-based tool using pair-wise correlations. Biotech Lett 26:509–515
Rabaoui L, Balti R, El Zrelli R, Tlig-Zouari S (2013) Assessment of heavy metal pollution in the Gulf of Gabes (Tunisia) using four mollusc species. Mediterr Mar Sci 15(1):45–58
Rainbow PS (1990) Heavy metal levels in marine invertebrates. In: Furness RW, Rainbow PS (eds) Heavy metals in marine environment. CRC Press, Boca Raton, pp 67–79
Ruiz Y, Suarez P, Alonso A, Longo E, Villaverde C, San Juan F (2011) Environmental quality of mussel farms in the Vigo estuary: pollution by PAHs, origin and effects on reproduction. Environ Pollut 159:250–265
Sasikumar G, Krishnakumar PK (2011) Aquaculture planning for suspended bivalve farming systems: The integration of physiological response of green mussel with environmental variability in site selection. Ecol Ind 11:734–740
Sellami F, Dammak R, Chafai A (2022) Analysis of daily and diurnal O3–NOx relationships and assessment of local/regional oxidant (OX = O3 + NO2) levels and associated human health risk at a coastal suburban site of Sfax (Tunisia). Arch Environ Contam Toxicol. https://doi.org/10.1007/s00244-022-00966-z
Siah A, Pellerin J, Amiard JC, Pelletier E, Viglino L (2003) Delayed gametogenesis and progesterone levels in soft-shell clams (Mya arenaria) in relation to in situ contamination to organotins and heavy metals in the St Lawrence River (Canada). Comp Biochem Physiol C 135:145–156
Smaoui-Damak W, Rebai T, Berthet B, Hamza-Chaffai A (2006) Does cadmium pollution affect reproduction in the clam Ruditapes decussatus? A one-year case study. Comp Biochem Physiol C 143:252–261
Smaoui-Damak W, Guebsi F, Karray S, Rebai T, Costil K, Hamza-Chaffai A (2011) Storage and reproductive strategy of the carpet-shell clam, Ruditapes decussatus in the Gulf of Gabès (Tunisia). Invertebr Reprod Dev 66:1–11
Stenyakina A, Walters LJ, Hoffman EA, Calestani C (2010) Food availability and sex reversal in Mytella charruana, an introduced bivalve in the southeastern United States. Mol Reprod Dev 77(3):222–230
Tankoua OF, Buffet PE, Amiard JC, Berthet B, Mouneyrac C, Amiard-Triquet C (2013) Integrated assessment of estuarine sediment quality based on a multi-biomarker approach in the bivalve Scrobicularia plana. Ecotoxicol Environ Saf 88:117–125
Tlili S, Métais I, Ayache N, Boussetta H, Mouneyrac C (2011) Is the reproductionof Donax trunculus affected by their sites of origin contrasted by their level of contamination? Chemosphere 84:136–1370
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Varotto L, Domeneghetti S, Rosani U, Manfrin C, Cajaraville MP, Alberto P, Paola V (2013) DNA damage and transcriptional changes in the gills of Mytilus galloprovincialis exposed to nanomolar doses of combined metal salts (Cd, Cu, Hg). PLoS ONE 8(1):e54602
Vázquez E, Woodin SA, Wethey DS, Peteiro LG, Olabarria C (2021) Reproduction under stress: acute effect of low salinities and heat waves on reproductive cycle of four ecologically and commercially important bivalves. Front Mar Sci 8:685282
Veiga K (2015) Assessment of metals contamination (Cd, Pb, Ni) at the Óbidos Lagoon using Cerastoderma edule as a biomonitoring tool, p 122
Viarengo A, Canesi L, Pertica M, Mancinelli G, Accomando A, Smaalb AC, Orunesu M (1999) Stress on stress response: a simple monitoring tool in the assessment of a general stress syndrome in mussels. Mar Environ Res 39:245–248
Zouch H, Cabrol L, Chifflet S, Tedetti M, Karray F, Zaghden H, Sayadi S, Quéméneur M (2018) Effect of acidic industrial effluent release on microbial diversity and trace metal dynamics during resuspension of coastal sediment front. Microbiology 9:3103
El Zrelli R, Courjault-Radé P, Rabaoui R, Castet, S, Michel S, Bejaoui, N (2015) Heavy metal contamination and ecological risk assessment in the surface sediments of the coastal area surrounding the industrial complex of Gabes city, Gulf of Gabes, SE Tunisia. Mar Pollut Bull 101:922–929
Zuykov M, Pelletier E, Harper DAT (2013) Bivalve mollusks in metal pollution studies: from bioaccumulation to biomonitoring. Chemosphere. https://doi.org/10.1016/j.chemosphere.2013.05.001
Acknowledgements
The authors thank all the participants.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Sahar Karray, Justine Marchand and Francoise Denis performed the lab experiments. Alain Geffard contributed to the metal analysis tests. Tarek Rebai contributed to the histological study. Sahar Karray prepared the manuscript draft. Amel Hamza Chaffai and Benoit Chenais revised, and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karray, S., Marchand, J., Geffard, A. et al. Metal Contamination and Biomarkers in Cerastoderma glaucum: A Multi-level Approach. Arch Environ Contam Toxicol 84, 484–503 (2023). https://doi.org/10.1007/s00244-023-00999-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-023-00999-y