Abstract
In this study, we measured various parameters of oxidative stress, immune response, and abnormalities in the erythrocyte nucleus of Labeo rohita inhabiting the polluted Kshipra River, India. The river water contains heavy metals in this order: Ni > Fe > Cd > Cr > Mn > Zn > Cu. Fe showed the highest accumulation in gills, liver, and gut, whereas Ni (gills and gut) and Cd (liver) were lowest accumulated. The superoxide dismutase (SOD) and catalase (CAT) were found to be increased significantly (p < 0.05) in the gills (SOD: 211%; CAT: 150%), liver (SOD: 447%; CAT: 304%), and gut (SOD: 98.11%; CAT: 58.69%) in comparison with the reference fish. However, glutathione S transferase (GST) showed significantly (p < 0.05) higher activity in the gills (25.5%) but lower activity in the liver (− 49.22%) and the gut (− 30.57%). Moreover, reduced glutathione (GSH) decreased significantly (p < 0.05) in the gills (− 46.66%), liver (− 33.20%), and gut (− 39.87%). Despite the active response of the antioxidant enzymes, the highest lipid peroxidation was observed in the liver (463%). The effect of heavy metals was also observed on the immunity of the fish, causing immunosuppression as evident by significantly (p < 0.05) lower values of acid phosphatase (− 50%), myeloperoxidase (− 48.33%), and nitric oxide synthase (− 50%) in serum. Histopathological findings showed gill lamellae shortening, hyperplasia, club-shaped lamellar tip in exposed gills and necrosis, vacuolization, and pyknosis in the exposed liver. Furthermore, polluted river water was also found to induce micronuclei (2.1%) and lobed nuclei (0.72%) in erythrocytes (0.65%). These results indicate the potential of heavy metal-induced oxidative stress and other forms of stress in inhabiting fish, highlighting the need to control the pollution of this river water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The issue of water pollution is one of great concern not only in developing countries but also in developed nations. It may be because freshwater is limited and is continuously contaminated by different anthropogenic sources. Heavy metals are one of the most common aquatic pollutants. They stay in the environment for a long time due to their persistent and nonbiodegradable nature. Recently, various studies reported changes in morphology, biochemistry, physiology, and behavior of fish and other aquatic fauna due to heavy metals pollution (Khan et al. 2020; Shah et al. 2020; Dhondiram 2021; Kong et al. 2021). Since fishes are at the top of the aquatic food chain, they are commonly used to monitor the effects of heavy metals in the underwater media. Several studies also reported the bioaccumulation of different heavy metals such as Ni, Cr, Cu, Fe, Pb, Co, Zn, Mn, Cd, and Hg in various tissues of fishes from different parts of the world (Rajeshkumar and Li 2018; Tamele and Loureiro 2020; Shah et al. 2020; Garai et al. 2021; Al-Taee et al. 2020; Harsij et al. 2018).
Besides bioaccumulation, other impacts have also been recorded in fishes. For example, Shah et al. (2020) reported genotoxicity and histopathology in the muscle and gills of Ctenopharyngodon idella as a result of Cu, Pb, and Cr exposure. Recently, Said et al. (2021) reported the adverse effects of heavy metal pollution on different enzymes and serum biochemical parameters in Oreochromis niloticus inhabiting the Nile River.
The present study was designed to investigate the pollution status of river Kshipra at Dewas (22.89 °N and 75.98 °E), Madhya Pradesh, India. It receives the untreated domestic waste, laundry waste, printing press waste, and also effluents from various industries. In an earlier study, Ganasan and Hughes (1998) reported its contamination by printing press, textile, steel, metal and other industries.
It is well known that heavy metals are redox-active metals and are known to generate free radicals and cause oxidative stress (Javed and Usmani 2019; Tabrez and Ahmad 2011). Therefore, in this study, the effect of these metals was studied on redox status, such as SOD, CAT, GST, GSH, and lipid peroxidation (LPO) in the gills, liver, and gut of Labeo rohita. L. rohita is an omnivore fish as it eats plants and phytoplankton. It is found in both flowing as well as in stagnant water bodies. It is a cyprinid, and so it cannot withstand harsh conditions. However, to resist and alleviate the toxicity caused by these heavy metals, fish also possess an extensive and well-developed immune system like mammals. The immunotoxicity was assessed by measuring the activities of myeloperoxidase (MPO), nitric oxide synthase (NOS), and acid phosphatase (ACP). The damage caused at the cell and tissue level was assessed by histopathological study. Heavy metals are also known to result in genotoxicity; hence, erythrocytic abnormalities such as micronuclei frequency and kidney-shaped and lobed nuclei were also investigated.
Material and Methods
Collection of Samples
The fish L. rohita (n = 10) were collected in September 2019 from three different nearby sites of the Kshipra River at Dewas, Madhya Pradesh, India. The average length was 15.6 ± 0.5 cm, and their weight was 155.62 ± 0.39 g. Conversely, the reference fish was collected (n = 10) from the fresh culture pond at Dewas, Madhya Pradesh. Their average length was 18 ± 0.4 cm, and their weight was 195 ± 0.86 g. They were caught using cast nets with the help of a professional fisherman and transported to the laboratory in large tanks where they were euthanized, and the tissue samples were preserved at − 20 °C for further analysis. Figure 1 shows the location of the Kshipra River. Samples of fish and water were collected from downstream of the river a few kilometers ahead of the domestic, laundry, and printing press outlets by following the standard American Public Health Association (APHA 2005) methods. Moreover, rain dilutes the pollution, so the samples were collected during the rainy season to know the water quality for drinking and its suitability for inhabitants. For heavy metal analysis, the water samples, exposed and reference fish tissues (gills, liver, gut) were digested in HClO4 and HNO3 (4:1), diluted to 50 ml with double distilled water as described earlier (Javed et al. 2016a, b). Then, samples were analyzed in atomic absorption spectrophotometer (Thermofisher, USA).
Redox Status/Oxidative Stress Assays
SOD activity was measured by the inhibition of pyrogallol autoxidation (Marklund and Marklund 1974). In brief, 100 μl of the sample was mixed with a 2.8 ml tris succinate buffer with 0.05 M and pH 0.05 and incubated at 25 °C for 20 min, and then, 100 μl (8 mM solution) was added to make the final 3 ml volume. Absorbance was taken at 412 nm for 3 min. Conversely, CAT activity was measured by H2O2 decomposition (Aebi 1984). GST estimation was done by following Habig et al. (1974). Briefly, a 100 μl sample was mixed with 2.7 ml GSH (pH 6.5, 0.1 M phosphate buffer) and 1-chloro 2,4 dinitrobenzene. It was then read at 340 nm for 3 min. GSH was done by the procedures of Jollow et al. (1974). The 1 ml of sample homogenate was added to the 1 ml of sulphosalicylic acid and incubated at 4 °C for 1 h, followed by centrifugation at 12,000 rpm for 15 min. From this, 0.4 ml supernatant was taken in a tube and was added 2.2 ml potassium phosphate buffer (pH 7.4, 0.1 M). To this, 0.4 ml 5,5′-dithiobis-2-nitrobenzoic acid was mixed and read at 412 nm. For LPO, thiobarbituric acid reactive substances were measured and quantitated as malondialdehyde equivalents in the homogenate of the sample as per the protocol of Buege and Aust (1978).
Immune Response
ACP and MPO were analyzed with the help of commercially available kits (LifeSpan BioSciences, USA, and Sigma-Aldrich, USA, respectively), strictly adhering to the manufacturer’s instructions. Similarly, NOS was measured as per the method described in our earlier study (Khan et al. 2020). Briefly, the tissue homogenate was prepared from 100 mg and 1 ml phosphate buffer (pH 7.4), centrifuged at 10,000g and 4 °C for approximately 20 min. To the 100 μl supernatant, 100 μl Griess reagent was added, followed by incubation (10 min) at room temperature then optical density was measured at 540 nm. All the analysis was performed using a UV–Vis spectrophotometer. For calibration, blanks were run along with samples.
Histopathology
Gill and liver of both the reference (n = 10) and the exposed fish (n = 10) were dissected out and fixed in Bouin’s solution, and the blocks were prepared as per the protocol of Javed et al. (2016a, b). The sections of the tissues were prepared in duplicates and, after the upgrade and downgrade dehydration, stained with hematoxylin and eosin and observed under the microscope (Leica DM 2500).
Erythrocytic Abnormalities
Blood smears of the reference and the exposed L. rohita fish were prepared to analyze nuclear abnormalities in the erythrocytes as per the procedure described by Ahmad and Ahmad (2016). A total of 1000 cells were scored to calculate the recurrence and frequency of micronuclei, lobed, and kidney-shaped nuclei.
Statistical Analysis
The water samples were collected from three different nearby locations of the Kshipra River. The analyzed parameters were assayed in triplicates, and values were reported as mean ± standard error of the mean. Statistical analysis was conducted using Student’s t test, and the significance was established at p < 0.05.
Results and Discussion
The heavy metals concentration in the Kshipra River water was measured in the following order Ni > Fe > Cd > Cr > Mn > Zn > Cu. Among them, Cr, Mn, Fe, Ni, and Cd were above the permissible limits of Indian standards (BIS 2012) and WHO (2006) (taken from UNEPGEMS) (Table 1). The high concentrations of these metals may be attributed to the fact that receives the wastes from printing press, untreated domestic wastes, and other municipal discharges. In the exposed fish, Fe was the highest accumulated metal in all tissues (gills, liver, and gut); also, it was several folds higher than the reference. Moreover, Ni showed the lowest accumulation in the exposed gills and gut, whereas in liver, it was Cd. In addition, Ni and Cd were below the detection limits in the reference fish (Table 2).
Oxidative Stress Parameters
Figure 2 shows oxidative stress parameters in L. rohita fish. SOD activity was found to be increased in all the studied tissues of the exposed fish compared to the reference fish. The percent change in SOD activity over reference was 211%, 447%, and 98.11% in gills, liver, and gut, respectively. Likewise, the activity of CAT was also found to be increased in the exposed fish, and the percent change over reference was 150%, 304%, and 58.69% in gills, liver, and gut, respectively. Furthermore, the GST activity was found to be elevated in the gills (25.5%); however, it was found to be declined in the liver (− 49.22%) and gut (− 30.57%). However, a decrease in the quantity of GSH was observed in the exposed L. rohita compared to the reference fish. Moreover, GSH showed a percentage decline in gills (− 46.66%), liver (− 33.20%), and gut (− 39.87%). Conversely, the LPO levels were found to be significantly increased in all the tissues of the exposed L. rohita. The percent increase over reference was 214%, 463%, and 254.5% in gills, liver, and gut, respectively.
The production of reactive oxygen species (ROS) is a part of normal cellular metabolism and physiological processes; however, their excessive production causes oxidative damage. Our earlier studies reported that heavy metals induce ROS production (Khan et al. 2020; Tabrez et al. 2011, 2021). To counteract ROS production, antioxidant defense is present in a living system, which includes both the enzymatic and the nonenzymatic systems. SOD, CAT, and GST are among the enzymatic parts, whereas GSH is nonenzymatic. SOD dismutates O2− and converts it to H2O2; furthermore, the CAT converts H2O2 to H2O and O2. We observed elevated levels of SOD and CAT in the gills, liver, and gut in L. rohita (Fig. 2a, b). Several studies reported significantly higher activities of SOD and CAT in different tissues of fishes such as L. rohita, Channa punctatus, and Anguilla japonica inhabiting polluted river waters (Mahamood et al. 2021; Ahn et al. 2020; Javed et al. 2016a, b). GST brings about the conjugation of GSH with electrophilic substances primarily produced from xenobiotics. In the present study, the activity of GST was found to be increased in gills. At the same time, a decreased level was observed in the liver and gut (Fig. 2c). The high GST level in the gills could explain that gills remain in direct contact with the surrounding medium. Our previous study recorded the highest metal load in the gills, followed by the liver and gut of exposed L. rohita (Tabrez et al. 2022). Furthermore, the GSH levels showed a decline in all the tissues because of its possible utilization by GST as it is a thiol-rich nucleophilic compound (Fig. 2d). Other studies also reported a significant decrease in the GSH levels in different tissues of fishes like Cyprinus carpio, L. rohita, A. japonicas, and Channa argus (Jindal et al. 2019; Mahamood et al. 2021; Ahn et al. 2020; Kong et al. 2021). Additionally, LPO levels were found to be significantly elevated in all studied tissues compared with the reference fish (Fig. 2e), indicating the possible damage caused by heavy metal-induced ROS even in the presence of an active antioxidant system. The minimum LPO level observed in the gills compared to the liver and gut of the exposed fish could be due to elevated GST and SOD, and CAT in this organ. Similar findings have also been reported in other fishes such as zebrafish, Cyprinus carpio, L. rohita, A. japonicas, and Channa argus (Awoyemi et al. 2019; Jindal et al. 2019; Mahamood et al. 2021; Ahn et al. 2020; Kong et al. 2021).
Immune Response
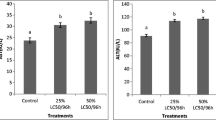
The effect of heavy metal-polluted water on the immune response of L. rohita fish is depicted in Fig. 3. The activities of enzymes MPO, NOS, and ACP in serum were found to be decreased in the exposed L. rohita compared to the reference fish. The percentage decline in MPO was − 48.33%, NOS was − 50%, and ACP was − 50% compared to control.
Innate immunity is the most crucial part and forms the first line of fish defense. It contains many important factors and enzymes such as MPO, ACP, ALP, and NOS. MPO has a significant role in advancing inflammatory processes and killing microbes (Kong et al. 2021). Similarly, ACP, ALP, and NOS also play an important role in regulating innate immunity and killing invading pathogens (Li et al. 2022, 2019; Kong et al. 2019). Moreover, NOS produces nitric oxide, a cell-signaling molecule that acts vigorously in fish's defense mechanism (Kumar et al. 2019; Khan et al. 2020). In the present study, the activities of these immune parameters (MPO, NOS, and ACP) in serum were found to be lower than the reference fish, indicating the suppressed immunity of the exposed fish. Earlier studies also reported that excessive production of ROS could reduce the immune function of immune cells (Fuente 2002; Li et al. 2011), which also supports our findings. Other researchers have also shown a decline in the activities of these immune factors (MPO, ACP, NOS, etc.), indicating the immunosuppression in Carassius auratus, Gobiocypris rarus, and Oreochromis niloticus (Kong et al. 2020; Zhang et al. 2020; Rahmana et al. 2019).
Histopathological Analysis
Histopathological data are shown in Fig. 4. The gills of the reference fish showed the normal structure of filament and lamellae, and lamellae are lined by epithelium and other cells. However, the gill of exposed fish lamellae gets shortened in length, hyperplasia on the filament, and swelling in the lamellae, and the tip of lamellae gets club-shaped. The liver of the reference fish showed normal histoarchitecture of the hepatocytes and the usual arrangement of the blood vessel. By contrast, the liver tissue of exposed fish showed necrosis, pyknotic nuclei, and vacuolization.
Histopathology of gill and liver of L. rohita inhabiting in reference water and polluted Kshipra River. a Reference gill; b Exposed gill; F (filament), L (lamellae), H (hyperplasia), S (swelling), SL (short lamellae), CSL (club-shaped lamellae); c Reference liver; d Liver of exposed fish; NH (normal hepatocyte), BV (blood vessel), N (necrosis), V (vacuolization), PN (pyknotic nuclei). Magnification = ×40; Scale bar = 20 μm
Histopathology is an important tool for assessing the damage caused by toxicants. In this analysis, the damage can be visualized by the investigator. The alterations found in the exposed gill were short lamellae, club-shaped lamellar tip, hyperplasia, and swelling speculated that gills are the first internal structure encountered in the surrounding medium. Moreover, they always remain immersed in polluted water. Furthermore, the liver is the main organ that detoxifies the toxicant. Therefore, it is imperative that it could also be affected by heavy metals. The damage observed in the exposed liver was necrosis, vacuolization, and pyknotic nuclei. Similar changes were reported in the gills and liver of Ictalurus punctatus living in the heavy metals polluted Chenab River (Shahid et al. 2021). Likewise, Onita et al. (2021) have also found lamellar disorganization, lamellar hyperplasia, epithelial damage in gills and vacuolation in the cytoplasm, nuclear damage, pyknosis, etc. in the liver of Barbus barbus, Squalius cephalus, and Chondrostoma nasus obtained from the Crisul Negru River, Romania.
Erythrocytic Abnormalities
The effect of heavy metals on the nuclear abnormalities in the exposed L. rohita is provided in Fig. 5. A significant increase in erythrocyte abnormality was recorded in the exposed fish. Micronuclei were found to have a higher frequency (2.1%) followed by lobed nuclei (0.72%) and kidney-shaped nuclei (0.65%).
Micronuclei are the small chromosome fragments that fail to be included in the daughter nuclei during cell division. We observed a higher frequency of micronuclei, lobed nuclei, and kidney-shaped nuclei in the exposed fish compared to the reference fish. This represents the genotoxic damage to the exposed fish under heavy metal stress. Shah et al. (2020) reported a high frequency of deformed nuclei, micronuclei, microcyte cells, nuclear shift, irregular nucleus, etc., in Ctenopharyngodon idella in response to Cu, Cr, and Pb exposure. Similarly, Khan et al. (2020) also observed a higher percentage of micronuclei, kidney-shaped nuclei, and lobed nuclei in O. niloticus inhabiting the heavy metal-polluted Yamuna River.
Conclusion
We observed the adverse effects on the antioxidant system, immunosuppression, and micronuclei induction in the L. rohita inhabiting the polluted Kshipra River, India. Furthermore, the immunotoxicity caused by heavy metal exposure demonstrates the weakened ability of the immune system to identify and kill pathogens, making the fish more vulnerable to diseases. Histopathology further confirms the damage to the target organs. Our study highlights the different forms of stress in inhabiting fish, pointing to the government regulating bodies’ need to control this river water pollution.
Availability of Data and Materials
Data will be available upon request to the corresponding author.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad I, Ahmad M (2016) Fresh water fish, Channa punctatus, as a model for pendimethalin genotoxicity testing: a new approach toward aquatic environmental contaminants. Environ Toxicol 31:1520–1529
Ahn TY, Park HJ, Kim JH, Kang JC (2020) Effects of antioxidant enzymes and bioaccumulation in eels (Anguilla japonica) by acute exposure of waterborne cadmium. Fish Aquat Sci 23:2–10. https://doi.org/10.1186/s41240-020-00166-7
Al-Taee SK, Al-Mallah KH, Ismail HK (2020) Review of some heavy metals toxicity on freshwater fishes. J Appl Vet Sci 5(3):78–86
APHA (2005) Standard methods for the examination of water and wastewater analysis, 21st 442. American Water Works Association/Water Environment Federation, Washington DC
Awoyemi OM, Kumar N, Schmitt C, Subbish S, Crago J (2019) Behavioral, molecular and physiological responses of embryo-larval zebra fish exposed to types I and II pyrethroids. Chemos 219:526–537
BIS (Bureau of Indian Standards) (2012) Indian standard drinking water-specification, 2 rev. Bureau of Indian Standards, New Delhi
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Dhondiram GS (2021) Antioxidant role of caffeine on arsenic induced alterations in the collagen of freshwater bivalve, Lamellidens corrianus (Lea). Int J Innov Res Multidiscip Field 22:34–38
Fuente DL (2002) Effects of antioxidants on immune system ageing. Eur J Clin Nutr 56:S5–S8
Ganasan V, Hughes RM (1998) Application of an index of biological integrity (IBI) to fish assemblages of the rivers Khan and Kshipra (Madhya Pradesh), India. Freshw Biol 40:367–383
Garai P, Banerjee P, Mondal P, Saha NC (2021) Effect of heavy metals on fishes: toxicity and bioaccumulation. J Clin Toxicol S 18:001
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Harsij M, Paknejad H, Khalili M, Jafarian H, Nazari S (2018) Histological study and evaluation of Hsp70 gene expression in gill and liver tissues of goldfish (Carassius auratus) exposed to Zinc oxide nanoparticles. Iran J Fish Sci 20(3):741–760
Javed M, Usmani N (2019) An overview of the adverse effects of heavy metal contamination on fish health. Proc Natl Acad Sci India Sect B Biol Sci 89:389–403
Javed M, Ahmad I, Usmani N, Ahmad M (2016a) Bioaccumulation, oxidative stress, and genotoxicity in fish (Channa punctatus) exposed to a thermal power plant effluent. Ecotoxicol Environ Saf 127:163–169
Javed M, Ahmad I, Usmani N, Ahmad M (2016b) Studies on biomarkers of oxidative stress and associated genotoxicity and histopathology in Channa punctatus from heavy metal polluted canal. Chemos 151:210–219
Jindal R, Sinha R, Brar P (2019) Evaluating the protective efficacy of Silybum marianum against deltamethrin induced hepatotoxicity in piscine model. Environ Toxicol Pharmacol 66:62–68
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Khan MS, Javed M, Rehman MT, Urooj M, Ahmad MI (2020) Heavy metal pollution and risk assessment by the battery of toxicity tests. Sci Rep 10:16593
Kong YD, Kang YH, Tian JX, Zhao LH, Zhang L, Wang GQ, Shan XF (2019) Oral immunization with recombinant Lactobacillus casei expressing flaB confers protection against Aeromonas veronii challenge in common carp, Cyprinus carpio. Fish Shellfish Immunol 87:627–637
Kong YD, Li M, Tian JX, Zhao LH, Kang YH, Zhang L, Wang GQ, Shan XF (2020) Effects of recombinant Lactobacillus casei on growth performance, immune response and disease resistance in crucian carp Carassius auratus. Fish Shellfish Immunol 99:73–85
Kong Y, Li M, Shan X, Wang G, Han G (2021) Effects of deltamethrin subacute exposure in snakehead fish, Channa argus: biochemicals, antioxidants and immune responses. Ecotoxicol Environ Saf 209:111821
Kumar N, Sharma JG, Singh SP, Singh A, Krishna VH, Chakrabarti R (2019) Validation of growth enhancing, immunostimulatory and disease resistance properties of Achyranthes aspera in Labeo rohita fry in pond conditions. Heliy 5:e01246
Li YY, Duan XD, Zhao J, Yu B, Mao X, Mao QB (2011) The effect of tea polyphenols on the growth performance and immune function of oxidatively stressed piglets. Chin J Anim Husb 47:53–57
Li M, Li L, Kong YD, Zhu R, Yu Z, Wu LF (2019) Effects of glycinin on growth performance, immunity and antioxidant capacity in juvenile golden crucian carp, Cyprinus carpio × Carassius auratus. Aquac Res 51(2):1–15
Li M, Kong Y, Wu X, Guo G, Sun L, Lai Y, Zhang J, Niu X, Wang G (2022) Effects of dietary curcumin on growth performance, lipopolysaccharide-induced immune responses, oxidative stress and cell apoptosis in snakehead fish (Channa argus). Aquacult Rep 22:100981
Mahamood M, Javed M, Alhewairini SS, Zahir F, Sah AK, Ahmad MI (2021) Labeo rohita, a bioindicator for water quality and associated biomarkers of heavy metal toxicity. Npj Clean Water 4:17. https://doi.org/10.1038/s41545-021-00107-4
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Onita B, Albu P, Herman H, Balta C, Lazar V, Fulop A, Baranyai E, Harangi S, Keki S, Nagy L et al (2021) Correlation between heavy metal-induced histopathological changes and trophic interactions between different fish species. Appl Sci 11:3760. https://doi.org/10.3390/app11093760
Rahmana ANA, ElHadya M, Shalaby SI (2019) Efficacy of the dehydrated lemon peels on the immunity, enzymatic antioxidant capacity and growth of Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus). Aquaculture 505:92–97
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep 5:288–295
Said REM, Ashry M, AbdAllah EM (2021) The use of biomarkers in the nile tilapia (Oreochromis niloticus) as biological signals to track Nile contamination in Egypt. Egypt J Aquat Biol Fish 25(5):203–214
Shah N, Ali R, Marimuthu K, NazirUddin M, Rizwan M, Rahman KU, Alam M, Adnan M et al (2020) Monitoring bioaccumulation (in gills and muscle tissues), hematology, and genotoxic alteration in Ctenopharyngodon idella exposed to selected heavy metals. BioMed Res Int Article ID 6185231:1–16. https://doi.org/10.1155/2020/6185231
Shahid S, Sultana T, Sultana S, Hussain B, Irfan M, Al-Ghanim KA, Misned FA, Mahboob S (2021) Histopathological alterations in gills, liver, kidney and muscles of Ictalurus punctatus collected from pollutes areas of River. Braz J Biol 81:814–821
Tabrez S, Ahmad M (2011) Oxidative stress mediated genotoxicity of wastewaters collected from two different stations in northern India. Mutat Res 726(1):15–20
Tabrez S, Shakil S, Urooj M, Damanhouri GA, Abuzenadah AM, Ahmad M (2011) Genotoxicity testing and biomarker studies on surface waters: an overview of the techniques and their efficacies. J Environ Sci Health Part C 29(3):250–275
Tabrez S, Zughaibi TA, Javed M (2021) Bioaccumulation of heavy metals and their toxicity assessment in Mystus species. Saudi J Biol Sci 28(2):1459–1464
Tabrez S, Zughaibi TA, Javed M (2022) Water quality index, Labeo rohita, and Eichhornia crassipes: suitable bio-indicators of river water pollution. Saudi J Biol Sci 29:75–82
Tamele IJ, Loureiro PV (2020) Lead, mercury and cadmium in fish and shellfish from the Indian Ocean and Red Sea (African Countries): public health challenges. J Mar Sci Eng 8:344. https://doi.org/10.3390/jmse8050344
WHO (2006) taken from UNEPGEMS (United Nations Environment Programme Global Environment Monitoring System/Water Programme). (2006) Adapted for water quality and ecosystem health 2006
Zhang L, Hong X, Zhao X, Yan S, Ma X, Zha J (2020) Exposure to environmentally relevant concentrations of deltamethrin renders the Chinese rare minnow (Gobiocypris rarus) vulnerable to Pseudomonas fluorescens infection. Sci Tot Environ 715:136943
Funding
M.S.K. acknowledges the generous support from the Research Supporting Project (RSP2023R352) by the King Saud University, Riyadh, Kingdom of Saudi Arabia.
Author information
Authors and Affiliations
Contributions
NA, GS, and MS conceived and designed the work. MT and MJ executed the work and did the statistical analysis and also made the first draft of this article. All authors reviewed the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Consent to Participate
Not applicable.
Consent to Publish
All authors have given their consent to publish this research article.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Altwaijry, N., Khan, M.S., Shaik, G.M. et al. Redox Status, Immune Alterations, Histopathology, and Micronuclei Induction in Labeo rohita Dwelling in Polluted River Water. Arch Environ Contam Toxicol 84, 179–187 (2023). https://doi.org/10.1007/s00244-022-00976-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-022-00976-x