Abstract
This study assessed the responses of molecular biomarkers and heavy metal levels in Cerastoderma glaucum exposed for 1 week to two industrial effluents (1 %) discharged into the Tunisian coastal area, F1 and F2, produced by different units of production of a phosphate treatment plant. A significant uptake of metals (Cd, Cu, Zn, and Ni) was observed in exposed cockles compared to controls, with an uptake higher for F1 than for F2. A decrease in LT50 (stress on stress test) was also observed after an exposure to the effluent F1. Treatments resulted in different patterns of messenger RNA (mRNA) expression of the different genes tested in this report. Gene transcription monitoring performed on seven genes potentially involved in the tolerance to metal exposure showed that for both exposures, mechanisms are rapidly and synchronically settled down to prevent damage to cellular components, by (1) handling and exporting out metal ions through the up-regulation of ATP-binding cassette xenobiotic transporter (ABCB1) and metallothionein (MT), (2) increasing the mRNA expression of antioxidant enzymes (catalase (CAT), superoxide dismutases, CuZnSOD and MnSOD), (3) protecting and/or repairing proteins through the expression of heat shock protein 70 (HSP70) mRNAs, and (4) increasing ATP production (through the up-regulation of cytochrome c oxidase 1 (CO1)) to provide energy for cells to tolerate stress exposure. The tools developed may be useful both for future control strategies and for the use of the cockle C. glaucum as a sentinel species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of modern industry, various contaminants have been discharged into marine environments and have caused instability and disorder of ecosystems (Cong et al. 2012; Ladhar-Chaabouni et al. 2012). Among these contaminants, effluents are continuously discharged from municipal and industrial treatment plants and have progressively affected more and more ecosystems (Jadeja and Tewari 2007). Treated or not, these effluents can release many pollutants such as polycyclic aromatic hydrocarbons, pharmaceuticals, and heavy metals (Sim et al. 2013). The latter are deemed to be serious pollutants because of their toxicity, persistence, and non-degradability in the environment (Gargouri et al. 2011). In general, exposure to effluents is known to cause a variety of stress-related changes in aquatic organism health such as immunotoxicity (Gagne et al. 2008; Salo et al. 2007), neurotoxicity and oxidative stress (Kamel et al. 2012), and physiological alterations (Douxfils et al. 2007). The impact of effluents on marine environments is often assessed by the measurement of pollutant body burdens in marine organisms (Haynes et al. 1997). This assessment can be done by passive biomonitoring (sampling organisms living in the investigated area) or by active biomonitoring (transplantation of organisms or laboratory experiments). However, laboratory experiences remain an alternative approach to evaluate the potential toxicity effects of a mixture of pollutants (effluents) to aquatic organisms. Using this methodology, it is possible to quantify the effects of contaminant in controlled conditions with precision. In Tunisia, the gulf of Gabès (localized on the southeastern coast) is influenced by an important industrial activity, especially a phosphate treatment plant (SIAPE: Société des Industries d’Acide Phosphoriques et d’Engrais), exposing coastal waters and marine organisms to increasing contamination (Ladhar-Chaabouni et al. 2009a). The main activity of this industry is the transformation of the crude phosphate to fertilizers. This activity generates wastewaters (effluent) rich in sulfate, fluoride, phosphorus, and heavy metals that will be discharged in the marine environment. Consequently, marine ecosystems around the crude phosphate treatment plant have been completely destroyed, and high levels of toxic metals were observed in the sediment and in bivalve species (Javied et al. 2009; Ketata et al. 2007; Mar and Okazaki 2012; Tayibi et al. 2009). Cerastoderma glaucum is one of the bivalves widely distributed in the gulf of Gabès, and this species has been validated as a bioindicator organism reflecting the pollution state of the Tunisian coast (Machreki-Ajmi and Hamza-Chaffai 2006; Szefer et al. 1999). This sedentary and filter-feeding organism lives in the superficial sediment and is known to be suitable for toxicology and risk assessment studies in the field (Ladhar-Chaabouni et al. 2009a; Machreki-Ajmi and Hamza-Chaffai 2006, 2008) or in laboratory experiments (Ladhar-Chaabouni et al. 2009b, c). Cockles are major preys for diverse animal groups such as crustaceans, fishes, and wading birds (Paul-Pont et al. 2010), and they may also contribute to reduce the particulate organic load (Derbali et al. 2012). Beyond these ecological roles, they are also commercially important resources (Paul-Pont et al. 2010). Studies carried out on this species typically focused on biochemical parameters (metal bioaccumulation and biomarker measures). In addition, experiments performed were usually conducted using a single contaminant (e.g., cadmium) (Ladhar-Chaabouni et al. 2009b, c). The originality of the present study consists of using molecular biomarkers to assess the response of cockles to a natural mixture of contaminants (effluent) of the gulf of Gabès. Given that the initial interaction between contaminant and aquatic organisms occurs at the cellular level, the molecular level is suitable for the early and sensitive detection of contaminant exposure and may allow the prediction of biological effects at higher biological organization levels. Moreover, changes in gene expression are likely to play critical roles in acclimation and/or adaptation of organisms (Schulte 2004).
The purpose of this study was therefore to investigate the responses of cockles’ C. glaucum exposed to two different effluents originated from the SIAPE of the gulf of Gabès, through the study of (1) metal bioaccumulation in cockles’ tissues, (2) the global physiological state of organisms, (3) the responses at the transcriptional level of several key genes, and (4) the relationships between gene expression and metal contents. The messenger RNA (mRNA) transcripts used encode proteins involved in diverse pathways such as metal and xenobiotic detoxification (metallothionein, MT; ATP-binding cassette xenobiotic transporter, ABCB1), protection against oxidative stress (superoxide dismutases, MnSOD and CuZnSOD; catalase, CAT), general stress (heat shock protein 70, HSP70), and mitochondrial alterations (cytochrome c oxidase 1, CO1). All transcriptional responses were measured in gills because this organ constitutes a key interface for the uptake, storage, and excretion of metals and has a high capacity to synthesize proteins (Al Kaddissi et al. 2012; Marigomez et al. 2002; Navarro et al. 2011; Paul-Pont et al. 2010). In contrast to most mRNA analyses that are performed on sample pools (Ciacci et al. 2012; Navarro et al. 2009), the present report analyzes gene expression at the individual scale to highlight all the variability between cockles experiencing the same exposition.
Materials and methods
Animals and treatments
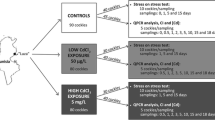
Cockles (C. glaucum) (28–32 mm) were collected in October from the “Luza” site (located 45 km in the north of Sfax, Tunisia; Fig. 1), which can be considered as a reference site with a low pollution level (Barhoumi et al. 2009; Kessabi et al. 2010). After a period of acclimation of 24 h, cockles were distributed in three polyethylene tanks (30 × 30 × 20 cm), each one containing a layer of autoclaved sediment and 6 l of aerated seawater. The first tank contained seawater from the reference site Luza (controls), the second tank contained seawater with the F1 effluent, and the third tank contained seawater with the effluent F2 (F1 and F2 effluents were collected at the exit of the SIAPE). Both F1 and F2 effluents were diluted to a final concentration of 1 % (sub-lethal concentration determined following a preliminary set of toxicity experiments, pers. comm.). Seventy to eighty-five cockles were held in each tank (experimental design schematized in Fig. 1), and exposure time varied between 12 h and 6 days. During the experiment, effluents were renewed twice a week.
Stress on stress test
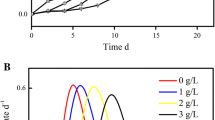
Ten animals of each of the three conditions of the experiment (controls, F1 and F2 groups) and at different times of exposure (after 0, 1, 3, and 6 days of exposure) were sampled and submitted to anoxia by air exposure at 15 °C (Fig. 1). For each sample, survival was assessed daily according to the method of Viarengo et al. (1995). Death symptoms were considered to be open valves and absence of muscular activity. Lethal time corresponding to 50 % of dead animals (LT50) was measured and the results expressed in days for each condition and at the four different times of exposure (0, 1, 3, and 6 days).
Condition index of cockles
Condition index was calculated individually on five cockles at each sampling time (Fig. 1). Before dissection, all cockles were weighted (total and soft weight), and the condition index (CI) was expressed as a percentage of the ratio of fresh weight of soft tissues to total weight (Lobel et al. 1991).
Determination of metal contents
Heavy metal analyses (Cd, Cu, Ni, and Zn) were carried out (1) on effluent waters directly from the effluent sampling site and (2) individually on five cockles from the laboratory experiment at each sampling time (Fig. 1) on the remaining animal tissues (including digestive gland, muscle, mantle, and foot). The determination of trace element concentration in effluent waters was performed using an inductively coupled plasma optical emission spectrometer (ICP-AOS, Thermo Scientific iCAP 6300 DUO), and measurement of metal concentrations in cockles was carried out as previously described (Dedourge-Geffard et al. 2009; Geffard et al. 2010) by flameless (Cd, Cu, and Ni) or flame (Zn) atomic absorption spectrophotometry with Zeeman correction, using a graphite furnace (SpectrAA Zeeman 220).
RNA extraction and cDNA synthesis
Gene transcription analyses were carried out individually on the same five individuals for which metal analyses and CI were measured and at each sampling time (Fig. 1). The gills of each sample were dissected, conserved in RNA later (Sigma-Aldrich), and stored at −20 °C. Total RNA was isolated from 100 mg of gills using Tri-Reagent (Invitrogen) following the manufacturer’s instructions. RNA concentration was determined spectrophotometrically at 260 nm, and RNA quality was checked using the Experion system (Bio-Rad). Three micrograms of total RNA were added to dTRace primer (5′-GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTTT-3′) and nuclease free water in a final volume of 12.25 μl. A denaturation at 70 °C was carried out for 5 min and then the mixture was kept on ice. First strand complementary DNAs (cDNAs) were then synthesized using dTRace, dNTPs, MMLV reverse transcriptase (Promega), RNAsin (Promega), and nuclease-free water in a final volume of 25 μl for 90 min at 42 °C.
Real-time quantitative PCR
Real-time PCR reactions were performed using a Step One plus apparatus (Applied Biosystems) with Fast SYBR Green Master Mix (Applied Biosystems) and specific primers designed (Table S1 in Supplementary Data) on the basis of previously characterized sequences as described in details in Supplementary Data. The best housekeeping gene was selected among four genes tested using the Bestkeeper Software (Pfaffl et al. 2004), and the relative expression was determined by the comparative Ct method (Livak and Schmittgen 2001) using control cockles at time 0 (T 0) as a calibrator (cf Supplementary Data).
Statistical analyses
All statistical analyses were performed using the R statistical Software version 2.14.1. In the stress on stress experiment, influence of metal concentration and exposure time on ability to survive under anoxia was analyzed using the SURVREG function (survival package, R). To test for significant differences between mRNA expression values, Kolmogorov-Smirnov tests were used (p < 0.05). After normalization of the data by log(x + 1) transformation and stabilization of their variances by square root transformation, influences of effluent treatment and exposure time on mRNA expression, metal bioaccumulation, and CI were assessed by independent ANOVA significance tests. A principal component analysis (PCA) was performed for each treatment on mRNA expression, metal bioaccumulation, and CI, and correlations between all these variables were tested statistically using Pearson’s test.
Results
Effects of effluents on the global physiological state
During the experiment, no mortality was recorded in the three groups of cockles. Survival under anoxia (i.e., stress on stress assay) is significantly affected by treatment (p < 0.05), with exposure causing a decrease in survival time in air relative to controls (significant for F1 with p < 0.05 but not significant for F2; Fig. 2). No difference in CI is observed with treatment (controls 11.7 ± 2.6; F1 12.1 ± 1.9; F2 13.3 ± 2.2) nor with time of exposure (data not shown).
Metal concentrations in F1 and F2 effluents and metal accumulation in cockles’ tissues
As indicated in Table 1, heavy metal concentrations are higher in F1 effluent than in F2: Cu (63-fold), Cd (29-fold), Cr (24-fold), Mn (13-fold), Ni (10-fold), Zn (6-fold), and Pb (5-fold). The concentration of Cd, Cu, Zn, and Ni in cockles’ soft tissues was also investigated during the time of the experiment (Fig. 3). Cd, Cu, Zn, and Ni concentrations (expressed with respect to dry weight) remain low and relatively constant in tissues of control cockles. Accumulation varied among metals; relative to their respective concentrations in the effluents, Cd was much less accumulated than the other metals (up to 100-fold). Their accumulation in treated cockles is dependent of the effluent treatment (p < 0.05), i.e., cockles exposed to F1 accumulate more Cd, Cu, Ni, and Zn than those exposed to F2 but only about 1.5-fold, except for Cd (about 24-fold). For Cu, Ni, and Zn, a similar bioaccumulation pattern is observed between cockles exposed to F1 and F2. The bioaccumulation of Ni and Zn is not time dependent. On the contrary, Cu accumulation is time dependent for both effluent experiments, i.e., treated cockles accumulate Cu between 1 and 3 days (bioaccumulation higher in F1 compared to F2). A similar pattern is also observed for Cd (for F1 only), with low variation during the two first days, followed by an increase in Cd concentration. This increase is quite moderate for F2, reaching 0.224 ± 0.106 μg/g dry weight (DW) after 5 days of exposure, while strong for F1, with a maximum value of 6.181 ± 1.276 μg/g DW after 6 days of exposure.
Effect of F1 and F2 effluent on relative gene expression
Relative expression of the seven stress-regulated genes with respect to the calibrator (controls at time 0) is represented as box plots in Figs. 4 and 5. In cockles exposed either to F1 or to F2, mRNA expression was globally higher than in controls for all the studied genes. The variability (represented by quartiles in boxplots) is also significantly higher in exposed cockles than controls (p < 0.001, Fligner-Killeen test of homogeneity of variances; Figs. 4 and 5).
For MT, CO1, and HSP70, expression increased with exposure time quite linearly in cockles exposed to F1 (Fig. 4; Table 2). In cockles exposed to F2, exposure time had no significant influence on mRNA expression for these genes. Despite fluctuations, the expression of ABCB1 is higher in treated cockles compared to controls, but exposure time has no significant influence on its expression whatever the effluent (Table 2). Concerning the enzymes involved in the response to oxidative stress (Fig. 5), mRNA expression increased with exposure time for MnSOD and CAT in cockles exposed to F1. In particular, the expression of these genes remained close to that of controls during the first two days of exposure and then expression increased strongly on the third day (+9- and +43-fold, the mean expression level of control samples for MnSOD and CAT, respectively) and remained high until the sixth day (Fig. 5). Exposure time has no significant influence on CuZnSOD mRNA expression (Fig. 5; Table 2). In cockles exposed to F2, on the contrary, the expression of the genes involved in response to oxidative stress (CAT, MnSOD, CuZnSOD) globally decreased with exposure time (Table 2). In details, the dynamics was the same for the three genes: expression increased during the first 24 h (+87-, +71-, and +58-fold, the mean expression level of control samples for CAT, MnSOD, and CuZnSOD, respectively) then decreased until the fifth day (−10-, −2-, and −3-fold for CAT, MnSOD, and CuZnSOD, respectively) (Fig. 5).
Principal component analyses (PCAs)
To highlight relationships between mRNA levels (ΔCt = Ctsample − CHKG), CI, and bioaccumulation of metals (Cd, Cu, Zn, and Ni), two different PCAs were carried out, one considering controls and cockles exposed to the effluent F1 and the other one considering controls and cockles exposed to the effluent F2 (Fig. 6).
Principal component analysis (PCA) on gene expressions, metal bioaccumulation (Ni, Zn, Cu, and Cd), and CI. a PCA performed on controls and cockles exposed to effluent F1; b PCA performed on controls and cockles exposed to effluent F2; CI condition index; [Ni], [Zn], [Cu], [Cd], and metal bioaccumulation in cockles
In the F1 PCA analysis (Fig. 6a), 59.3 % of the total variance is explained by the two main axes. All parameters appear on the same side relative to the first axis (i.e., on the left of the factorial map). Two groups of correlated parameters (p < 0.05, Pearson’s test) appear according to their location relative to the second axis (in dotted circle on the map, Fig. 6a). The first one, appearing on the top of the factorial map, is composed of Zn bioaccumulation, MT, MnSOD, CuZnSOD, CAT, and ABCB1 mRNA levels. The second one, appearing on the bottom of the factorial map, contains Ni, Cu, and Cd bioaccumulation, CO1 and HSP70 mRNA levels, and CI (Fig. 6a). The two groups are in fact overlapping with significant correlations found between some parameters of each group (Table S2 in Supplementary Data). The projection of the samples on the two main axes of the PCA (Fig. 7a) indicates that all the control samples are found quite clustered on the right of the figure with the lowest metal bioaccumulations (Ni, Zn, Cu, and Cd) and the lowest mRNA levels of the seven genes studied. On the contrary, treated cockles are found mostly on the left of the figure with the highest metal bioaccumulations and the highest mRNA expression of the biomarkers cited above (Fig. 7a). A few exposed cockles are however found clustered with the control group. The projection of the samples according to the time of exposure (Fig. 7c) indicates that the samples corresponding to the three first times of exposure (12 hours, 1 day, and 2 days) are located on the right near the control group with low mRNA expression of all genes tested and low bioaccumulation of heavy metals compared to the other samples. Samples subjected to 3, 5, and 6 days of F1 effluent are projected on the left of the figure with the highest mRNA expression for the different biomarkers and the highest metal bioaccumulations. It can also be noted that cockles at 3 days of exposure are located at the top of Fig. 7c with the highest expression of oxidative markers (MnSOD, CuZnSOD, CAT), MT, and ABCB1, whereas cockles at 6 days of exposure are located in the lower part of the figure with the highest expression of HSP70 and CO1.
a Projections of samples according to the different quantitative data presented in Fig. 5a (F1 exposure); b projections of samples according to the different quantitative data presented in Fig. 5b (F2 exposure); c projections of samples according to the time of exposure to F1; d projections of samples according to the time of exposure to F2
In the F2 PCA analysis (Fig. 6b), 62.8 % of the total variance is explained by the two main axes. A first group of correlated parameters (p < 0.05, Pearson’s test, Table S3 in Supplementary Data) appears on the top of the factorial map (in dotted circle) and is composed of CAT, MnSOD, CuZnSOD, and MT mRNA expression (Pearson’s correlation coefficients >0.517, p < 0.05). The second one appears on the bottom of the factorial map (in dotted circle) and contains bioaccumulation of metals (Cd, Cu, Zn, and Ni) and HSP70 and ABCB1 mRNA expression (Pearson’s correlation coefficients >0.341, p < 0.05, Table S3). The mRNA expression of CO1, appearing along the first axis, is highly correlated to the two groups (Table S3). The projection of the samples on the two main axes of the PCA (Fig. 7b) indicates that all the control samples are found quite clustered on the right of the figure with the lowest metal bioaccumulations, the lowest mRNA levels of the seven genes, and the lowest CI. On the contrary, treated cockles are again found mostly on the left of the figure with the highest metal bioaccumulations and the highest mRNA expression of the biomarkers cited above (Fig. 7b). The projection of the samples according to the time of exposure (Fig. 7d) indicates that most of the samples corresponding to the three first times of exposure (12 h, 1 day, and 2 days) are located on the top left of the factorial map (in dotted circle) with high levels of mRNA coding for antioxidant enzymes (CAT, MnSOD, CuZnSOD) and MT. In contrast, most of the samples subjected to 3, 5, and 6 days of F2 effluent are projected on the bottom left of the factorial map (in dotted circle), i.e., with high accumulation of metals (Cd, Cu, Zn, and Ni) and high mRNA expression of HSP70 and ABCB1 genes (Fig. 7d).
Discussion
Metal concentrations in effluents
The effluents (F1 and F2) used in this study were discharged by a phosphate treatment plant localized near Skhira in the gulf of Gabès. F1 was a global effluent produced by the three different units of production (sulfuric acid, phosphoric acid, and fertilizers) of the SIAPE factory, while F2 was a product of a single unit (phosphoric acid). Therefore, it was not surprising to find higher metal concentration in effluent F1 compared to F2. F1 and F2 effluents appear in the high concentration range of industrial effluents (Hajem et al. 2007; Petala et al. 2009; Sancey et al. 2011; Santos Yabe and de Oliveira 2003), especially concerning Cd which concentration exceeds Tunisian and French norms (T.N. 106.002 and French Ministerial Decree of 22 February 1998, respectively). At 1 % of dilution (sub-lethal concentration used for the experimentation), the Cd concentration of F1 still exceeds the Tunisian norm.
Metal accumulation in cockles’ tissues
Cockles exposed continuously for 1 week to effluent F1 or F2 at a concentration of 1 % accumulated higher concentrations of all studied heavy metals (Cd, Cu, Zn, and Ni) than control cockles. The accumulation of metals has often been highlighted in different bivalve species (see Zuykov et al. 2013 for a review). Accumulation was higher in the cockles exposed to F1 than in the ones exposed to F2, in accordance with the metal concentration of the two effluents. However, accumulation did not increase proportionally with concentration, which is probably due to a saturation of the influx rate at very high concentrations (Luoma and Rainbow 2005). Cd was much less accumulated than the other metals, relative to their respective concentrations in the effluents, suggesting a lower uptake rate of this metal (Luoma and Rainbow 2005). For Ni and Zn, accumulation in cockles has been relatively constant during the length of the experiment (6 days). For Cu and Cd, accumulation increased with time. This increase was slight, except for Cd in cockles exposed to F1 where it was exponential. According to Luoma and Rainbow (2005), low variation with exposure time may be due to a rapid rate of metal excretion, while a rapid increase in body metal concentration would be due to the slow rate of excretion relative to the ingestion rate. Our results therefore suggest that excretion is more effective for Ni and Zn than for Cu and especially Cd in C. glaucum.
The global physiological state of cockles
Our data clearly demonstrate that exposure to effluent F1 significantly affects LT50 data, reflecting metabolic perturbations and a general lower health status of cockles following effluent exposure. This result may suggest that protection and/or detoxification mechanisms as possible responses to toxicants could imply energetic costs and therefore could reduce the capacity to resist to an additional change, i.e., air exposure in our study (Viarengo et al. 1995). Such trade-offs have already been described in the literature (Forbes 2000; Handy et al. 1999; Van Straalen and Hoffman 2000). For effluent F2, the same trend is detected though not significant. This difference may be explained by the highest concentrations in metals found in effluent F1 compared to effluent F2.
Measures of bivalve’s condition based on weight are thought to be reliable indicators of the energetic health status and energy reserves in mollusks. The results obtained in our study do not show any difference in the condition index between controls and exposed cockles. This finding probably highlights that this parameter is too much integrative and that only 6 days of exposition might not be enough to produce perceptible variations between exposed cockles and control ones. This result is corroborated by the results of the two PCA analyses, which indicate that CI is a weak structuring parameter (small arrows).
Effects of effluent exposure on mRNA expression
In cockles exposed either to F1 or F2, mRNA expression is significantly higher than in controls for all the studied genes (Table 2; Figs. 4 and 5). Moreover, the much higher variability observed in exposed individuals (large box plots) suggests the existence of different types of responses among cockles experiencing the same exposition. Elements confirming this hypothesis may be found with the projections of individuals into the PCAs (Figs. 6 and 7). Indeed, for both effluents, control individuals are found quite grouped while exposed individuals are more scattered (Figs. 6 and 7).
The ATP-binding cassette xenobiotic transporter ABCB1 gene transcription seems to follow a wave pattern in the two effluent exposures. ABCB1 shows a rapid increase in expression (days 0.5 and 1) followed by a decrease and an additional peak at day 3 (for F1) or 5 (for F2). This marker is significantly correlated to Cd, Zn, Ni, and Cu accumulation in tissues following exposure to F2 effluent. The ABCB1 transmembrane protein acts by actively exporting toxic compounds out of the cell (Pain and Parant 2003; Minier et al. 2006), and our results show that it is an actor of the response to metal exposure, its mRNA expression being modulated by the level of metals. Previous studies already highlighted a strong ABCB1 mRNA increase in marine organisms following exposure to Cd and other metal exposure (Della Torre et al. 2014; Zucchi et al. 2010).
Considering both effluent exposures, antioxidative defenses are triggered through the mRNA expression of gene coding for CAT, CuZnSOD, and MnSOD. SODs (CuZnSOD and MnSOD being respectively the cytosolic and the mitochondrial form) are known to catalyze the dismutation of superoxide anions into hydrogen peroxides, which are in turn reduced by CAT into water and molecular oxygen. The increase of these antioxidant enzymes in response to effluent exposure seems to constitute an adaptation in gills to prevent and/or repair metal-induced damage in cellular component (Fernandez et al. 2010). Several studies have shown that metals can promote oxidative stress in cells (Sabatini et al. 2011; Trevisan et al. 2014; Wang et al. 2010), and in particular, Kamel et al. (2012) found a correlation between CAT, MnSOD, and metal accumulations in clams treated with municipal effluents.
MT was also induced in response to the uptake of metals in both exposures, with an inverted U shape and a significant higher expression of +35-fold at day 2 for the F2 exposure while quite linear for the F1 exposure, reaching a significant expression of +20 at day 6. The up-regulation of this gene is in accordance with previous experiments performed with different invertebrates following metal exposure (Al Kaddissi et al. 2012; Dondero et al. 2005; Lecoeur et al. 2004; Navarro et al. 2011; Zorita et al. 2007). It may reflect the role of MTs in the uptake and handling of metals, as well as their role as antioxidants reacting with free radicals and reactive oxygen species (ROS), reducing significantly the toxicity of ROS and preventing interactions with critical cellular components such as enzymes, structural proteins, DNA, and membrane lipids (Amiard et al. 2006; Andrews 2000; Coyle et al. 2002; Ivanina et al. 2009; Kamel et al. 2012; Tanguy et al. 2001).
The biomarkers coding for CO1 and HSP70 showed a remarkably higher mRNA expression in cockles exposed to F2, with in addition a high correlation to metal concentrations in cockles exposed to both effluents. For F1 exposed cockles, this up-regulation is more observed in the second part of the experiment (3 to 6 days). The CO1 (cytochrome oxidase 1) transfers electrons from reduced cytochrome c to molecular oxygen in the mitochondrial respiratory chain and contributes to the production of an electrochemical proton gradient across the mitochondrial inner membrane that drives the synthesis of ATP (Capaldi 1990; Capaldi et al. 1983). Its increase in mRNA expression following effluent exposure indicates a higher metabolic activity in exposed cockles. This could be a cellular strategy (1) to compensate for the decrease in the number of functional mitochondria (through inhibition phenomenon or membrane potential decrease) by increasing ATP production, so as to provide enough energy for cellular needs, especially to tolerate metals (Al Kaddissi et al. 2012), and/or (2) to efficiently consume O2 to limit metal-induced oxidative damage in cells (Chen 2003; Wang et al. 2004). It has to be noted that the gene transcription level of CO1 correlated well with those of antioxidant enzymes (CuZnSOD, MnSOD, and CAT) in cockles exposed to F2 effluent. Previous studies have highlighted an up-regulation of the CO1 gene in invertebrates following Cd and other metals exposure (Achard-Joris et al. 2006; Al Kaddissi et al. 2012; Navarro et al. 2011). The response of HSP70 is quite similar to that of CO1 after exposure to F1 and F2. HSP70 are involved in folding/refolding of newly synthesized and damaged proteins as well as in sequestering and degradation of proteins that are damaged beyond repair (Mayer and Bukau 2005). Studies have already reported an increase in HSP70 expression in bivalves such as Dreissena polymorpha and Crassostrea virginica (Gmelin) upon the presence of metals like Cu, Cd, Pd, Pt, and Rt (Ivanina et al. 2008, 2009; Navarro et al. 2011). Even if HSP70 are known to be synthesized intensively in response to a variety of harmful stimuli (heat, metals, organic contaminants, injuries, disease, …), the strong up-regulation of its gene (reaching +15- and +75-fold, the mean expression level of controls for F1 and F2, respectively) reflects a mechanism highly settled to protect cells against stress-induced damage.
Finally, it has to be noted that exposure concentration (F1 versus F2) seems to have an impact on the global gene transcription profile over time. Indeed, mRNA up-regulations for the different genes studied (excepted ABCB1) are faster and higher in F2 compared to F1, though F1 effluent contains higher concentrations of the different metals than F2 and leads to higher metal accumulation in tissues. Similarly, recent studies on gene expression in daphnia, zebrafish, and fathead minnows following exposure to several metals over a range of concentrations highlighted that the transcription of several genes occurred at the lower metal concentrations (even though the accumulation was greater at higher concentrations). A high stress level (which is the case for F1) may cause several transcriptional inhibitions and/or mRNA degradations that may impede or delay defense mechanisms (Klaper et al. 2008; Lerebours et al. 2010; Poynton et al. 2008). Moreover, at the end of the F1 experiment, RNA copy number seems to be in an increasing phase for most of the seven genes studied.
Conclusion
Gene transcription monitoring performed on seven genes potentially involved in the tolerance to metal exposure showed that for both exposures (though different in concentrations), mechanisms are rapidly and synchronically settled down to prevent damage to cellular components, by (1) handling and exporting out metal ions through the up-regulation of ABCB1 and MT, (2) reducing the toxicity of ROS through the expression of antioxidant enzymes (CAT, CuZnSOD, and MnSOD), (3) protecting and/or repairing proteins through the expression of HSP70 mRNAs, and (4) increasing ATP production (through the up-regulation of CO1) to provide energy for cells to tolerate stress exposure. These molecular responses imply energetic costs and therefore might explain the reduced tolerance of cockles to anoxia observed with the stress on stress test. The tools developed may be useful both for future control strategies and for the use of the cockle C. glaucum as a sentinel species. However, future investigations should be performed to validate these biomarkers in field conditions. Moreover, parallel biochemical, physiological, immunological, and/or morphological/pathological data should complement this study to better assess metal toxicities and their mechanism of action.
References
Achard-Joris M, Gonzalez P, Marie V, Baudrimont M, Bourdineaud JP (2006) Cytochrome c oxidase subunit I gene is up-regulated by cadmium in freshwater and marine bivalves. Biometals 19:237–244. doi:10.1007/s10534-005-5671-9
Al Kaddissi S, Legeay A, Elia AC, Gonzalez P, Floriani M, Cavalie I, Massabuau JC, Gilbin R, Simon O (2012) Mitochondrial gene expression, antioxidant responses, and histopathology after cadmium exposure. Environ Toxicol. doi:10.1002/tox.21817
Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76:160–202. doi:10.1016/j.aquatox.2005.08.015
Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59:95–104
Barhoumi S, Messaoudi I, Deli T, Said K, Kerkeni A (2009) Cadmium bioaccumulation in three benthic fish species, Salaria basilisca, Zosterisessor ophiocephalus and Solea vulgaris collected from the Gulf of Gabes in Tunisia. J Environ Sci (China) 21:980–984
Capaldi RA (1990) Structure and function of cytochrome c oxidase. Ann Rev Biochem 59:569–596. doi:10.1146/annurev.bi.59.070190.003033
Capaldi RA, Malatesta F, Darley-Usmar VM (1983) Structure of cytochrome c oxidase. Biochim Biophys Acta 726:135–148
Chen Q (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36027–36031. doi:10.1074/jbc.M304854200
Ciacci C, Barmo C, Gallo G, Maisano M, Cappello T, D'Agata A, Leonzio C, Mauceri A, Fasulo S, Canesi L (2012) Effects of sublethal, environmentally relevant concentrations of hexavalent chromium in the gills of Mytilus galloprovincialis. Aquat Toxicol 120–121:109–118. doi:10.1016/j.aquatox.2012.04.015
Cong M, Wu H, Liu X, Zhao J, Wang X, Lv J, Hou L (2012) Effects of heavy metals on the expression of a zinc-inducible metallothionein-III gene and antioxidant enzyme activities in Crassostrea gigas. Ecotoxicology 21:1928–1936. doi:10.1007/s10646-012-0926-z
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59:627–647
Dedourge-Geffard O, Palais F, Biagianti-Risbourg S, Geffard O, Geffard A (2009) Effects of metals on feeding rate and digestive enzymes in Gammarus fossarum: an in situ experiment. Chemosphere 77:1569–1576. doi:10.1016/j.chemosphere.2009.09.042
Della Torre C, Bocci E, Focardi SE, Corsi I (2014) Differential ABCB and ABCC gene expression and efflux activities in gills and hemocytes of Mytilus galloprovincialis and their involvement in cadmium response. Mar Environ Res 93:56–63. doi:10.1016/j.marenvres.2013.06.005
Derbali A, El Hasni K, Jarboui O, Ghorbel M (2012) Distribution, abundance and biological parameters of Cerastoderma glaucum (Mollusca: Bivalvia) along the Gabes coasts (Tunisia, Central Mediterranean). Acta Adriat 53(3):363–374
Dondero F, Piacentini L, Banni M, Rebelo M, Burlando B, Viarengo A (2005) Quantitative PCR analysis of two molluscan metallothionein genes unveils differential expression and regulation. Gene 345:259–270. doi:10.1016/j.gene.2004.11.031
Douxfils J, Mandiki R, Silvestre F, Bertrand A, Leroy D, Thome JP, Kestemont P (2007) Do sewage treatment plant discharges substantially impair fish reproduction in polluted rivers? Sci Total Environ 372:497–514. doi:10.1016/j.scitotenv.2006.08.020
Fernandez B, Campillo JA, Martinez-Gomez C, Benedicto J (2010) Antioxidant responses in gills of mussel (Mytilus galloprovincialis) as biomarkers of environmental stress along the Spanish Mediterranean coast. Aquat Toxicol 99:186–197. doi:10.1016/j.aquatox.2010.04.013
Forbes VE (2000) Is hormesis an evolutionary expectation? Funct Ecol 14:12–24
Gagne F, Andre C, Cejka P, Hausler R, Fournier M, Blaise C (2008) Immunotoxic effects on freshwater mussels of a primary-treated wastewater before and after ozonation: a pilot plant study. Ecotoxicol Environ Saf 69:366–373. doi:10.1016/j.ecoenv.2007.10.027
Gargouri D, Azri C, Serbaji MM, Jedoui Y, Montacer M (2011) Heavy metal concentrations in the surface marine sediments of Sfax Coast, Tunisia. Environ Monit Assess 175:519–530. doi:10.1007/s10661-010-1548-7
Geffard A, Sartelet H, Garric J, Biagianti-Risbourg S, Delahaut L, Geffard O (2010) Subcellular compartmentalization of cadmium, nickel, and lead in Gammarus fossarum: comparison of methods. Chemosphere 78:822–829. doi:10.1016/j.chemosphere.2009.11.051
Hajem B, Hamzaoui H, M'Nif A (2007) Chemical interaction between industrial acid effluents and the hydrous medium. Desalination 206:154–162. doi:10.1016/j.desal.2006.03.565
Handy RD, Sims DW, Giles A, Campbell HA, Musonda MM (1999) Metabolic trade-off between locomotion and detoxification for maintenance of blood chemistry and growth parameters by rainbow trout (Oncorhynchus mykiss) during chronic dietary exposure to copper. Aquat Toxicol 47:23–41
Haynes D, Leeder J, Rayment P (1997) A comparison of the bivalve species Donax deltoides and Mytilus edulis as monitors of metal exposure from effluent discharges along the Ninety Mile beach, Victoria, Australia. Mar Pollut Bull 34:326–331
Ivanina AV, Sokolova IM, Sukhotin AA (2008) Oxidative stress and expression of chaperones in aging mollusks. Comp Biochem Physiol B Biochem Mol Biol 150:53–61. doi:10.1016/j.cbpb.2008.01.005
Ivanina AV, Taylor C, Sokolova IM (2009) Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin). Aquat Toxicol 91:245–254. doi:10.1016/j.aquatox.2008.11.016
Jadeja RN, Tewari A (2007) Effect of soda ash industry effluent on bioaccumulation of metals by seaweeds of coastal region of Gujarat, India. J Hazard Mater 147:148–154. doi:10.1016/j.jhazmat.2006.12.060
Javied S, Mehmood T, Chaudhry MM, Tufail M, Irfan N (2009) Heavy metal pollution from phosphate rock used for the production of fertilizer in Pakistan. Microchem J 91:94–99. doi:10.1016/j.microc.2008.08.009
Kamel N, Jebali J, Banni M, Ben Khedher S, Chouba L, Boussetta H (2012) Biochemical responses and metals levels in Ruditapes decussatus after exposure to treated municipal effluents. Ecotoxicol Environ Saf 82:40–46. doi:10.1016/j.ecoenv.2012.05.008
Kessabi K, Navarro A, Casado M, Said K, Messaoudi I, Pina B (2010) Evaluation of environmental impact on natural populations of the Mediterranean killifish Aphanius fasciatus by quantitative RNA biomarkers. Mar Environ Res 70:327–333. doi:10.1016/j.marenvres.2010.06.005
Ketata I, Smaoui-Damak W, Guermazi F, Rebai T, Hamza-Chaffai A (2007) In situ endocrine disrupting effects of cadmium on the reproduction of Ruditapes decussatus. Comp Biochem Physiol C Toxicol Pharmacol 146:415–430. doi:10.1016/j.cbpc.2007.05.002
Klaper R, Carter BJ, Richter CA, Drevnick PE, Sandheinrich MB, Tillitt DE (2008) Use of a 15 k gene microarray to determine gene expression changes in response to acute and chronic methylmercury exposure in the fathead minnow Pimephales promelas Rafinesque. J Fish Biol 72:2207–2280. doi:10.1111/j.1095-8649.2008.01899.x
Ladhar-Chaabouni R, Machreki-Ajmi M, Hamza-Chaffai A (2009a) Spatial distribution of cadmium and some biomarkers in Cerastoderma glaucumliving in a polluted area. Mar Biol Res 5:478–486. doi:10.1080/17451000802683985
Ladhar-Chaabouni R, Mokdad-Gargouri R, Denis F, Hamza-Chaffai A (2009b) Cloning and characterization of cDNA probes for the analysis of metallothionein gene expression in the Mediterranean bivalves: Ruditapes decussatus and Cerastoderma glaucum. Mol Biol Rep 36:1007–1014. doi:10.1007/s11033-008-9274-8
Ladhar-Chaabouni R, Smaoui-Damak W, Hamza-Chaffai A (2009c) In vivo variation of some biomarkers with time and cadmium concentration in the cockle Cerastoderma glaucum. Mar Biol Res 5:487–495. doi:10.1080/17451000802534881
Ladhar-Chaabouni R, Machreki-Ajmi M, Hamza-Chaffai A (2012) Use of metallothioneins as biomarkers for environmental quality assessment in the Gulf of Gabès (Tunisia). Environ Monit Assess 184:2177–2192. doi:10.1007/s10661-011-2108-5
Lecoeur S, Videmann B, Berny P (2004) Evaluation of metallothionein as a biomarker of single and combined Cd/Cu exposure in Dreissena polymorpha. Environ Res 94:184–191
Lerebours A, Bourdineaud J-P, Kvd V, Vandenbrouck T, Gonzalez P, Camilleri V, Floriani M, Garnier-Laplace J, Adam-Guillermin C (2010) Sublethal effects of waterborne uranium exposures on the zebrafish brain: transcriptional responses and alterations of the olfactory bulb ultrastructure. Environ Sci Technol 44:1438–1443. doi:10.1021/es902550x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Lobel PB, Bajdik CD, Belkhode SP, Jackson SE, Longerich HP (1991) Improved protocol for collecting mussel watch specimens taking into account sex, size, condition, shell shape, and chronological age. Arch Environ Contam Toxicol 21:409–414. doi:10.1007/bf01060364
Luoma SN, Rainbow PS (2005) Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ Sci Technol 39:1921–1931
Machreki-Ajmi M, Hamza-Chaffai A (2006) Accumulation of cadmium and lead in Cerastoderma glaucum originating from the Gulf of Gabes, Tunisia. Bull Environ Contam Toxicol 76:529–537. doi:10.1007/s00128-006-0952-8
Machreki-Ajmi M, Hamza-Chaffai A (2008) Assessment of sediment/water contamination by in vivo transplantation of the cockles Cerastoderma glaucum from a non contaminated to a contaminated area by cadmium. Ecotoxicology 17:802–810. doi:10.1007/s10646-008-0238-5
Mar SS, Okazaki M (2012) Investigation of Cd contents in several phosphate rocks used for the production of fertilizer. Microchem J 104:17–21. doi:10.1016/j.microc.2012.03.020
Marigomez I, Soto M, Cajaraville MP, Angulo E, Giamberini L (2002) Cellular and subcellular distribution of metals in molluscs. Microsc Res Tech 56:358–392. doi:10.1002/jemt.10040
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684. doi:10.1007/s00018-004-4464-6
Minier C, Abarnou A, Jaouen-Madoulet A, Le Guellec AM, Tutundjian R, Bocquene G, Leboulenger F (2006) A pollution-monitoring pilot study involving contaminant and biomarker measurements in the Seine Estuary, France, using zebra mussels (Dreissena polymorpha). Environ Toxicol Chem 25:112–119
Navarro A, Quiros L, Casado M, Faria M, Carrasco L, Benejam L, Benito J, Diez S, Raldua D, Barata C, Bayona JM, Pina B (2009) Physiological responses to mercury in feral carp populations inhabiting the low Ebro River (NE Spain), a historically contaminated site. Aquat Toxicol 93:150–157. doi:10.1016/j.aquatox.2009.04.009
Navarro A, Faria M, Barata C, Pina B (2011) Transcriptional response of stress genes to metal exposure in zebra mussel larvae and adults. Environ Pollut 159:100–107. doi:10.1016/j.envpol.2010.09.018
Pain S, Parant (2003) Response of multixenobiotic defence mechanism in Dreissena polymorpha exposed to environmental stress. Chemosphere 52(7):1105–1113
Paul-Pont I, Gonzalez P, Baudrimont M, Nili H, de Montaudouin X (2010) Short-term metallothionein inductions in the edible cockle Cerastoderma edule after cadmium or mercury exposure: discrepancy between mRNA and protein responses. Aquat Toxicol 97:260–267. doi:10.1016/j.aquatox.2009.12.007
Petala M, Kokokiris L, Samaras P, Papadopoulos A, Zouboulis A (2009) Toxicological and ecotoxic impact of secondary and tertiary treated sewage effluents. Water Res 43:5063–5074. doi:10.1016/j.watres.2009.08.043
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Poynton HC, Loguinov AV, Varshavsky JR, Chan S, Perkins EJ, Vulpe CD (2008) Gene expression profiling in Daphnia magna part I: concentration-dependent profiles provide support for the no observed transcriptional effect level. Environ Sci Technol 42:6250–6256
Sabatini SE, Rocchetta I, Nahabedian D, Luquet C, Maria R, Eppis M, Bianchi L, Rios de Molina MC (2011) Oxidative stress and histological alterations produced by dietary copper in the fresh water bivalves Diplodon chilensis. Comp Biochem Physiol C 154:391–398
Salo HM, Hebert N, Dautremepuits C, Cejka P, Cyr DG, Fournier M (2007) Effects of Montreal municipal sewage effluents on immune responses of juvenile female rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 84:406–414. doi:10.1016/j.aquatox.2007.06.014
Sancey B, Trunfio G, Charles J, Minary J-F, Gavoille S, Badot P-M, Crini G (2011) Heavy metal removal from industrial effluents by sorption on cross-linked starch: chemical study and impact on water toxicity. J Environ Manag 92:765–772. doi:10.1016/j.jenvman.2010.10.033
Santos Yabe MJ, de Oliveira E (2003) Heavy metals removal in industrial effluents by sequential adsorbent treatment. Adv Environ Res 7:263–272
Schulte PM (2004) Changes in gene expression as biochemical adaptations to environmental change: a tribute to Peter Hochachka. Comp Biochem Physiol B: Biochem Mol Biol 139:519–529. doi:10.1016/j.cbpc.2004.06.001
Sim CH, Quek BS, Shutes RB, Goh KH (2013) Management and treatment of landfill leachate by a system of constructed wetlands and ponds in Singapore. Water Sci Technol 68:1114–1122. doi:10.2166/wst.2013.352
Szefer P, Wolowicz M, Kusak A, Deslous-Paoli J, Czarnowski W, Frelek K, Belzunce M (1999) Distribution of mercury and other trace metals in the cockle Cerastoderma glaucum from the Mediterranean Lagoon Etang de Thau. Arch Environ Contam Toxicol 36:56–63
Tanguy A, Mura C, Moraga D (2001) Cloning of a metallothionein gene and characterization of two other cDNA sequences in the Pacific oyster Crassostrea gigas (CgMT1). Aquat Toxicol 55:35–47
Tayibi H, Choura M, López FA, Alguacil FJ, López-Delgado A (2009) Environmental impact and management of phosphogypsum. J Environ Manag 90:2377–2386. doi:10.1016/j.jenvman.2009.03.007
Trevisan R, Flesch S, Mattos JJ, Milani MR, Bainy ACD, Dafre AL (2014) Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna. Comp Biochem Physiol C Toxicol Pharmacol 159:22–30. doi:10.1016/j.cbpc.2013.09.007
Van Straalen NM, Hoffman AA (2000) Review of experimental evidence for physiological costs of tolerance to toxicants. In: Kammenga J, Laskowski R (eds) Demography in ecotoxicology. John Wiley & Sons, New York, pp 147–161
Viarengo A, Canesi L, Pertica M, Mancinelli G, Accomando A, Smaalb AC, Orunesu M (1995) Stress on stress response: a simple monitoring tool in the assessment of a general stress syndrome in mussels. Mar Environ Res 39:245–248
Wang Y, Fang J, Leonard SS, Rao KM (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 36:1434–1443. doi:10.1016/j.freeradbiomed.2004.03.010
Wang Q, Wang X, Yang H, Liu B (2010) Analysis of metallotionein expression and antioxidant enzyme activities in Meretrix meretrix larvae under sublethal cadmium exposure. Aquat Toxicol 100:321–328. doi:10.1016/j.aquatox.2010.08.012
Zorita I, Bilbao E, Schad A, Cancio I, Soto M, Cajaraville MP (2007) Tissue- and cell-specific expression of metallothionein genes in cadmium- and copper-exposed mussels analyzed by in situ hybridization and RT-PCR. Toxicol Appl Pharmacol 220:186–196. doi:10.1016/j.taap.2007.01.003
Zucchi S, Corsi I, Luckenbach T, Bard SM, Regoli F, Focardi S (2010) Identification of five partial ABC genes in the liver of the Antarctic fish Trematomus bernacchii and sensitivity of ABCB1 and ABCC2 to Cd exposure. Environ Pollut 158:2746–2756. doi:10.1016/j.envpol.2010.04.012
Zuykov M, Pelletier E, Harper DA (2013) Bivalve mollusks in metal pollution studies: from bioaccumulation to biomonitoring. Chemosphere 93:201–208. doi:10.1016/j.chemosphere.2013.05.001
Acknowledgments
This research was financially supported by the European Program FP7 PEOPLE-IRSES GENERA (use of genomic and proteomic tools for the development of contaminant specific biomarkers for the environmental risk assessment of aquatic ecosystems). A. Goupille is acknowledged for the ICP-OES measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Karray, S., Tastard, E., Moreau, B. et al. Transcriptional response of stress-regulated genes to industrial effluent exposure in the cockle Cerastoderma glaucum . Environ Sci Pollut Res 22, 17303–17316 (2015). https://doi.org/10.1007/s11356-015-4108-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4108-4