Abstract
Burn survival has improved significantly over the last few decades reflecting the major advances in burn surgery and care including improvements in the management of the initial resuscitation, inhalation injury, nutrition, and early excision and grafting. Burns however still represent a reconstructive challenge for the plastic surgeon, both in terms of initial adequate tissue coverage, and in the long-term prevention of scar contractures, restoration of function, biomechanical strength, and aesthetic quality, all of which can significantly affect the quality of life and societal reintegration of the burn survivor.
Despite the myriad of reconstructive options available, no ideal treatment exists and thus it is important to explore new treatment options.
Dermal skin substitutes (also known as dermal regeneration templates) such as Integra were first introduced in burns and have now gained a wide usage even in other fields.
The use of skin substitutes has expanded the reconstruction options in burns, as they allow the regeneration of better quality soft tissue even on poorly vascularized wound beds such as bone or tendon.
In our experience, the use of a dermal skin substitutes has allowed us to achieve easy, safe, durable reconstructions with low complication rates. These skin substitute reconstructed tissues have also been shown to be of better quality in terms of pliability, texture, and robustness with lack of contracture and hypertrophic scarring compared to reconstruction with skin graft alone. Additionally, we have also found skin substitutes useful in the reconstruction of scars that require surgical release.
Lipofilling, prepared and performed in many different ways, is nowadays one of the effective therapies for scars, but significant research is still required and currently underway even in acute burns to evaluate its effectiveness.
Further research is required to further study and expand the role and application of stem cells and growth factors even in acute burns, and it is important not to lose our focus on deepening our understanding of the mechanisms behind the regenerative phenomenon.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Regeneration in acute burns
- Tissue engineering
- Cell cultures
- Keratinocytes
- Fibroblasts
- Skin grafts
- Biomaterials
- Lipofilling in burns
- PRP
- Stem cells
-
Burns still represent a reconstructive challenge in terms of adequate tissue coverage, prevention of scar contractures, restoration of skin functions, biomechanical strength, and aesthetic quality.
-
The aim of tissue bioengineering is to create a scaffold that resembles as much as possible the original extracellular matrix (ECM), the natural support.
-
A high resemblance between the engineered and the native tissues leads to the creation of a phenotype that follows the natural skin tone and is associated with healing process improvement.
-
The poor mechanical performance of some biological skin substitutes limits their use, whereas synthetic ones have a passive role of cellular support but also one active in signal transmission.
-
There are few studies on platelet-rich plasma application in burns showing it can be beneficial in reducing inflammation and the amount of cell death in the zone of stasis, but its use is controversial.
-
Adipose-derived stem cells (ADCs) have been shown to improve healing even in acute burns in various models although most of the studies have been limited to animal subjects.
-
As ASCs have shown not to be significantly negatively affected by the post-burn inflammatory process, it may be possible to improve wound healing deficits with the use of topical stem cells.
-
Peripheral blood mononuclear cells (PBMNC) have shown to enhance the regenerative effect of mesenchymal stem cells (MSCs), which on the other side can regulate the function of macrophages.
-
MSCs regenerative capacity is influenced and regulated by the local immune response, particularly from the macrophages, as coordinators of tissue repair and regeneration.
-
The next generation of regenerative therapies may evolve from typical biomaterial-, stem cell-, or growth factor-centric approaches to an immune-centric approach.
1 Introduction
Burn survival has improved significantly over the last few decades reflecting the major advances in burn surgery and care including improvements in the management of the initial resuscitation, inhalation injury, nutrition, and early excision and grafting.
Burns however still represent a reconstructive challenge for plastic surgeons, both in terms of initial adequate tissue coverage and, in the long term, of scar contractures prevention, restoration of function, biomechanical strength, and aesthetic quality, all of which can significantly affect the quality of life and societal reintegration of the burn survivor.
Traditional methods of ameliorating the pathological burn scarring are limited but the advances in biomaterials and future potential regenerative techniques in the reconstruction and improvement of burn scars are exciting developments that harbor rich possibilities.
The purpose of this chapter, more than showing techniques and clinical results, aim to stimulate the debate and new studies, taking into consideration different cellular therapies already used for other indications.
1.1 Depth Classification
Burns are injuries that can result in partial or complete destruction of the involved area and commonly are due to thermal injuries (most commonly hot liquids, flame, steam, or contact with hot surfaces) but may also be due to chemicals, electricity, and radiation.
Before embarking on any therapeutic procedure, it is advisable to take into account all the parameters that may affect the severity of the burn.
The most important criteria for the classification of burns and therefore, for the pronosis and the management of the burn patient, are the extent and depth of the burn.
The extent of the burn can be calculated on the basis of specific charts that codify in percentage the surface area of the various parts of the body. These percentages differ from child to adult: the most accurate and most commonly used chart is the Lund and Browder.
The surface area can be easily calculated, but in a more approximate way, with the “Wallace rule of nines.”
The classification of the severity of the burn injury also includes the depth of the burn, which is particularly important for determining the required management. When examining a burn, there are four components required to assess depth: appearance, pressure blanching, pain, and sensation. Burns can be classified according to thickness according to the American Burn Association criteria using these four elements.
Burn injuries however are in a dynamic state and can evolve over time. In fact, some burns, in particular with partial thickness, can progress within 2–4 days.
Burns depth classification is as follows:
-
Superficial (first degree) are characterized by vasodilation and edema and by an increase in permeability of capillaries with leakage of water, salts, and proteins, thus producing a diffuse erythema. The skin flaking that follows usually resolves in a few days.
-
Partial superficial thickness (second degree) burns involves the superficial, strongly hyperemic dermis, presenting vesicles or blisters. Healing generally occurs within 3 weeks with minimal scarring [1].
-
In deep partial thickness or deep dermal burns (second degree), complete destruction of the epidermis and deep dermis are present. Healing times vary from 15 to 21 days for re-epithelialization starting from the bottom of the annexes.
-
Burns of the full-thickness skin (third degree) are characterized by the formation of eschar, with very little possibility of spontaneous re-epithelialization and usually require surgical treatment.
With deep dermal and full-thickness burns, the dermal layer of the skin which contributes significantly to the appearance, robustness, pliability, and functionality of skin is mostly or completely lost. Solely split skin grafting does not regenerate the dermis and results in both in poor aesthetic and functional outcomes but the use of biomaterials and stem cells can potentially restore the dermal layer and lead to greatly improved outcomes.
1.2 Traditional Treatments
The emergency period, which corresponds to the first 48–72 h from the accident, represents the most critical moment for the burn patient: short- and long-term prognosis is closely related to the treatment to which the patient is subjected to during this crucial time frame.
First aid treatment of burns—Current recommendations for the initial first aid treatment of burns includes keeping the burn wounds cool at around 15 °C for 20 min. Water from 2 °C to 15 °C reduces pain, helps prevent damage to tissue in the stasis zone, and improves the appearance and thickness of the resulting scar.
Initial assessment—After the establishment of both the extent and depth of burns, the initial assessment is based upon the examination of the patient’s cardiocirculatory and respiratory status.
Urgent therapy—It is therefore primarily addressed to airway treatment and immediate fluid replacement. It is essential to treat, where present, possible inhalational damage. These can be characterized by upper airway edema, acute respiratory failure, and carbon monoxide intoxication.
Protein Loss—Significant intravascular protein loss occurs in the burn patient due to endothelial rupture of damaged vessels. Endothelial integrity is restored in the first 24 h, and thereafter the use of 5% albumin according to the 0.5 mL/kg/% TBSA formula may be useful to maintain the balance between the extracellular and intravascular space and reduce mortality and compartment syndrome [2].
Infection risk—Burn injury damages the skin which is the first barrier against infection. Additionally, necrotic tissues are a fertile ground for bacterial growth, which together with the immunosuppressed state of the burn patient contribute greatly to the risk of wound infection and sepsis which, if not optimally managed, can lead to the death of the patient. Infections are in most cases caused by streptococci and in particular by Streptococcus pyogenes and Streptococcus agalactiae, which are fortunately responsive to Penicillin. However, infections and consequent sepsis today are the most common cause of death (about 50–84%) in burn patients [3].
Treatment of local injuries—The only absolute surgical procedure that needs to be performed in an acute burn patient is a decompression escharotomy. These are indicated in all circumferential full-thickness burns that are present on the trunk and extremities: the presence of burn edema in the interstitial spaces in these anatomical areas can lead to respiratory failure or ischemia at the extremities.
Treatment of superficial burns—Epidermal or first-degree burns: These do not require special therapies. Emulsions and ointments based on cortisone, antihistamines, and including anesthetic agents can be used for anti-inflammatory or symptomatic purposes to relieve itching and pain.
Superficial or superficial grade dermal burns: it is possible to clean burnt surfaces with disinfectants that are not overly toxic to cells and followed subsequently with the application of a thin layer of vaseline gauze covered with normal gauzes and bandages. It is advisable to refrain from using ointments containing anesthetic agents or non-sterile preparations.
Treatment of deep burns—The treatment of burns by early removal of necrotic tissue and immediate coverage with skin grafts is the current standard of care for the treatment of deep burns. The technique of tangential excision, when first introduced in 1970 by Zora Janzekovic, revolutionized burn surgery [4]. Her technique involved the removal of most of the necrotic tissue, by means of tangential excision which was more precise compared to previous surgical excision techniques and preserved a greater amount of underlying vital tissue, and the immediate coverage of wounds with skin grafts. A fundamental principle was to abandon the idea that the burn wounds could heal on its own and that early removal of necrotic tissue was instead necessary, without waiting for the sequelae of infection. The covered and grafted areas recover in a few days, unlike those left uncovered that tended to dry out and not heal. This innovative therapeutic approach showed that, when the excision and closure of the wound was carried out early, survival increased and hospitalization decreased, particularly in children (2–4 years).
The rationale behind the early surgical treatment was the drastic decrease in the release of inflammatory mediators and bacterial colonization of wounds led to a decrease in the systemic inflammatory response by reducing metabolic alterations, sepsis, and the risk of multi-organ failure.
The most important limitation of this new approach was the massive blood loss associated with early surgical intervention, the difficulty of an accurate early diagnosis of burn depths, and the availability of adequate wound cover such as with donor skin. However, the successive and notable improvements in terms of resuscitation therapy, the development of epidermal substitutes, and advanced medications to be used as temporary coverage of the lesions, have allowed, over the last 30 years, a wide and universal spread of this new approach which, to date, represents the standard of care in the treatment of burns.
Moreover, to prevent surgery-related complications, several non-surgical (e.g., mechanical) and enzymatic debriding were introduced and nowadays their usage is spreading in burns surgery.
The enzymatic debridement uses proteolytic enzymes derived from microorganisms or plants, sometimes enriched with other substances and with rapid and selective action. The advantage of enzymatic debridement lies in the possibility of not sacrificing large areas of vitality and in the efficacy and rapidity of the intervention; however, it does not represent the gold standard in the treatment of burns due to the variability of the response.
Recently, the use of bromelain has been introduced for the chemical debridement of deep burns through the application of a gel kept in place with a compressive dressing for 4 h. This treatment can be performed at patient’s side, after analgesia with sedation. After this treatment, all wounds are subjected to an evaluation of the effectiveness (% of eschar removed) and a new evaluation of the % of deep and superficial burns, on the basis of the direct visualization of the vital structures possible after enzymatic debridement. More independent research and adequate reporting of adverse events are necessary in order to address issues like indications, pain management and anesthesia, timing and technique of application, after-intervention care and others.
2 Tissue Engineering
Introduced by the Washington National Science Foundation at a meeting in the 1987, the term “Tissue Engineering” refers to a multidisciplinary area of research that aims to regenerate damaged tissues and organs, with the assumption that almost all animal cells can be grown in the laboratory [5]. Wound healing was the first application of the field of tissue engineering techniques and is still one of the most studied areas clinically.
The general principle of tissue engineering is to harvest stem cells from the same patient who is in need of a transplant, and allowing the cells to grow and differentiate on a synthetic matrix in order to reproduce faithfully and three-dimensionally the tissue or organ that is required to be replaced; and finally to implant the newly grown tissue or organ into the patient.
The cells used for regeneration and tissue repair can come from:
-
embryonic stem cells (up to the eighth week of gestation)
-
fetal stem cells (from the eighth week)
-
umbilical cord stem cells
-
adult stem cells (e.g., from bone marrow).
These cells have “plasticity” that is the ability to give rise to cells and tissues different from the original. An example of this would be stem cells present in the bone marrow that can differentiate into hepatocytes and bile duct cells.
Stem cells can be divided into different categories based on their ability to differentiate:
-
Totipotent: potentially capable of creating any type of tissue. They can potentially create a complete organism.
-
Pluripotent or multipotent: they can develop a wide range of tissues and specific cells, but they are unable to develop into a complete organism.
-
Unipotent: partially differentiated cells that can develop a single cell line.
Stem cells can also be divided according to the source of the cells. Autologous cells are from the same person (autografts); allogenic or homologous cells are from the same species (allographs), and heterologous cells are from different species (xenografts, e.g., porcine for humans). When possible, the first choice for donors is autologous cells as allografts and xenografts may present infection, rejection, and sample safety problems.
2.1 Skin Substitutes
Biomaterials are defined as substances that have been engineered, either alone or part of a complex system, to interact with and direct biological systems for medical purposes be it diagnostic or therapeutic [6]. Skin substitutes are an example of biomaterials that have been developed over the past few decades mainly in the field of burns due to the need of burn victims of large amounts of tissue to be able to cover large burn wound surfaces and immediate availability. The development of skin substitutes can be traced back to 1979, where Rheinwald and Green proposed to use keratinocytic lamina cultures, cultivated according to the method rigorously developed by them 4 years before, for the treatment of skin tissue defects [7].
Although skin substitutes can vary greatly in terms of their composition and structure, they typically have dual functions; the immediate cover of wound beds to replicate the barrier function of the skin and prevent infections and to act as a matrix and scaffold for proliferating cells that allow faster healing and better structured replacement tissue.
A scaffold is a three-dimensional matrix that acts as a support for anchor-dependent cells, i.e., these cells perform their function only after having adhered to a substrate and is dependent on the substrate for their proliferation and differentiation, thanks to specific mechanical characteristics which are variable specific to different tissues. Isolated cells lack the ability to maintain the architecture of the tissue because they have no support to guide their growth. Furthermore, using such a scaffold, it is sufficient for a lower number of cells to cover even very large areas, an important aspect especially in the harvesting of autologous cells.
The aim is to create a scaffold or support that resembles as much as possible the natural and original extracellular matrix (ECM). The ECM is a complex porous structure consisting of gelatinous and viscous material. In fact, it is believed that an increased resemblance between the engineered tissue and the native tissue leads to the creation of a phenotype that follows the natural skin and is associated with an increase of the healing process.
Native ECM is formed of a network of heteropolysaccharides and fibrous proteins where significant amounts of interstitial liquid, especially water, are retained. These fiber weaves act as a support and protection for the cells and allow the tissue to retain its form. Pores in the ECM allow nutrients, metabolism products, and oxygen to diffuse to and from each cell. The structural role of the ECM is accompanied by an active task in signal transmission: the matrix, in fact, regulates the development, migration, proliferation, shape, and function of cells in close contact with it.
From a general point of view, skin substitutes can be classified as biological, semi-synthetic, and synthetic skin substitutes:
-
Biological skin substitutes are represented by autografts, allografts, and xenotransplantation. An example of this would be laminae cultures of keratinocytes.
-
Semi-synthetic skin substitutes consist of an engineered extracellular matrix that acts as a scaffold for tissue regeneration and incorporates cells or purified elements of animal and/or human origin.
-
Synthetic skin substitutes, also known as non-biological skin substitutes, consist exclusively of fully engineered matrices. Synthetic scaffolds can contain both natural components (such as polypeptides, hydroxyapatite, hyaluronic acid, glycosaminoglycans (GAGs), fibronectin, collagen) and synthetic components (such as polyglycolic acid, polylactic acid, polyurethane (PUR)).
Biological and synthetic skin substitutes have their advantages and disadvantages. The poor mechanical performance of some biological skin substitutes limits their use as scaffolds. Whereas synthetic substrates, on the other hand, can be customized to maximize mechanical performance and can be enhanced further with the addition of growth and adhesion factors that direct the neoformation of the tissue, so as to create a support characterized not only by a passive role of cellular support, but also one active in signal transmission.
Skin substitutes can also be distinguished via the presence or absence of cells (cellular and acellular):
-
Acellular skin substitutes are substitutes in which, by removing the cellular component, we obtain a scaffold of collagen, hyaluronic acid, and fibronectin.
-
Cellular substitutes contain living cells, such as keratinocytes and fibroblasts, inside the matrix. These cells can be autologous, allogeneic, or obtained from other species.
Finally, according to the skin layer that is replaced, skin substitutes can be divided into three major categories: dermal, epidermal, and dermo-epidermal. Epidermal substitutes require a biopsy from which keratinocytes are isolated and cultured on fibroblasts. A large number of dermal substitutes are currently available on the market or in the experimentation phase. Dermal substitutes give greater stability to the wound, preventing it from contracting. Dermo-epidermal substitutes are also called full-thickness substitutes and are composed of both the epidermal and dermal layers. Autologous fibroblasts and keratinocytes are used in their preparation allogeneic.
With its useful properties of immediate wound coverage and protection, cell recruitment and survival, scaffolding function and advantages that endear them to clinical usage (e.g., ease of use, tear resistance), the field of use of skin substitutes have expanded considerably and have been expanded beyond their use in burns to other areas such as the treatment of chronic arterial or venous ulcers. Table 36.1 shows the structure and composition of the main skin substitutes available on the market. The various biomaterials differ greatly from one to another in terms of template composition, sizes available, also the relative cost of the material.
2.2 Author’s Practice
In our practice, we use a semi-synthetic acellular dermal substitute which is INTEGRA® Dermal Regeneration Template. It can be meshed and non-meshed and it is available in two types:
-
Integra® Dermal Regeneration Template Single Layer (Integra® DRT SL) is a porous matrix of cross-linked bovine tendon collagen fibers and a glycosaminoglycan (chondroitin-6-sulfate), manufactured with a controlled porosity and defined degradation rate. It acts as a scaffold for proliferating cells, and it is used as a one-staged procedure with skin graft apposition.
-
Integra® Dermal Regeneration Template Double Layer (Integra® DRT DL) is a bi-laminar dermal substitute that consists of the same wound facing layer of IDRT-DL and a top removable layer of silicone (polysiloxane) which mimics the epidermal layer of skin. The silicone sheet acts as a protective barrier against infection and controls moisture loss from the wound.
Integra® facilitates the formation of functional neo-dermis. It acts as a support for proliferating cells promoting the addition of collagen and elastin for the formation of a new dermis and new vessels. It guides the formation of an autologous matrix replacing the synthetic one, which is completely biodegradable. The regeneration process is showed in Figs. 36.1–36.7.
INTEGRA®DRT REGENERATION PROCESS—DAY 0: initial situation. DAY 1: Debridement and application. Immediately fluids start to invade the matrix. DAY 7–14: New dermis and vascular network formation. Fibroblasts, lymphocytes, and macrophages begin migrating into the matrix followed by endothelial cells and the template collagen gradually biodegrades, and thus it is replaced by organic collagen. DAY 21: Silicon removal and thin epidermal Autograft. (0.1016–0.1524 mm). DAY 25–56: Regenerated skin
We apply Integra® DRT SL and skin graft in a single surgical step at the time of the debridement whereas Integra® DRT DL application is a two-stage process wherein the first step, after scar excision, it is rinsed with saline and then inset onto the wound and secured. Hence, the collagen matrix action starts with fibroblasts and endothelial cells migration and growth, the bovine collagen is replaced by endogenous collagen, which forms the new dermal layer. Once this occurs (usually within 3–4 weeks), in the second surgical step the silicone layer is removed and an autologous skin graft is applied to the surface of the new dermis.
Compared to other biomaterials as well as traditional skin grafts, the benefit of the use of INTEGRA® template in reconstruction includes:
-
More physiological wound closure with thicker dermal layer rebuilds and higher skin elasticity results.
-
More permanent regeneration of dermal skin.
-
Thinner skin grafts required, almost half thickness of a standard split thickness skin graft, resulting in less scarring and faster healing at the donor site which can be harvested more frequently and in a second surgical step as for no immediate need.
-
It is possible to check the regeneration progress through the silicon layer color change (Fig. 36.2).
-
Patients can begin rehabilitation with Integra® template in place.
-
No reports of rejection. The results have been confirmed by frequent patient follow-up over a 10-year period. Risks with Integra® include take root failure of the matrix or of the skin graft and infection.
3 Regenerative Challenges—New Frontiers
Significant research is underway to further unlock and expand the use of different technologies and different cells are being investigated for their potential use in wound healing. Here, we propose three regenerative approaches already object of study and clinically used in some fields, that can be interesting to be considered also in acute burns for different reasons correlating some characteristics of burns with specific cellular therapies effects (Figs. 36.3, 36.4, 36.5, 36.6, and 36.7).
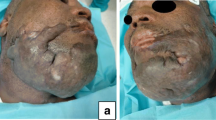
CLINICAL CASE—10% third-degree chemical burn of the face in a 36-year-old female patient. (a) Preoperative view: Initial situation. (b) Intraoperative view: early surgical debridement and skin grafts in 1 step. (c) Postoperative view: result at 3 weeks. We can see there were already scar contractures of the eyelids with closing difficulties of the rim and of the lower lip with labial incontinence. Scars were treated with excision and use of dermal skin substitute in further steps
CLINICAL CASE—30% third-degree chemical burn of the head and the neck in a 70-year-old cardiac patient. (a) Preoperative view: Initial situation. (b) Intraoperative view: early surgical debridement of the necrotic tissue showed a bone exposure. (c) Intraoperative view: INTEGRA® DRT fixation in 1 step at the debridement time; at day 21 the biomaterial was removed and a split thickness skin graft was placed in a second surgical step. (d) Postoperative view: result at 2 months. Unlike the previous clinical case (Fig. 36.1) we achieved the skin closure with no scar contractures, thus no further surgical treatment was required
CLINICAL CASE—20% third/fourth-degree flame burn of the lower limb in a 73-year-old heart patient in anticoagulant therapy. Flap-based reconstructions were not possible in this patient. (a) Preoperative view: the burn presented exposure and necrosis of the tibial crest. (b) Intraoperative view: early surgical debridement including removing of the devitalized bone and necrotic tissue and INTEGRA® DRT placement in one step; at day 21 the biomaterial was removed and a split thickness skin graft was placed in a second surgical step. (c) Postoperative view: result at 2 months. Good epithelialization was achieved even on exposed structures
CLINICAL CASE—Post-flame 4% third-degree burn of the right hand in a 60-year-old male patient. (a) Preoperative view: Initial situation. (b) Intraoperative view: early surgical debridement. (c) Intraoperative view: INTEGRA® DRT apposition in 1 step at the debridement time; At day 21, the biomaterial was removed and a split thickness skin graft was placed in a second surgical step. (d) Postoperative view: Follow up at 6 months. We can appreciate the elasticity and softness of the skin rebuilt by the dermal skin substitute on a difficult area like the hand
CLINICAL CASE—2% second-degree burn of the scalp in a 65-year-old male polytrauma patient. (a) Preoperative view: initial situation. (b) Intraoperative view 1: we proceed with surgical debridement and coverage with INTEGRA® DRT in order to recreate a scaffold suitable for the subsequent direct hair bulb transplantation. (c) Intraoperative view 2: follicular units donor site. (d) Intraoperative view 2: follicular units harvested ready to be grafted. (e) (f) Intraoperative view 2: follicular units grafted. (g) (h) Follow ups: follicular units engrafted. We can appreciate the current state of the wound through the color change of the neo-dermis under the silicon layer over time. The purpose of the procedure was to allow re-epithelialization from the grafted new bulbs and avoid subsequent grafts
3.1 Platelet-Rich Plasma (PRP)
PRP is a suspension of platelets and leucocytes which also contains various growth factors (including TGF-β1, EGF, etc.) and is prepared by the way of multiple centrifuges of autologous blood. PRP has the potential to stimulate and enhance healing as it can stimulate angiogenesis and promote fibroblast proliferation; therefore, it has its application in wound healing. However, there are currently few studies on its application in burn patients. Animal studies however have shown that PRP can be beneficial in reducing inflammation and limiting the amount of cell death in the zone of stasis [8]. Its benefits are not limited to the acute wound healing phase either as studies have shown benefits with PRP in post-burn scars such as the relief of post-burn neuropathic pain [9].
Autologous platelet-rich gel (APG) is a modification of PRP and is prepared by mixing up PRP, thrombin and calcification factors in different ratios to form a gel which then can be applied to wounds [10]. At present however, the clinical benefits of APG on patients with deep grade II burn wounds is still controversial.
A great obstacle in the advancement of PRP use in burns is the high variety in available PRP products, preparation procedures and the content of the PRP itself [11]. Furthermore, platelets in burn patients are affected by the significant inflammatory response these patients tend to exhibit [12] but it is not known if this affects the efficacy of the PRP. Thus, further research is required to advance the use of PRP in burns.
3.2 Adipose-Derived Stem Cells (ASCs)
Fat tissue has been widely used in wounds and burn scars management, considering the fact that it is an ideal source of stem cells, it is available in large quantities and that the harvesting procedure is relatively simple and safe even in patients with extensive burns. Furthermore, some studies have also shown that ASCs are not significantly negatively affected by the post-burn inflammatory process [13].
Enzymatic digestion with collagenase separates the harvested fat tissue into two fractions: a top layer of mature adipocytes and a bottom layer of cells termed the stromal vascular fraction (SVF). This SVF fraction is a rich source of human adipose-derived stem cells (hASCs) which are mesenchymal stem cells (hMSC) with considerable proliferation rates and pluripotent potential. The hMSCs have shown a vasculogenic, angiogenic, antiapoptotic, and antifibrotic induction in vitro and in vivo through trophic factor production mediation (further information about stem cells therapy is available in the chapter: Regenerative Surgery Choices in Burns Sequelae Management).
ASCs have been shown to improve healing even in acute burns in various models although most of the studies have been limited to animal studies. For example, studies have shown that injected ASCs improved wound healing in rats compared to controls; however, this is only the case with autologous but not allogenic stem cells [14].
The mechanism whereby ASCs improves wound healing in burns is unclear. Several mechanisms have been postulated. ASCs have been hypothesized to improve wound healing via a paracrine function as ASCs are known to secrete numerous growth factors such as keratinocyte growth factor, vascular endothelial growth factor, and enzymes such as matrix metallothionein-9 (MMP-9) [15].
The grafted ASCs could also potentially improve wound healing by accumulation at the wound site and differentiating into cells involved in the process of wound healing such as keratinocytes and fibroblasts or into endothelial cells that improve the circulation to the wound. Several studies have shown that it is possible for ASCs to differentiate into stratified epidermis, and it has even been shown that fibroblasts newly differentiated from human ASCs migrate well and produced a superior ECM compared to primary fibroblasts which could translate to improved wound healing [16]. Further studies however are needed to optimize use of fat or ASC grafts in acute burns and reconstructive surgery.
In the future, it may be possible to improve wound healing deficits with the use of topical stem cells. In addition, scar modulation and manipulation therapies will likely become more available as well as we increasingly better understand the complexities of the wound and scarring process.
3.3 Peripheral Blood Mononuclear Cells (PBMNC)
In recent years, many studies have shown the promising potential of peripheral blood mononuclear cells (PBMNC) concentrate, with its regenerative and high angiogenic stimulating capacity, in chronic non-healing ulcers and critical limb ischemia and even in the treatment of scleroderma. A considerable advantage of PBMNC is that it can be produced from peripheral blood without the need for the significantly more invasive procedures required to extract bone marrow and fat concentrates.
Macrophages have been shown to be critical during most phases of the tissue healing process, but the mechanisms by which they change phenotypes to stimulate tissue repair, fibrosis, or full regeneration remain unclear. Thus, further effort is required to understand the contributions of the different macrophage populations and activation states in multiple organ systems. For instance, inflammatory macrophages mature (M1) into anti-inflammatory macrophages (M2) in certain type of tissues, while a distinct population of anti-inflammatory macrophages is mobilized in others.
Depending on the tissue or organ targeted, one could develop regenerative strategies based on the use of the immuno-modulatory effect of ASCs to stimulate macrophage polarization or recruit pro-wound healing macrophage subsets. Mesenchymal stem cells (MSC, which include ASCs) can be utilized to modulate the immune system through the secretion of different molecules including anti-inflammatory and growth factors. Studies in recent years have shown that MSCs can regulate the function of macrophages such as inducing them to adapt an enhanced regulatory phenotype [17] with higher phagocytic activity a more regenerative and immuno-modulatory function [18]. But the effect seems to be bi-directional as the PBMNC have also been shown to enhance the regenerative effect of MSCs, as their regeneration capacity is influenced and regulated by the local immune response, particularly from the macrophages as coordinators of tissue repair and regeneration [19].
As the ultimate goal in improving wound healing in burn patients is to prevent or reduce subsequent pathological scar formation, it is important to note that studies have found that macrophage depletion in experimental in vivo studies reduces hypertrophic scar formation [20] thus treatment may be geared towards reducing the number of PBMNCs in wounds or direct them towards anti-inflammatory phenotypes.
There are currently no studies that the authors are aware of that utilize PBMNC alone or in conjunction with ASCs in the treatment of acute burns; however, in consideration of the systemic inflammatory state and immune alterations in burns patients, we believe it holds a great potential, probably mainly for deep burns where there might be also a vascularity issue and small burns where the quality of the PMBNC can be unaltered. The next generation of regenerative therapies may evolve from typical biomaterial-, stem cell-, or growth factor-centric approaches to an immune-centric approach.
4 Conclusion
Basic science research and translational findings continue to advance our knowledge of burns and assist in the development of novel treatment approaches. Unfortunately, many of these products are market and industry driven, with little prospective randomized comparative studies evaluating their efficacy. However, the concept of wound care can still benefit patients, even if it has been aggressively marketed by centers which are companies not always staffed by personnel with sufficient background in surgery and/or wound healing. Future investigations and studies should focus on the combination of cellular therapies and standard procedures such as skin autografting, allografting, and xenografting. These improvements are necessary to provide better functional and visual outcomes for burned patients.
References
Chipp E, Charles L, Thomas C, Whiting K, Moiemen N, Wilson Y. A prospective study of time to healing and hypertrophic scarring in paediatric burns: everyday counts. Burns Trauma. 2017;5:3.
Navickis RJ, Greenhalgh DG, Wilkes MM. Albumin in burn shock resuscitation: a meta-analysis of controlled clinical studies. J Burn Care Res. 2016;37(3):e268–e78.
Nunez Lopez O, Cambiaso-Daniel J, Branski LK, Norbury WB, Herndon DN. Predicting and managing sepsis in burn patients: current perspectives. Ther Clin Risk Manag. 2017;13:1107–17.
Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10(12):1103–8.
Parveen S, Krishnakumar K, Sahoo S. New era in health care: tissue engineering. J Stem Cells Regen Med. 2006;1(1):8–24.
Bergmann CP, Stumpf A. Biomaterials. In: Bergmann C, Stumpf A, editors. Dental ceramics: microstructure, properties and degradation. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. p. 9–13.
Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–43.
Uraloglu M, Ural A, Efe G, Yulug E, Livaoglu M, Karacal N. The effect of platelet-rich plasma on the zone of stasis and apoptosis in an experimental burn model. Plast Surg (Oakville, ON). 2019;27(2):173–81.
Huang S-H, Wu S-H, Lee S-S, Lin Y-N, Chai C-Y, Lai C-S, et al. Platelet-rich plasma injection in burn scar areas alleviates neuropathic scar pain. Int J Med Sci. 2018;15(3):238–47.
Liu J, Qu W, Li R, Zheng C, Zhang L. Efficacy of autologous platelet-rich gel in the treatment of deep grade II burn wounds. Int J Clin Exp Med. 2018;11(3):2654–9.
Marck RE, Middelkoop E, Breederveld RS. Considerations on the use of platelet-rich plasma, specifically for burn treatment. J Burn Care Res. 2014;35(3):219–27.
Marck RE, van der Bijl I, Korsten H, Lorinser J, de Korte D, Middelkoop E. Activation, function and content of platelets in burn patients. Platelets. 2019;30(3):396–402.
Prasai A, El Ayadi A, Mifflin RC, Wetzel MD, Andersen CR, Redl H, et al. Characterization of adipose-derived stem cells following burn injury. Stem Cell Rev Rep. 2017;13(6):781–92.
Chang YW, Wu YC, Huang SH, Wang HD, Kuo YR, Lee SS. Autologous and not allogeneic adipose-derived stem cells improve acute burn wound healing. PLoS One. 2018;13(5):e0197744.
Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol. 2012;24(2):136–43.
Gersch RP, Raum JC, Calvert C, Percec I. Fibroblasts derived from human adipose stem cells produce more effective extracellular matrix and migrate faster compared to primary dermal fibroblasts. Aesthet Surg J. 2019;40(1):108–17.
Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–53. https://doi.org/10.1016/j.exphem.2009.09.004.
Lu W, Fu C, Song L, Yao Y, Zhang X, Chen Z, Li Y, Ma G, Shen C. Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. Int J Cardiol. 2012;165:333–40.
Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration : from mechanism to therapy. Nat Med. 2014;20:857–69.
Feng Y, Sun Z-L, Liu S-Y, Wu J-J, Zhao B-H, Lv G-Z, Du Y, Yu S, Yang M-L, Yuan F-L, Zhou X-J. Direct and indirect roles of macrophages in hypertrophic scar formation. Front Physiol. 2019;10:1101. https://doi.org/10.3389/fphys.2019.01101.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Agovino, A., d’Alessio, M., Lee, K., Bloanca, V., Crainiceanu, Z., d’Alessio, R. (2022). Acute Burns Management: The Current Role of Regenerative Surgery and its Challenges. In: Kalaaji, A. (eds) Plastic and Aesthetic Regenerative Surgery and Fat Grafting. Springer, Cham. https://doi.org/10.1007/978-3-030-77455-4_36
Download citation
DOI: https://doi.org/10.1007/978-3-030-77455-4_36
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77454-7
Online ISBN: 978-3-030-77455-4
eBook Packages: MedicineMedicine (R0)