Abstract
Our previous study has shown that lime powder (LP) had an inhibitory effect against calcium oxalate stone formation. However, the precise mechanisms underlying such beneficial effect remained unclear. Our present study thus aimed to address the effect of LP on excretory level and compositions of urinary proteins using a proteomics approach. From a total of 80 calcium oxalate stone formers recruited into our 2-year randomized clinical trial of LP effect, 10 patients with comparable age and clinical parameters were selected for this proteomic study. 24-h urine specimens were collected from all subjects, at baseline (before) and after LP treatment for 6 months, and then subjected to quantitative proteomics analysis and subsequent validation by ELISA. Total urinary protein excretion was significantly decreased by LP treatment, but unaffected by placebo. Nanoflow liquid chromatography coupled to tandem mass spectrometry (nanoLC–MS/MS) followed by quantitative analysis revealed 17 proteins whose levels were significantly altered (16 decreased and 1 increased) exclusively by LP treatment. Among these, the decrease of transferrin and increase of uromodulin were validated by ELISA. Moreover, there was a significant correlation between microalbuminuria and urinary transferrin level by Pearson’s correlation test. In summary, LP treatment caused significant reduction in total urinary protein excretion and changes in urinary protein compositions that could be linked to stone inhibitory effects and might be relevant mechanisms responsible for the beneficial effects of LP to prevent kidney stone formation and recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis [the presence of stone(s) along urinary tract] is a common urological disease worldwide. The lifetime prevalence of this disease is up to 12% in the United States [1] and even higher in some other areas (e.g., up to 17% in Thailand) [2]. Furthermore, the recurrence rate is approximately 50% after 10 years of the stone removal [3]. Formation of the stone within urinary system can cause ureteral obstruction and renal tubular cell damage induced by oxidative stress and inflammation [4, 5]. Our previous study has shown that urolithiatic patients manifested low-grade intrarenal inflammation and increased intrarenal mRNA expression levels of MCP-1 and IL-6 that were associated with impaired renal function [6]. In addition, urolithiasis is one of the risk factors for chronic kidney disease (CKD) [7] and end-stage renal disease (ESRD) [8], as evidenced by the decrease of creatinine clearance and estimated glomerular filtration rate (eGFR) in the stone formers [9, 10].
The most common type of stones found in patients worldwide is calcium oxalate, of which aggravating factors include hypercalciuria, hyperoxaluria, and hypocitraturia. Among these, hypocitraturia is the most frequent risk factor found in >80% of calcium oxalate stone formers in Thailand [11]. Lime—a common citrus fruit in Southeast Asia—contains high amounts of citric acid and some antioxidants (e.g., polyphenol, flavonoids, and ascorbic acid) [11]. Interestingly, our previous study has found that lime powder (LP) exerted stone preventive properties by its citraturic and alkalinizing effects [11]. However, whether LP also has any other beneficial effects to the stone formers remained unknown.
It is well known that some urinary proteins may play important roles in modulation of the stone formation. For example, nephrocalcin, trefoil factor-1 and Tamm–Horsfall protein (THP) have been demonstrated to serve as inhibitors for calcium oxalate crystal growth, whereas albumin and annexin II have an opposite activity and serve as the stone promoters [12, 13]. Boyce et al. [14] has previously reported that protein excretion in urolithiatic patients was increased 3–13 times when compared to the normal controls. Therefore, it may be possible that the stone preventive properties of LP are partly due to its effect on urinary protein excretion in the stone formers. Our present study thus aimed to address the effect of LP on excretory level and compositions of urinary proteins using a proteomics approach.
Materials and methods
Subjects
This study was performed in concordance to the guidelines detailed in Declaration of Helsinki and was approved by the ethical committee for research in human subjects in the fields of Thai traditional and alternative medicine, Ministry of Public Health, Thailand and by the institutional ethical committee for research in humans at Sappasit Prasong Hospital. The written informed consents were obtained from all the participants. From a total of 80 calcium oxalate stone formers recruited into our 2-year randomized clinical trial of LP effect, 10 patients with comparable age and clinical parameters were selected for this proteomic study to reduce potential biases for subsequent data interpretation.
Specimen collection and sample preparation
24-h urine specimens were collected from all subjects, at baseline (before) and after LP treatment for 6 months. The samples were preserved in thymol and frozen at −80 °C until ready for protein precipitation by 75% ice-cold ethanol in an icebox for 30 min. The precipitants were collected by a low-speed centrifugation for 5 min and solubilized with SDT lysis buffer (4% SDS, 100 mM DTT, and 100 mM Tris-HCl; pH 7.6). Protein concentrations were measured using Bradford’s method.
SDS-PAGE
Equal amount of proteins derived from the same group were pooled and a total of 10 µg proteins derived from each pooled sample were subjected to SDS-PAGE in a precast gel (12% separating with 4% stacking gels) using Mini-Protean III electrophoresis system (Bio-Rad Laboratories, Hercules, CA) at 220 V for 2 h. The separated proteins were then visualized by Coomassie Brilliant Blue staining.
Protein identification by nanoflow liquid chromatography coupled to tandem mass spectrometry (nanoLC–MS/MS)
Equal amount of proteins solubilized in SDT lysis buffer derived from the same group were pooled, reduced by heating at 95 °C for 5 min, and then alkylated with 50 mM iodoacetamide at room temperature in the dark for 20 min. Thereafter, the proteins were then digested with sequencing-grade modified trypsin (Promega; Madison, WI) at a ratio of 1:50 (w/w) trypsin/protein at 37 °C for 16–18 h. Thereafter, 5% formic acid in 80% acetonitrile was added to stop the enzymatic activity. The digested peptides were dried and finally resuspended in 0.1% formic acid prior to MS/MS analysis.
Peptide mixture was separated by two-column system consisted of the 3 cm long pre-column containing 5-µm C18 resin and the 15 cm long analytical column packed with 3-µm C18 resin. The 160-min gradient elution of 0.1% formic acid in acetonitrile was performed using nanoflow liquid chromatography (nano-LC system, Thermo Fisher Scientific; Waltham, MA). The eluted peptides were directly electrosprayed into the mass spectrometer (LTQ-Orbitrap, Thermo Fisher Scientific). Each sample was made to run in technical triplicates.
MS/MS data processing and protein quantitative analysis
The MS raw files were analyzed by integrated MaxQuant software suite (version 1.5.3.30) (http://www.biochem.mpg.de/5111795/maxquant). Proteins were identified by searching against the UniprotKB/Swiss-Prot Homo sapiens database (downloaded on March 2016, containing 20,193 protein entries). The search parameters were set as follows: maximal number of missed cleavages = 1, minimum peptide length = 7 amino acids, fixed modification = cysteine carbamidomethylation, variable modification = methionine oxidation, mass deviation for the main search of precursor masses <4.5 ppm, and de novo mass tolerance for tandem mass (MS/MS) spectra <0.25 Da. The false discovery rate (FDR) was filtered at <0.01 against a decoy database. For quantitation, label-free quantification (LFQ) intensities were created and the output files were subsequently examined by Perseus software version 1.5.3.2 (http://www.biochem.mpg.de/5111810/perseus) or manually processed using Microsoft Excel.
Validation of the proteomic data
The LP-induced changes in levels of urinary proteins identified by quantitative proteomics analysis were confirmed by a conventional method. Transferrin and uromodulin (also known as Tamm–Horsfall protein or THP) were measured using corresponding ELISA kits (Mybiosource; CA, USA). For the correlation study, microalbumin level was analyzed by immunoturbidimetric assay, using Architect analyzer (Abbott).
Statistics
The data are reported as mean ± SD. Comparisons between the two groups of samples were performed using Student’s t test, whereas correlation between microalbuminuria and transferrin levels was analyzed by Pearson’s correlation test. p values less than 0.05 were considered statistically significant.
Results
From a total of 10 stone formers, 5 were from the LP-treated group, whereas another 5 were from the placebo group. Their age, body mass index (BMI), gender, and several baseline clinical chemistry characteristics before treatment, including urine volume, pH, protein, calcium, citrate, magnesium, sodium, oxalate, phosphate, creatinine, and uric acid levels were comparable (Table 1).
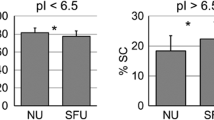
With comparable level of total urinary protein before treatment (Table 1), we also monitored this laboratory parameter after treatment with LP or placebo for 6 months. The data showed that the total urinary protein level was markedly decreased after LP treatment, but remained unchanged in the placebo group (Fig. 1). SDS-PAGE showed obvious change of protein band pattern only in the LP group after the treatment (Fig. 2). These data indicated that there should be some obvious alterations in urinary proteome profile in the LP group after the treatment.
Quantitative proteomics analysis of the samples derived from LP (before vs. after treatment) and placebo (before vs. after treatment) groups revealed some common and unique changes by each treatment. There were 20 common proteins whose levels were significantly altered in both LP and placebo groups after 6-month treatment. Among these, 17 were decreased, whereas 3 were increased by both LP and placebo treatments (Table 2). These altered proteins might thus be the non-specific changes. Interestingly, there were the other 17 proteins whose levels were significantly altered (16 decreased and 1 increased) only by LP treatment (Table 3).
From these data, we selected two altered proteins for subsequent validation using conventional methods. For the unique changes induced by LP treatment, serotransferrin (or transferrin) was decreased with a fold-change of 0.09, whereas uromodulin (or Tamm–Horsfall protein; THP) was the only increased protein with a fold-change of 3.73. We thus measured urine transferrin and THP using corresponding commercial ELISA kits. The data showed that urinary excretion of transferrin tended to decrease after 6-month LP treatment (with a marginal p value), but remained unchanged in the placebo group (Fig. 3a). Moreover, urinary THP excretion was significantly increased by the LP treatment, but no significant change was observed in the placebo group (Fig. 3b) consistent with the proteomic data.
Finally, we analyzed the correlation between microalbuminuria and transferrin levels in all the urine samples by Pearson’s correlation test. The scatter plot showed a positive correlation between microalbumin and transferrin levels with a coefficient of correlation (r) = 0.589 (p value =0.006) (Fig. 4).
Discussion
We have previously reported that LP treatment for 3 months decreased the stone-forming potential, improved antioxidative status, and reduced renal tubular damage in the stone formers [11, 15]. Moreover, LP could inhibit calcium oxalate crystal growth and provided citraturic, kaliuric, and antioxidative effects in healthy individuals without any adverse events [11, 15]. The present study further explored potential mechanisms underlying LP beneficial effects in the stone formers by demonstrating changes in urinary protein excretion and compositions using a proteomics approach, which has been demonstrated as the powerful tool in kidney stone research by several previous studies [16,17,18,19].
Protein is a major component of the stone matrix [20, 21] and is excreted with greater level in urolithiatic patients as compared to the healthy individuals [14]. We found that treatment with LP, not placebo, for 6 months significantly decreased total urinary protein excretion in urolithiatic patients (Fig. 1). The decreased level of urinary protein excretion is a good prognosis to avoid development of impaired renal function and chronic kidney disease (CKD), which are very common in urolithiatic patients [7]. Protein distribution pattern derived from SDS-PAGE presented several different bands in LP group of the urine samples (Fig. 2), indicating that not only the level, but also compositions of the urinary proteins were also altered by LP treatment.
Quantitative proteomics revealed a number of common proteins altered by both LP and placebo groups that could be considered as non-specific changes (Table 2). Most of these common decreased proteins were involved in immunity (Ig gamma/mu chains and complement factors C3/C4), inflammation (alpha-1-antitrypsin and lactotransferrin) and fibrotic process (fibrinogen beta/alpha/gamma chains), whereas the others were carrier proteins (albumin, haptoglobin, alpha-1-acid glycoprotein 1 and ceruloplasmin).
There were 16 proteins that were decreased in levels exclusively after LP treatment (Table 3). These proteins were involved in various processes, such as immunity (Ig gamma/kappa/heavy chains and complement factor H), inflammation (peroxiredoxin-2 and alpha-1-antichymotrypsin), carrier proteins (serotransferrin and hemopexin), fibrosis or coagulation (fibronectin), and cellular proteins (hemoglobin subunits). In previous proteomic reports, inflammatory and fibrotic proteins were abundantly found in both urine and stone matrix from urolithiatic patients [12, 22, 23]. Their decreases might suggest the reduced immune, inflammatory and fibrotic processes by LP.
Among these unique changes after LP treatment, we selected transferrin and uromodulin as the representatives for LP-induced decreases and LP-induced increases, respectively, for subsequent validation. ELISA confirmed the decrease of transferrin and increase of THP levels, exclusive after LP treatment, whereas there were no significant changes observed after placebo treatment (Fig. 3).
Transferrin (or serotransferrin, which can be used interchangeably) is an iron carrier protein in the plasma with a molecular mass of 76.5 kDa that also serves as a sensitive biomarker for glomerular damage. A previous study has demonstrated that transferrin could be found in both urine and stone matrix from urolithiatic patients [24]. In other disease models, transferrin excretion has been found to be correlated with microalbuminuria and other vascular complications in Type 2 diabetes and diabetic nephropathy [25]. We thus performed a correlation study of urinary transferrin and microalbuminuria in all the stone formers included in this study. The data revealed a positive correlation between urinary transferrin and microalbuminuria (Fig. 4), implicating that LP not only reduced protein excretion, but also improved kidney function as determined by the reduced excretion of both total urinary protein (Fig. 1) as well as microalbuminuria (Fig. 4).
Interestingly, uromodulin (or THP) was the only one protein exclusively increased after LP treatment. THP is high abundant protein in the normal urine and its defects have been demonstrated to involve in many urological conditions, including tubular damage and urolithiasis [26, 27]. Many studies have reported that THP has a stone inhibitory effect [28, 29]. We observed the increase of THP only after LP treatment, consistent with the findings reported by Harold et al. [30] demonstrating the increased level of urinary THP in stone formers after potassium citrate treatment. Additionally, Ganter et al. [31] also found that urinary THP level was correlated with urinary citrate level in urolithiatic patients, and low excretion rate of THP might be due to tubular dysfunction. Citrate is a well known potent inhibitor for kidney stone formation and has an anti-inflammatory effect in endothelial cells and macrophages [32, 33]. Moreover, our previous studies have shown that urinary citrate level was increased after LP treatment in the stone formers [11, 15]. Therefore, the increase of urinary THP is most likely another relevant beneficial effect of LP to prevent kidney stone formation or recurrence.
It should be noted that the number of subject included in this study was relatively small. Increasing such sample size would yield a more confident statistical power for defining significant changes as well as validation. Additionally, duration of LP treatment was limited to 6 months in the present study. Varying such duration or evaluation of spatial changes of the urinary proteome would enhance our understanding on the beneficial effects of LP against kidney stone formation. Nevertheless, our data report herein at least in part implicate that LP could prevent kidney stone formation via alterations in excretion of the stone modulators into the urine.
In summary, we reported herein a decrease of total urinary protein excretion and changes in excretion of 17 urinary proteins induced by LP treatment using a proteomics approach. Among these, the decrease of urinary transferrin and increase of THP were validated by ELISA. The changes of these two urinary proteins could be linked to stone inhibitory effects and may be relevant mechanisms responsible for the beneficial effects of LP to prevent kidney stone formation and recurrence.
References
Ferrari P, Piazza R, Ghidini N, Bisi M, Galizia G, Ferrari G (2007) Lithiasis and risk factors. Urol Int 79(Suppl 1):8–15
Yanagawa M, Kawamura J, Onishi T, Soga N, Kameda K, Sriboonlue P, Prasongwattana V, Borwornpadungkitti S (1997) Incidence of urolithiasis in northeast Thailand. Int J Urol 4:537–540
Uribarri J, Oh MS, Carroll HJ (1989) The first kidney stone. Ann Intern Med 111:1006–1009
Khan SR (2006) Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res 34:86–91
Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P (2005) Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res 33:65–69
Boonla C, Wunsuwan R, Tungsanga K, Tosukhowong P (2007) Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res 35:185–191
Rule AD, Bergstralh EJ, Melton LJ III, Li X, Weaver AL, Lieske JC (2009) Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4:804–811
Jungers P, Joly D, Barbey F, Choukroun G, Daudon M (2004) ESRD caused by nephrolithiasis: prevalence, mechanisms, and prevention. Am J Kidney Dis 44:799–805
Worcester EM, Parks JH, Evan AP, Coe FL (2006) Renal function in patients with nephrolithiasis. J Urol 176:600–603
Gillen DL, Worcester EM, Coe FL (2005) Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int 67:685–690
Tosukhowong P, Yachantha C, Sasivongsbhakdi T, Ratchanon S, Chaisawasdi S, Boonla C, Tungsanga K (2008) Citraturic, alkalinizing and antioxidative effects of limeade-based regimen in nephrolithiasis patients. Urol Res 36:149–155
Boonla C, Tosukhowong P, Spittau B, Schlosser A, Pimratana C, Krieglstein K (2014) Inflammatory and fibrotic proteins proteomically identified as key protein constituents in urine and stone matrix of patients with kidney calculi. Clin Chim Acta 429:81–89
Thongboonkerd V, Chutipongtanate S, Semangoen T, Malasit P (2008) Urinary trefoil factor 1 is a novel potent inhibitor of calcium oxalate crystal growth and aggregation. J Urol 179:1615–1619
Boyce WH, Garvey FK, Norfleet CM Jr (1954) Proteins and other biocolloids of urine in health and in calculous disease. I. Electrophoretic studies at pH 4.5 and 8.6 of those components soluble in molar sodium chloride. J Clin Invest 33:1287–1297
Chariyavilaskul P, Poungpairoj P, Chaisawadi S, Boonla C, Dissayabutra T, Prapunwattana P, Tosukhowong P (2015) In vitro anti-lithogenic activity of lime powder regimen (LPR) and the effect of LPR on urinary risk factors for kidney stone formation in healthy volunteers. Urolithiasis 43:125–134
Chutipongtanate S, Nakagawa Y, Sritippayawan S, Pittayamateekul J, Parichatikanond P, Westley BR, May FE, Malasit P, Thongboonkerd V (2005) Identification of human urinary trefoil factor 1 as a novel calcium oxalate crystal growth inhibitor. J Clin Invest 115:3613–3622
Thongboonkerd V, Semangoen T, Sinchaikul S, Chen ST (2008) Proteomic analysis of calcium oxalate monohydrate crystal-induced cytotoxicity in distal renal tubular cells. J Proteome Res 7:4689–4700
Tavichakorntrakool R, Boonsiri P, Prasongwatana V, Lulitanond A, Wongkham C, Thongboonkerd V (2017) Differential colony size, cell length, and cellular proteome of Escherichia coli isolated from urine vs. stone nidus of kidney stone patients. Clin Chim Acta 466:112–119
Vinaiphat A, Thongboonkerd V (2017) Prospects for proteomics in kidney stone disease. Expert Rev Proteom 14:185–187
Moe OW (2006) Kidney stones: pathophysiology and medical management. Lancet 367:333–344
Davies RJ, Steele RD, Kourambas J (2009) Management of kidney stone disease in New South Wales. Med J Aust 190:339
Merchant ML, Cummins TD, Wilkey DW, Salyer SA, Powell DW, Klein JB, Lederer ED (2008) Proteomic analysis of renal calculi indicates an important role for inflammatory processes in calcium stone formation. Am J Physiol Renal Physiol 295:F1254–F1258
Canales BK, Anderson L, Higgins L, Slaton J, Roberts KP, Liu N, Monga M (2008) Second prize: comprehensive proteomic analysis of human calcium oxalate monohydrate kidney stone matrix. J Endourol 22:1161–1167
Brooks ER, Hoppe B, Milliner DS, Salido E, Rim J, Krevitt LM, Olson JB, Price HE, Vural G, Langman CB (2016) Assessment of urine proteomics in Type 1 primary hyperoxaluria. Am J Nephrol 43:293–303
Gluhovschi C, Gluhovschi G, Petrica L, Timar R, Velciov S, Ionita I, Kaycsa A, Timar B (2016) Urinary biomarkers in the assessment of early diabetic nephropathy. J Diabetes Res 2016:4626125
Serafini-Cessi F, Malagolini N, Cavallone D (2003) Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 42:658–676
Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR (2004) Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66:1159–1166
Drach GW, Thorson S, Randolph A (1980) Effects of urinary organic macromolecules on crystallization of calcium oxalate: enhancement of nucleation. J Urol 123:519–523
Grover PK, Ryall RL, Marshall VR (1990) Does Tamm-Horsfall mucoprotein inhibit or promote calcium oxalate crystallization in human urine? Clin Chim Acta 190:223–238
Fuselier HA, Ward DM, Lindberg JS, Allen JM, Husserl FE, Marcucci PA, Cole FE, Turnipseed J, Alam J, Kok DJ (1995) Urinary Tamm-Horsfall protein increased after potassium citrate therapy in calcium stone formers. Urology 45:942–946
Ganter K, Bongartz D, Hesse A (1999) Tamm-Horsfall protein excretion and its relation to citrate in urine of stone-forming patients. Urology 53:492–495
Bryland A, Wieslander A, Carlsson O, Hellmark T, Godaly G (2012) Citrate treatment reduces endothelial death and inflammation under hyperglycaemic conditions. Diab Vasc Dis Res 9:42–51
Choi EY, Kim HJ, Han JS (2015) Anti-inflammatory effects of calcium citrate in RAW 264.7cells via suppression of NF-kappaB activation. Environ Toxicol Pharmacol 39:27–34
Author information
Authors and Affiliations
Contributions
PT, PK, SC, WU, NK, SR and VT designed research; PK, SC, WU, NK and SR performed experiments; PT, PK, SC, WU, NK, SR and VT analyzed data; PT, PK and VT wrote the manuscript; All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Funding
This study was supported by National Research Council of Thailand, Mahidol University research grant, Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative, and the Thailand Research Fund (RTA5680004 and IRG5980006). SC and VT are also supported by Faculty of Medicine Siriraj Hospital.
Conflict of interest
All Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tosukhowong, P., Kulpradit, P., Chaiyarit, S. et al. Lime powder treatment reduces urinary excretion of total protein and transferrin but increases uromodulin excretion in patients with urolithiasis. Urolithiasis 46, 257–264 (2018). https://doi.org/10.1007/s00240-017-0986-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-017-0986-x