Abstract
Background
Using a proteomic approach, we aimed to identify and compare the urinary excretion of proteins involved in lipid transport and metabolism in children with kidney stones and hypercalciuria (CAL), hypocitraturia (CIT), and normal metabolic work-up (NM), and in healthy controls (HCs). Additionally, we aimed to confirm these results using ELISA, and to examine the relationship between the urinary excretion of selected proteins with demographic, dietary, blood, and urinary parameters.
Methods
Prospective, controlled, pilot study of pooled urine from CAL, CIT, and NM versus age- and gender-matched HCs, using liquid chromatography-mass spectrometry. Relative protein abundance was estimated using spectral counting. Results were confirmed by ELISA performed on individual samples.
Results
Of the 1,813 proteins identified, 230 met the above criteria. Of those, 5 proteins (apolipoprotein A-II [APOA2]; apolipoprotein A-IV [APOA4]; apolipoprotein C-III [APOA3]; fatty acid-binding protein, liver [FABPL]; fatty acid-binding protein, adipocyte [FABP4]) involved in lipid metabolism and transport were found in the CAL group, with significant differences compared with HCs. ELISA analysis indicated statistically significant differences in the urinary excretion of APOC3, APOA4, and FABPL in the CAL group compared with HCs. Twenty-four-hour urinary calcium excretion correlated significantly with concentrations of ApoC3 (r = 0.77, p < 0.001), and FABPL (r = 0.80, p = 0.005).
Conclusions
We provide proteomic data showing increased urinary excretion of lipid metabolism/transport-related proteins in children with kidney stones and hypercalciuria. These findings suggest that abnormalities in lipid metabolism might play a role in kidney stone formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A link between dyslipidemia and the risk of kidney stones has been reported in several experimental and human studies [1, 2]. Animal studies have shown that rats fed a high-cholesterol diet develop dyslipidemia and hypercalciuria, hyperoxaluria, and calcium phosphate nephrocalcinosis [3]. Atorvastatin administration decreases renal crystal retention and growth [4]. Increased triglycerides levels resulted in elevated urinary excretion of calcium and urinary uric acid, leading to renal stone formation [5]. Kadlec et al. showed that about one third of stone-forming adults were on cholesterol-lowering therapy, and 70% of them had calcium oxalate stones [6]. Higher cholesterol levels were found in adult stone formers compared with controls, with a pronounced difference seen in those with calcium oxalate and uric acid stones [7].
There is no evidence, to date, of an association between the risk of renal stones and dyslipidemia in the pediatric population. We hypothesized that children with kidney stones might have a higher urinary excretion of proteins involved in lipid metabolism, based upon proteomic profiling. Using a proteomic approach and pooled samples, we aimed to identify and compare the urinary excretion of proteins involved in lipid transport and metabolism between children with kidney stones and hypercalciuria (CAL), hypocitraturia (CIT), and normal metabolic work-up (NM), and their age- and gender-matched healthy controls (HCs). Furthermore, we aimed to test these proteins in individual samples by ELISA, and to correlate the results with demographic, clinical, and laboratory data.
Materials and methods
We prospectively collected the second morning mid-stream fresh urine samples from children with renal calculi and healthy controls between April 2011 and December 2014. No child was on treatment for nephrolithiasis at the time of urine collection. We performed an initial screening using quantitative proteomic comparison of pooled urine from children with CAL, CIT, and NM versus age- and gender-matched HCs, using liquid chromatography-mass spectrometry (LC-MS/MS). The use of pooled samples was intended to reduce the intra- and inter-variability across urine specimens, and to test expense. Confirmatory enzyme-linked immunosorbent assay (ELISA) was performed in individual samples for four selected proteins (apolipoprotein A-IV [APOA4]; apolipoprotein C-III [APOC3]; fatty acid-binding protein, liver [FABPL]; fatty acid-binding protein, adipocyte [FABP4]). Additionally, we examined the relationship between the urinary excretion of these proteins with demographic, dietary, serum, and urinary parameters. This study was approved by the Institutional Review Board (IRB; 075511MP4E).
Patient selection
Patient inclusion criteria consisted of children with kidney stones, aged 5–18 years, who were presenting for their initial evaluation of a stone, had a satisfactory 24-h urine collection (urinary creatinine more than 15 mg/kg/day), and had normal renal function. At the time of urine collection, these children were asymptomatic (no flank or abdominal pain, no urinary symptoms), were on a regular diet and were not on medication for renal stones. Hypercalciuria was defined as excretion of calcium greater than 4 mg/kg/day [8] and hypocitraturia was diagnosed when urinary citrate was less than 310 mg/1.73 m2/day in girls and 365 mg/1.73 m2/day in boys [9]. The normal metabolic work-up group included children in whom no metabolic abnormality was identified in the 24-h urine collection (i.e., an absence of hypercalciuria, hyperoxaluria, hyperuricosuria, hypocitraturia, and cystinuria). One-day dietary records were completed during the initial 24-h urine collection, while the child was on a regular diet and fluid intake, before initiation of medical treatment. Nutrients were calculated by using the ESHA Research Food Processor computer program. Children who were not toilet-trained, or those with hematuria, pyuria, bladder stones, nephrocalcinosis, neuropathic bladder, major congenital bladder abnormality, active urinary tract infection, hematuria, chronic kidney disease, previous reconstructive bladder surgery requiring catheterization, and significant cardiac, pulmonary, gastro-intestinal, and neurological problems, were excluded. Additionally, we excluded children who were on dietary restriction or medical treatment for kidney stones.
The control group consisted of age- and gender-matched healthy children seen in our clinic for monosymptomatic primary nocturnal enuresis that resolved at the time of urine collection. Inclusion criteria consisted of a normal renal bladder ultrasound when available, normal urine dipstick, and a normal calcium-to-creatinine ratio in a spot urine, when available. The 24-h urine collection was not performed in these children for technical reasons and because of the cost. Exclusion criteria included lack of toilet training, bladder and/or kidney stones, and chronic medical conditions.
Sample collection and preparation
Second morning mid-stream fresh urine samples from both patients and controls were obtained in sterile cups, prepared within 3 h of collection (centrifuged at 2,500 rpm for 15 min) and stored as recommended by standardized protocols (developed by the Human Urine and Kidney Proteome Project [HUKPP] and the European Urine and Kidney Proteomics [EuroKUP] initiatives) until use [10]. Proteins in each sample were concentrated in a Centricon-type filter. Albumin and IgG were removed using anti-HSA/IgG resin (Sartorius). Protein concentration in each sample was measured using the BCA protein assay (Pierce).
2D LC-MS/MS and protein quantification
Pooled samples were used in patients and controls. Ten individual samples of 10 μg each were pooled for a total of 100 μg protein per group. Pooled samples were digested with trypsin and analyzed using two-dimensional (2D) LC-MS/MS. When comparing control groups with patient groups, proteins were specified as differentially abundant if they met the following criteria: ≥5 spectral counts; ≤0.05 p value for Fisher’s exact test; and ≥2-fold difference in spectral counts. These criteria were chosen to be at or above the threshold used for most label-free proteomic analyses [11].
ELISA validation
Based on the findings from MS, we selected five proteins: APOA4, APOC3, APOA2, FABPL, and FABP4, for validation using ELISA analysis in individual samples obtained from the CAL group (Human APO A-IV ELISA kit from Millipore, Human APO C3 from Novus Biologicals, Human FABPL ELISA kit from R&D Systems). An additional reason for selection was their involvement in lipid transport and metabolism. The intra-assay and inter-assay coefficients of variation (respectively) were 11–13% and 4–6% for ApoA4, 7.5% and 4.9% for ApoC3, and 7.6% and 11.3% for FABPL.
Statistical analysis
Continuously scaled data variables were presented descriptively using means and standard deviations. Categorically scaled data were presented using proportions and ratios. Two group comparisons of means were conducted using a parametric independent samples t test. Homogeneity of variance was checked and verified using Levene’s test. Data variables with significant skewness were transformed using a square root procedure. Correlations were conducted using both Pearson’s and Spearman’s rho to examine strength and direction. Statistical significance was set at ≤0.05, two-tailed. All procedures were conducted using SPSS Version 23.0, IBM. The p values for the spectral count data comparing patients with controls were calculated using Fisher’s exact test.
Results
Demographics and characteristics of the groups for proteomic analysis
The hypercalciuria group used for proteomic analysis consisted of a pooled sample of 10 children (6 girls), mean age 11.64 ± 4.46 years. There were 10 children (8 girls, mean age 14.83 ± 2.61 years) in the hypocitraturia pool and 10 children in the normal metabolic pool (8 girls, mean age 12.37 ± 4.4 years).
Proteomic analysis
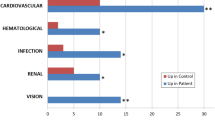
Of the 1,813 proteins identified, 230 met the above criteria. Of these, 12 proteins involved in lipid metabolism and transport were found in higher concentrations in the urine of children with stones compared with HCs. Subgroup analysis showed statistically significant differences between the CAL and HC groups in the urinary excretion of 5 proteins (Table 1). APOA2 and APOA4 were significantly increased in the CIT group versus HCs, with no statistically significant difference between NM and HCs
.
Demographics and characteristics of the groups for ELISA testing
Demographics and characteristics of the CAL and HC groups used for ELISA testing are presented in Table 2. All children had normal urinary dipstick at the time of urine collection, and all but 6 patients were stone-free on the ultrasound carried out around the time of urine collection. One third of the control group had available spot urinary calcium and creatinine, and the calcium-to-creatinine ratios were normal. Renal bladder ultrasound in HCs was available in 70% of children, and was normal in all. Fasting serum cholesterol and triglyceride values were collected from available stone patient data and were within the normal range.
ELISA analysis
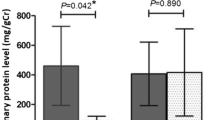
Analysis using ELISA was carried out in individual samples and indicated statistically significant differences in the urinary excretion of APOC3, APOA4, and FABPL in the CAL group compared with HCs (Table 3). Urinary ApoA4 correlated positively with urinary ApoC3 (r = 0.68, p < 0.001), and intake of meat (r = 0.65, p < 0.011). Twenty-four-hour urinary calcium excretion significantly positively correlated with concentrations of ApoC3 (r = 0.77, p < 0.001), and FABPL (r = 0.80, p = 0.005).
Discussion
In this study, initial proteomic screening revealed marked elevation of several urinary proteins involved in lipid metabolism and transport in children with CAL compared with controls. Confirmatory ELISA tests detected significantly elevated urinary levels of APOC3, APOA4, FABPL and nearly significant FABP4 in children with CAL compared with their age- and gender-matched HCs.
Apolipoproteins are protein constituents of plasma lipid transport particles [12, 13]. Apolipoproteins C3 and A4 play important roles in triglyceride transport and homeostasis [14]. FABPs are small (15 kDa) cytosolic proteins that bind unesterified long-chain fatty acids [15]. Both L-FABP and FABP4 control fatty acid transport, metabolism and storage, and modulate inflammatory and metabolic responses [16]. Because of their small molecular size, these proteins may escape the glomerular sieves and be excreted in the urine if not reabsorbed by the renal tubules. They may also be produced by the tubular epithelial cells or adjacent tissue.
The significance of excessive urinary excretion of apolipoproteins and fatty acids in children with CAL and nephrolithiasis is unclear. However, we can speculate that these proteins play a role in the induction of mineralization, and therefore potentially represent a stone nidus. Indeed, lipids are present in calcified tissue and have the ability to trigger calcium phosphate and calcium oxalate precipitation in vitro [17]. In vivo studies have demonstrated increased lipid affinity for calcium, with the lipid–calcium interaction initiating calcification [18]. Moreover, lipoproteins can interact with mucoproteins, leading to the production of organic matrix in the renal interstitium [19]. Whether this is also true for apolipoproteins requires further investigation.
Our study indicates an association between alterations in lipid metabolism and/or transport and kidney stones, implying that these two conditions might share common pathophysiological mechanisms. Adult stone formers have been found to have increased urinary excretion of lipids, mainly phospholipids and glycolipids, both products of cellular membrane degradation [20]. Lipids were present in the matrix of all stones, irrespective of their predominant inorganic component, with calcium oxalate and calcium phosphate stones containing 2 to 4 times more lipids than proteins [20].
The major limitation of this study is the inability to establish a cause–effect relationship between the urinary lipid excretion and kidney stone, owing to the cross-sectional study design. Other limitations include a small study sample, and the lack of serum and 24-h urinary data in controls. Additionally, in a few children in the control group no calcium and creatinine measurements in spot urine were available.
In conclusion, we provide proteomic and ELISA data showing marked increases in urinary excretion of lipid metabolism-/transport-related proteins in children with kidney stones and hypercalciuria. These findings should be validated using a larger sample. Furthermore, the involvement of lipid metabolism-/transport-related proteins in kidney stone formation and the impact of dietary change on the urinary excretion of lipoproteins should be investigated further.
References
Torricelli FC, De SK, Gebreselassie S, Li I, Sarkissian C, Monga M (2014) Dyslipidemia and kidney stone risk. J Urol 191(3):667–672
Kajikawa H (1998) The influence of dietary lipids on nephrolithiasis in rats. Nippon Hinyokika Gakkai Zasshi 89:931–938
Schmiedl A, Schwille PO, Bonucci E, Erben RG, Grayczyk A, Sharma V (2000) Nephrocalcinosis and hyperlipidemia in rats fed a cholesterol- and fat-rich diet: association with hyperoxaluria, altered kidney and bone minerals, and renal tissue phospholipid-calcium interaction. Urol Res 28(6):404–415
Tsujihata M, Momohara C, Yoshioka I, Tsujimura A, Nonomura N, Okuyama A (2008) Atorvastatin inhibits renal crystal retention in a rat stone forming model. J Urol 180:2212–2217
Iba A, Kohjimoto Y, Mori T, Kuramoto T, Nishizawa S, Fujii R, Nanpo Y, Matsumura N, Shintani Y, Inagaki T, Hara I (2010) Insulin resistance increases the risk of urinary stone formation in a rat model of metabolic syndrome. BJU Int 106:1550–1554
Kadlec AO, Greco K, Fridirici ZC, Hart ST, Vellos T, Turk TM (2012) Metabolic syndrome and urinary stone composition: what factors matter most? Urology 80(4):805–810
Inci M, Demirtas A, Sarli B, Akinsal E, Baydilli N (2012) Association between body mass index, lipid profiles, and types of urinary stones. Ren Fail 34(9):1140–1143
Cameron MA, Sakhaee K, Moe OW (2005) Nephrolithiasis in children. Pediatr Nephrol 20:1587–1592
Hoppe B, Kemper MJ (2010) Diagnostic examination of the child with nephrolithiasis or nephrocalcinosis. Pediatr Nephrol 25:403–413
Thongboonkerd V (2007) Practical points in urinary proteomics. J Proteome Res 6(10):3881–3890
Neilson KA, Ali NA, Muralidharan S, Mirzaei M, Mariani M, Assadourian G, Lee A, van Sluyter SC, Haynes PA (2011) Less label, more free: approaches in label-free quantitative mass spectrometry. Proteomics 11:535–553
Brewer HB Jr, Shulman R, Herbert P, Ronan R, Wehrly K (1974) The complete amino acid sequence of alanine apolipoprotein (apoC-3), and apolipoprotein from human plasma very low density lipoproteins. J Biol Chem 249(15):4975–4984
Utermann G, Beisiegel U (1979) Apolipoprotein A-IV: a protein occurring in human mesenteric lymph chylomicrons and free in plasma. Isolation and quantification. Eur J Biochem 99(2):333–343
Zheng C (2014) Updates on apolipoprotein CIII: fulfilling promise as a therapeutic target for hypertriglyceridemia and cardiovascular disease. Curr Opin Lipidol 25(1):35–39
Zhang M, Zhu W, Li Y (2013) Small molecule inhibitors of human adipocyte Fatty Acid Binding Protein (FABP4). Med Chem 10(4):339–347
Thumser AE, Moore JB, Plant NJ (2014) Fatty acid binding proteins: tissue-specific functions in health and disease. Curr Opin Clin Nutr Metab Care 17(2):124–129
Eanes ED (1989) Biophysical aspects of lipid interaction with mineral: liposome model studies. Anat Rec 224(2):220–225
Khan SR, Shevock PN, Hackett RL (1988) Presence of lipids in urinary stones: results of preliminary studies. Calcif Tissue Int 42(2):91–96
Moorhead JF, Chan MK, El-Nahas M, Varghese Z (1982) Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2(8311):1309–1311
Khan SR, Glenton PA, Backov R, Talham DR (2002) Presence of lipids in urine, crystals and stones: implications for the formation of kidney stones. Kidney Int 62(6):2062–2072
Acknowledgements
This research is being funded by the Children’s Hospital of Michigan Foundation (R1-2012-19 and R2-2014-31). We acknowledge the assistance of the Wayne State University Proteomics Core, which is supported through NIH grants P30 ES020957, P30 CA 022453, and S10 OD010700
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethics statement
This study was IRB-approved (IRB number: 075511MP4E). Informed consent was obtained from parents and patients 13 years of age or older.
Rights and permissions
About this article
Cite this article
Kovacevic, L., Lu, H., Caruso, J.A. et al. Marked increase in urinary excretion of apolipoproteins in children with nephrolithiasis associated with hypercalciuria. Pediatr Nephrol 32, 1029–1033 (2017). https://doi.org/10.1007/s00467-016-3576-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3576-1