Abstract
Background
Dermal regeneration template (DRT) has been well implicated in the reconstruction of full-thickness burn injury. This case series specifically presents our experience and our clinical application of Pelnac® to achieve wound closure with complex acute full-thickness defect.
Methods
A retrospective review of patients treated with Pelnac for complex wound defects from 2008 to 2014 at Concord Burns Unit was carried out. Variables such as wound aetiology, wound size and complications were considered.
Results
Five patients (four females and one male with a mean age 54 ± 20) all had full-thickness defects (mean defect size 4.3 ± 2.0 % TBSA), some with exposed tendon and bone. The wounds were treated with Pelnac®; the silicone layer was removed at postoperative day 14 and a split-thickness skin graft (0.2 to 0.3 mm) was applied. Clinically, the reconstructed areas demonstrated good granulation tissue at 14 days with good take of the skin graft. There were no major acute graft loss, rejection or associated infection. However, there were small areas of graft loss which did not require re-grafting.
Conclusions
DRT provides a safe and efficacious alternative when dealing with acute contaminated full-thickness wounds. Pelnac® seems versatile in obtaining wound coverage in difficult complex wounds, especially in critically ill patients where free or pedicle flap reconstruction would be problematic.
Level of Evidence: Level V, therapeutic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of dermal regeneration templates (DRTs) was first described by Yannas and Burke in 1980 [1–3]. The introduction of DRTs has revolutionised the management of major burn injuries as well as the reconstruction of complex open wounds. Classical teaching of Gillies’ principal, “tissue losses should be replaced in a kind,” can sometimes be challenging and not always beneficial due to patient factors, size and location of defect. DRT provides an alternative option for wound closure, in the acute setting, in critically ill patients with extensive injuries where otherwise major reconstructive flaps would be considered risky and morbid. Furthermore, DRTs are able to provide long-term functional and cosmetic outcomes with improved skin texture and pliability, results that are comparable to that of autologous skin graft [4–6].
Integra® (Integra Life Sciences Corp., Plainsborg, NJ) is one of the widely used DRTs, particularly for the purpose of sizeable burn injuries and resurfacing of scar contractures. Integra® consists of a bilayer artificial dermis made up of bovine collagen and glycosaminoglycan matrix covered with an inert layer of silicone. It has the ability to facilitate organised infiltration of host’s fibroblast and endothelial cells and regenerate a neodermis in 14–21 days.
Pelnac® (Gunze Corp., Osaka, Japan) is another DRT which was first described in Japan by Suzuki et al., with the aim of expanding the indications and applications of DRTs [7, 8]. It works on similar principles with several distinct properties with regards to composition; the details will be further discussed later.
There is a fair amount of literature on the use of Integra® for achieving acute wound closure secondary to trauma, oncological defects and necrotising infections [9–12]. The results are encouraging as Integra® is able to provide immediate wound coverage with few graft associated infections or graft failures. However, there is limited evidence available for the clinical application and efficacy of Pelnac®. In Australia, Concord Burns Unit is the only centre that has started trialling Pelnac®; hence, in this series, we present our experience with Pelnac® on a variety of complex wounds to achieve wound closure, which was otherwise deemed problematic with flap reconstruction.
Material and methods
Patients
A retrospective review of five patients treated with Pelnac® for complex wound defects from January 2008 to October 2014 was performed. Patient medical records were reviewed, and the following variables were considered: wound size and depth, wound aetiology, wound location, amount of Pelnac® used, time to auto-grafting, acute complications, functional outcomes and patient satisfaction. Before and after photos were also included, and clinical photography was obtained with informed consent from all participants in the study. All photos included have been de-identified so as to retain patient confidentiality.
Surgical technique
The preparation of Pelnac® was in accordance to manufacturer’s guidelines. Pelnac® was submerged into sterile saline for 20 min. With serial debridement, all wounds were thoroughly debrided to viable bleeding tissue and washed with hydrogen peroxide and normal saline. Meticulous haemostasis was achieved prior to application of Pelnac®. Pelnac® was meshed at a ratio of 1:1 using a non-crushing Brennen Mesher (Molnlycke Health Care., Gothenburg, Sweden) and then secured to the wound with skin staples. Negative wound pressure therapy (NWPT) was applied with black foam at a pressure of 125 mmHg to all patients undisturbed for 14 days, with the exception of patient 4 as seal was difficult to achieve and patient was non-compliant. At day 14, the silicone layer was removed and thin split-thickness skin graft (STSG) ranging from 0.02 to 0.03 mm was applied to the neodermis and was dressed with non-adherent dressing, gauze and crepe bandage which was left intact for 5 days.
Results
A total of five patients’ medical records were examined in the study. The average age of patients was 53.8 years (range 26–79 years). There were four females and one male. Additional demographic and wound details are displayed in Table 1.
Pelnac® was used in complex wound defects from varying aetiologies, including necrotising fasciitis, necrotising vasculitis and thermal and chemical burns. The mean size of 4.3 ± 2.0 % TBSA was covered with Pelnac®. Four out of five patients in this series were treated with NWPT following the application of Pelnac®. In this group of patients, NWPT was left undisturbed for a period of 14 days at which point skin grafting was performed.
There were no major infections, failures or rejections of Pelnac® in all the patients except patient 4 where a second round of Pelnac® was required as a consequence of poor take. However, after 14 days, there was well-vascularised neodermis for auto-grafting. Patient 5 did have areas of moderate STSG failure 3 weeks post STSG, despite it being grafted on vascularised neodermis; this was a consequence of poor compliance and ongoing smoking. All patients received meticulous wound care which included aseptic dressing changes with appropriate choice of dressing as the wounds evolved. All patients were managed with a multi-disciplinary approach with constant allied health care support including intense physiotherapy and occupational therapy. Follow-up, some at 3 months and some at 1 year, showed that no patients developed hypertrophic scarring in areas of Pelnac® reconstruction with STSG. All donor sites healed without hypertrophic scaring. Patients 1, 2, 3 and 4 had good functional outcomes. Unfortunately, patient 5 was lost to follow-up.
Case presentations
Patient 1, 65-year-old female, developed necrotising vasculitis secondary to cryoglobulinemia to bilateral lower limbs with full-thickness soft tissue loss requiring aggressive surgical debridement. Pelnac® was applied to her left dorsum foot and ankle onto exposed Achilles tendon, distal tibia and fibula. NWPT was administered prior to and after Pelnac® application. Her progress was complicated with ongoing vasculopathy which required a significant immunosuppression which resulted in delayed wound healing with small areas of graft breakdown but did not require re-grafting. Six-month follow-up (Fig. 1e) showed satisfactory healing.
Patient 2, 43-year-old female, developed necrotising fasciitis to chest and right upper limb. After aggressive surgical debridement, she had a loss of 75 % of the posterior compartment of the right arm. Pelnac® was applied circumferentially to the right arm and shoulder. She received NWPT prior to and after application of Pelnac®. Three-month follow-up (Fig. 2c) showed satisfactory wound healing.
Patient 3, 79-year-old female, had a chemical burn to bilateral forearms, thighs and anterior chest. Following surgical debridement, full-thickness defect to the deep compartment of forearms with exposed tendon, bone and neurovascular structures remained. Pelnac® applied to the bilateral forearm. She received NWPT after Pelnac® application. She had small wound breakdown over the left extensor elbow and right wrist, which was managed conservatively; further follow-up at 1 year (Figs. 3d and 4c) shows satisfactory wound healing.
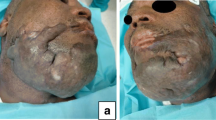
Patient 4 had a notable reconstruction. She was a 26-year-old female with self-inflicted full-thickness flame burn to scalp, neck, anterior chest and right upper limb. Following surgical debridement, there was 4 % TBSA full-thickness defect over the scalp with exposed calvarium and non-viable periosteum. This patient did not receive NWPT on the site of Pelnac® as a seal was not able to be achieved due to burns and healing skin grafts in the surrounding wounds. It was observed with subsequent dressing change that the initial colour change of Pelnac® and granulation tissue was seen to develop around the wound edges. Due to the large exposed calvarium defect, a second application of Pelnac® was required (Fig. 6). However, it is important to note that the area covered on the second application was smaller; the wound had contracted from the peripheral vascularised Pelnac®. There were no histological studies performed, but from clinical observation, it supports the suggestion of Baynosa et.al and Hulsan et.al that ingrowth of vessels from the peripheral tissue allows integration of DRT [13, 14]. Follow-up at 3 months (Fig. 5c, d) showed satisfactory result.
Figure 6 illustrates the success on the second application of Pelnac® on a smaller contracted wound. Vascularised Pelnac® had small areas of graft failure which were left to heal by secondary intention. Follow-up photo at 1 year showed satisfactory healing.
Patient 5, 56-year-old male, had a less than ideal reconstruction. He had a delayed presentation of thermal burns from an electric heater to his right dorsal hand and developed necrotising fasciitis into the deep compartments of the hand requiring extensive debridement. Pelnac® was applied to full-thickness defect in the dorsal hand with exposed neurovascular structures and extensor tendons. NWPT was administered prior and after application of Pelnac®. Three weeks post STSG (Fig. 3c), he had localised areas of graft loss over extensor tendons which was multifactorial, grafting over avascular structure compounded by significant hand infection and septic arthritis of the fifth metacarpal joint, and this was on a background of significant mental health issues and ongoing heavy smoking. His graft loss was considered borderline, and decision was made to monitor wound progress; however, there were ongoing issues with compliance and follow-up. Unfortunately, there was no 3-month follow-up available for this patient (Fig. 7c).
Discussion
Complex full-thickness wounds pose challenging clinical scenarios. This case series demonstrates the success of Pelnac® on its application and its ability to be incorporated in a wide range of full-thickness wounds with exposed tendons and bones. Pelnac® provided wound coverage in the acute setting with excellent graft take without the complications of prolonged surgery, detrimental wound infection or significant donor site morbidity. In the cases described, alternative reconstruction options for defects with underlying avascular wound beds would be pedicle or free tissue transfer, which itself poses risks in critically ill patient, or major amputation of threatened limb. Both options, however, in the patient cohort presented were not ideal. It is important to acknowledge that dermal regeneration template provides an alternative option of acute wound closure in the acute setting, especially when dealing with critically ill patient, and prolonged reconstruction surgery is not ideal. However, according to the fundamental of Gillis’s principal, no reconstruction is as durable as “like replacing like.” But, this can be revisited when the patient is haemodynamically stable and pre-operative modifiable risk factors such as smoking, cardiopulmonary status and medications have been optimised to ensure success of reconstruction.
The success of Pelnac® on contaminated wounds can be attributed to its unique properties. Pelnac® is a DRT made from soluble atelocollagen which is a highly purified type 1 collagen from porcine tendon. Atelocollagen is almost identical to endogenous collagen composing of stable repeating blocks of amino acid without the highly antigenic telopeptides. During its production, pepsin is used to remove telopeptides in the collagen which results in a reduced antigenic matrix, consequently lowering the rates of immune response, rejection and failure [8]. Therefore, when applied to contaminated wounds it eliminates further inflammatory response, resulting in better wound healing. When compared to Integra®, the average pore size in Pelnac® is larger, ranging from 70 to 110 μm as opposed to 30 to 120 μm. Larger average pore size prevents the formation of a tissue capsule and facilitates cell migration into the matrix, allowing the formation of a consistent and elastic neodermis [3]. This provides a final result which mimics endogenous dermis reducing disabling scar contractures and improved cosmetic outcome [15]. In addition, unlike Integra®, the matrix in Pelnac® is not chemically cross-linked with glycosaminoglycan (GAG) and is still able to provide similar resistance to degradation against fibroblast collagenases. It allows a durable construct without the added cost; the cost of Pelnac® is US$4/cm2, while the cost of Integra® is US$8/cm2 [7, 16, 17].
The ability of Pelnac® to provide coverage on avascular wound beds relies on peripheral revascularisation into the matrix highlighting the importance of aggressive debridement to well-vascularised tissue [13]. Coverage of sizeable exposed bone and tendon defects are made possible with lateral neovascularisation into the dermal matrix, which would otherwise require flap reconstruction and potentially lengthy microsurgery. A case report which follows the long-term efficacy of Pelnac® supports its ability to produce a dermis that is safe and histologically resembling true dermis in large full-thickness defects [18].
Negative wound pressure therapy (NWPT) has proven to be a valuable adjunct to graft take. Multiple randomised control trials support the use of NWPT dressings for split-thickness skin grafts [19, 20]. The application of NWPT helps to control local infection and decrease haematoma and seroma formation which are common reasons for graft failure [19]. It also improves the direct contact of graft to host bed, allowing improved plasmic imbibition and neovascularisation and eliminating physical shearing of the graft. The same can be said with our experience of NWPT on Pelnac® in this case series. V.A.C.® therapy (KCI Licensing, Inc. San Antonio, TX) has been shown to decrease the time taken for neovascularisation of neodermis when applied to avascular wound beds, which implies a decreased risk of infection and reduced length of stay in a hospital [21].
The study is limited due to the small number of patients and the lack of randomisation; therefore, it is difficult to draw significant conclusions. Moreover, we acknowledge that there is a lack of histological evaluation in regards to the integration of Pelnac® into the wound beds and objective assessment of scar outcome. However, the study serves as a pilot to describe our clinical experience with Pelnac® and its applications which are limited in the literature. The paper prompts future research comparing the use of DRT versus other forms of reconstruction in obtaining wound closure with avascular wounds. It also prompts studies to examine histological and functional outcomes between DRTs and the conventional STSG in order to provide substantial evidence for the efficacious use of Pelnac®.
Conclusion
Our case series provides additional support to the current literature in the application of DRTs on reconstruction of extensive soft tissue defects from multiple aetiologies. Specifically, we have demonstrated success of extending the use of Pelnac® to both burn- and non-burn-related injuries. The implications of expanding the indication for DRTs are numerous. To be able to provide a simple straightforward surgical technique in the closure of complex wound has a considerable impact on the patient’s outcome, morbidity and accessibility. In certain cases, it allows us to “buy time” for definitive closure, without subjecting critically ill patients to prolong surgery. DRT could be considered as an acceptable alternative to provide wound closure on avascular wound beds. The results of our case series prompt future randomised control trial to establish efficacy of Pelnac® over traditional techniques for wound closure.
References
Yannas IV, Burke JF (1980) Design of an artificial skin. I. Basic design principles. J Biomed Mater Res 14(1):65–81
Yannas IV, Burke JF, Gordon PL, Huang C, Rubenstein RH (1980) Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res 14(2):107–32
Dagalakis N, Flink J, Stasikelis P, Burke JF, Yannas IV (1980) Design of an artificial skin. Part III. Control of pore structure. J Biomed Mater Res 14(4):511–28
Moiemen N, Yarrow J, Hodgson E, Constantinides J, Chipp E, Oakley H et al (2011) Long-term clinical and histological analysis of Integra dermal regeneration template. Plast Reconstr Surg 127(3):1149–54
Bloemen MCT, van Leeuwen MCE, van Vucht NE, van Zuijlen PPM, Middelkoop E (2010) Dermal substitution in acute burns and reconstructive surgery: a 12-year follow-up. Plast Reconstr Surg 125(5):1450–9
Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung WK (1981) Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 194(4):413–28
Suzuki S, Matsuda K, Nishimura Y, Maruguchi Y, Maruguchi T, Ikada Y et al (1996) Review of acellular and cellular artificial skins. Tissue Eng 2(4):267–75
Suzuki S, Matsuda K, Isshiki N, Tamada Y, Ikada Y (1990) Experimental study of a newly developed bilayer artificial skin. Biomaterials 11(5):356–60
Muangman P, Engrav LH, Heimbach DM, Harunari N, Honari S, Gibran NS et al (2006) Complex wound management utilizing an artificial dermal matrix. Ann Plast Surg 57(2):199–202
Koenen W, Felcht M, Vockenroth K, Sassmann G, Goerdt S, Faulhaber J (2011) One-stage reconstruction of deep facial defects with a single layer dermal regeneration template: single layer dermal regeneration template in the face. J Eur Acad Dermatol Venereol 25(7):788–93
Demiri E, Papaconstantinou A, Dionyssiou D, Dionyssopoulos A, Kaidoglou K, Efstratiou I (2013) Reconstruction of skin avulsion injuries of the upper extremity with Integra® dermal regeneration template and skin grafts in a single-stage procedure. Arch Orthop Trauma Surg 133(11):1521–6
Abbas Khan MA, Chipp E, Hardwicke J, Srinivasan K, Shaw S, Rayatt S (2010) The use of dermal regeneration template (Integra®) for reconstruction of a large full-thickness scalp and calvarial defect with exposed dura. J Plast Reconstr Aesthet Surg 63(12):2168–71
Baynosa RC, Browder LK, Jones SR, Oliver JA, Van Der Harten CA, Stephenson LL et al (2009) Evaluation of artificial dermis neovascularization in an avascular wound. J Reconstr Microsurg 25(7):405–10
Hulsen J, Diederich R, Neumeister MW, Bueno RA (2014) Integra® dermal regenerative template application on exposed tendon. Hand (NY) 9(4):539–42
Suzuki S, Matsuda K, Isshiki N, Tamada Y, Yoshioka K, Ikada Y (1990) Clinical evaluation of a new bilayer “artificial skin” composed of collagen sponge and silicone layer. Br J Plast Surg 43(1):47–54
Brusselaers N, Pirayesh A, Hoeksema H, Richters CD, Verbelen J, Beele H et al (2010) Skin replacement in burn wounds. J Trauma Inj Infect Crit Care 68(2):490–501
Matsuda K, Suzuki S, Isshiki N, Yoshioka K, Okada T, Ikada Y (1990) Influence of glycosaminoglycans on the collagen sponge component of a bilayer artificial skin. Biomaterials 11(5):351–5
Suzuki S, Morimoto N, Yamawaki S, Fujitaka J, Kawai K (2013) A case of giant naevus followed up for 22 years after treatment with artificial dermis. J Plast Reconstr Aesthet Surg 66(8):e229–e233
Llanos S, Danilla S, Barraza C, Armijo E, Piñeros JL, Quintas M et al (2006) Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg 244(5):700–5
Moisidis E, Heath T, Boorer C, Ho K, Deva AK (2004) A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 114(4):917–22
Eo S, Kim Y, Cho S (2011) Vacuum-assisted closure improves the incorporation of artificial dermis in soft tissue defects: Terudermis® and Pelnac®. Int Wound J 8(3):261–7
Conflict of interest
Winy Widjaja and Peter Maitz declare that they have no conflict of interest.
Funding
This study is not funded.
Ethical standards
For this retrospective study formal consent from a local ethics committee is not required.
Patient consent
All participants of the study have given informed consent for clinical photography and patient data prior to the study. All patients’ data have been de-identified.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Widjaja, W., Maitz, P. The use of dermal regeneration template (Pelnac®) in acute full-thickness wound closure: A case series. Eur J Plast Surg 39, 125–132 (2016). https://doi.org/10.1007/s00238-015-1131-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-015-1131-0