Abstract
Purpose
Aim of this study was to compare the use of split-thickness skin graft (STSG) with and without additional MatriDerm® application in a predominantly one-step procedure for the treatment of severe traumatic soft tissue defects of the lower limb.
Methods
This retrospective study included patients treated in a European level I trauma center between June 2013 and July 2018 in terms of a severe traumatic soft tissue defect of the lower extremity using STSG alone or in combination with the acellular dermal substitute MatriDerm®. The healing of the soft tissue defect was measured by assessment of the take rate. Outcome quality of the scar tissue was assessed using the Vancouver Scar Scale.
Results
A total of 147 cases were included in this study. The overall healing rate (number of patients with take rate ≥ 75%) was 88/147 (60%) and did not demonstrate significant differences between the treatment groups (p = 0.15). Despite the difference in wound complexity between the treatment groups, there was no difference regarding the scar tissue quality 12 months postoperatively. In about 25% of all cases, a post-operative event was mentioned that had to be revised surgically.
Conclusion
Surgical treatment with STSG and additional MatriDerm® application can be recommended as satisfactory alternative for dermis replacement in patients with severe skin defects, independent of age. The additional MatriDerm® use allows for bridging of exposed ligaments, tendons, vessels or bones without relevant differences in cosmetical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are several treatment options for the treatment of complex soft tissue defects of the lower leg of various causes, such as split-thickness skin graft (STSG), local or free flaps [1,2,3]. STSG is considered the first step of the reconstructive ladder, however nowadays a common approach is to combine the use of STSG with a dermal substitute. Dermal substitutes can bridge the steps of the reconstructive ladder, by replacing the missing dermis without the need to perform a flap procedure. MatriDerm® is a collagen-elastin dermal matrix used for dermis replacement providing a basic framework for the regeneration of the dermis [4,5,6].
There are several publications on the use of MatriDerm® in the clinical literature. However, only a few large case series or controlled studies are available for defects in the lower extremities: a retrospective analysis of 56 soft tissue defects treated with one-step reconstruction using dermal skin substitutes demonstrated, that in cases where flap surgery is unavailable or undesirable, the use of dermal skin substitutes in combination with split-thickness skin grafting can be a promising alternative for covering severe soft tissue defects [7]. In a retrospective study of 34 patients with defects of the lower extremities, the acellular dermal matrix presented an improvement in skin quality with elastin and collagen. A skin graft along with a simultaneous application of MatriDerm® demonstrated to be safe and effective and led to a significantly better outcome from the perspective of skin elasticity [8]. A recently provided prospective study in 60 patients with diabetic foot ulcer demonstrated that MatriDerm® enabled effective healing and improved elasticity [9].
The purpose of this study was to compare the use of STSG with and without additional MatriDerm® application for the treatment of severe traumatic soft tissue defects of the lower limb and to evaluate the occurrence of adverse events and the outcome quality of the scar tissue.
Methods
This retrospective chart review included patients who were treated surgically in a level I trauma center between June 2013 and July 2018 for severe traumatic soft tissue defects with exposed structures, such as tendons, ligaments, vessels, or bone of the lower extremities, using single autologous STSG (group 1) or in combination with the application of the dermal substitute MatriDerm® (group 2) (MedSkin Solutions Dr. Suwelack AG, Billerbeck, Germany). MatriDerm® was used based on manufacturer’s recommendations in all cases. Data collection was performed between January 2019 and March 2020. During this range of time, all available documents for these cases were retrieved from the electronic medical records and from the hospital archives. Patients who met the following inclusion criteria were eligible for participation: Age over 18 years, severe soft tissue defect of the lower extremities. Patients with tumorous soft tissue defect were excluded from the study.
Standard surgical procedure
In group 1, debridement of the soft tissue defect was performed followed by hemostasis. Then, STSG (thickness 0.2 mm) was adapted to the size of the wound and placed on the defect zone. The skin graft was used in mesh or sheet version. After a non-adherent contact layer was placed on top of the STSG, either conventional wound dressing, or a negative pressure wound therapy (NPWT) device with a pressure setting of 80 mmHg was applied (RENASYS TOUCH, Smith & Nephew GmbH, Hamburg, Germany) [10]. After 5 days, NPWT was removed at bedside.
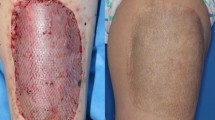
Also in group 2, debridement of the wound was done followed by hemostasis (Fig. 1a–c). MatriDerm® was placed either in a one-step procedure in dry condition directly in the wound base of the defect (Fig. 1d, e) and moistened in situ with sterile saline or ringer solution. STSG was placed on top MatriDerm® (thickness 1 mm, size 52 × 74 mm) in the same procedure in the one-step approach. In the two-step procedure, MatriDerm® was placed on the wound base, covered with a non-adherent contact layer, and NPWT was applied for 5 days [10]. Coverage with STSG was performed in a second surgical step after 5 days.
a–c Forty-five-year-old major trauma patient secondary transferred from another hospital with decollement injury and extensive necrosis of the left lower leg. Debridement of the soft tissue defect was performed followed by hemostasis. d, e MatriDerm® was placed in dry condition directly in the wound base of the defect. STSG was placed above MatriDerm®. f, g Clinical results with almost free range of motion of the ankle joint and excellent VSS of 0 points
All surgical procedures were performed by the same four senior surgeons according to the above described surgical treatment concept.
Outcome measures
The take rate of the soft tissue defect was measured as part of a routine protocol by assessment at day 7 postoperatively. Subsequently, photo documentation was performed every two days until day 18 as part of the wound checks. The healing rate was defined as number of patients with a take rate ≥ 75%. Assessment was performed by two independent investigators without knowledge of the treatment. The duration of hospitalization was assessed. Safety was analyzed by recording of post-operative complications and documented surgery-related adverse events. Clinical outcome (Fig. 1f, g) was assessed using the Vancouver Scar Scale (VSS) one month and 12 months postoperatively in two treatment groups (STSG: n = 44; STSG + MatriDerm®: n = 69) including all cases with complete documentation of treatment progress (n = 113). The VSS detects the four variables vascularity, height/thickness, pliability, and pigmentation demonstrating the quality of the scar. This scoring system ranges between 0 and 13 with 0 as the best result, representing healthy skin [11].
Statistical analysis
Given the nature of this research, a statistical sample size calculation using an unpaired t test has not been performed. Data included in this research were collected as a consecutive case series from the Investigator’s standard patient population in an Excel data sheet (Excel 2018, Microsoft Corp., Redmond, WA, USA). Rates were compared by chi-squared test. Continuous and metric data were presented by mean and standard deviation and compared by Wilcoxon signed-rank test.
Results
A total of 147 cases (134 patients, 13 patients with both legs involved) were included in this evaluation. Sixty-three soft tissue defects in 55 patients treated with STSG alone (group 1) and 84 severe soft tissue defects, such as dermis, subcutaneous tissues, tendons, ligaments, fascia, vessels or bone, in 79 patients treated with STSG in combination with MatriDerm® (group 2) met the eligibility criteria. Patients with a severe soft tissue defect of the lower extremities were predominantly male with a gender ratio of approximately 3/1 (99 male/ 35 female). Patients’ age ranged from 18 to 90 years with a mean age of 52 ± 17 years. The mean age was 53 ± 18 years in group 1 and 52 ± 16 years in group 2. Frequencies of relevant medical history as documented in patients’ medical reports are listed in Table 1.
Soft tissue defects consisted of 18 open fractures with extensive decollement, 43 thermic and chemical burns, 78 severe soft tissue lesions, and 8 ulcers. Overall, soft tissue defects were more severe in group 2 (Fig. 2). The majority of open fractures were treated using MatriDerm® in combination with STSG. In contrast, injuries due to burns were treated with STSG alone in 31 cases of second-degree deep partial thickness burns, and with STSG plus MatriDerm® in 12 third-degree full-thickness burns. There was no difference in terms of frequency between left and right leg. The defect was predominately located in the distal lower leg and foot. About one-third of all patients demonstrated germ colonization in the intra-operative samples. Staphylococcus aureus and Staphylococcus epidermidis were found most frequently (Table 2).
The number of scheduled surgical revisions until definitive coverage was higher in group 2 (3.4 ± 2.4) than in group 1 (2.6 ± 1.5; p = 0.03), indicating that the treatment was used in complex wounds following more severe injuries. In a subgroup of 92 patients with wounds without exposed anatomical structures, the number of prior surgical revisions was 2.5 ± 1.4 in group 1 and 3.0 ± 2.2 in group 2. A two-step procedure of MatriDerm® treatment was recorded in 15 patients with mean hospitalization of 39 ± 25 days. NWPT was the post-operative treatment in 140 cases (Table 3). Immobilization with a cast was used in one-third of the cases.
The overall healing rate (number of patients with take rate ≥ 75%) was 88/147 (60%). In group 1, healing rate was 42/63 (67%) compared to group 2 with a healing rate of 46/84 (55%) (p = 0.15). The results regarding the VSS of two treatment groups one month and 12 months postoperatively are presented in Fig. 3. In an additional subgroup analysis depending on different exposed soft tissues, in the largest subgroup with exposed muscle, there was a trend toward better VSS results after use of STSG in combination with additional MatriDerm® (VSS = 4.4 points) than after use of STSG only (VSS = 5.3 points) (p = 0.112).
Scar quality assessment according to the Vancouver Scar Scale [11]
The number of days in hospital was significantly less in group 1 (26 days ± 17 days) than in group 2 (36 ± 19 days) (p = 0.001).
In about 25% of the cases, a post-operative event was mentioned that had to be revised surgically. The majority of the events included healing disturbances, such as remaining defects, necrosis, or delayed healing (Table 4). Complications were recorded after 5–285 days postoperatively. The events recorded after more than 100 days included scar instability, fistula, and swelling. The number of cases with at least one necessary surgical revision was 4 in group 1 versus 18 in group 2 (p = 0.02).
Discussion
The use of STSG may result in clinically relevant complications, such as hypertrophic scaring, keloids or disabling contractures, especially across joint surfaces [12, 13]. This has led to the development of dermal templates to improve the reconstruction of the dermis, which is very important for the quality and functionality of the reconstructed skin [14,15,16,17,18].
In this study, a large collective of 147 cases was included of whom almost two-thirds were treated using STSG in combination with MatriDerm®. Soft tissue defects were predominantly located at distal lower leg and foot. Deeper wounds with exposed tendons, ligaments and bone were treated with additional MatriDerm®. While these wounds were found to be clinically complicated, covering with a flap was not possible due to concomitant diseases. Especially in patients with disturbances of arterial blood flow treatment with MatriDerm® in addition to STSG seemed to be the best alternative therapeutic option prior to amputation, when skin flaps were not possible due to reduced circulation. Interestingly, one-third of all patients demonstrated presence of bacteria at the time of surgery. More patients with germ colonization were treated with STSG only.
The healing rate was slightly lower in the group treated with STSG plus MatriDerm® compared to the group with STSG. After detailed processing of these cases, this difference seemed to be related to the fact that complex wounds with exposed ligaments were predominately treated with additional MatriDerm®. A relevant difference could not be found between the treatment groups in terms of the scar quality according to the VSS. Improved skin elasticity as seen in other studies [7, 8] was found in some patients treated with STSG combined with MatriDerm® demonstrating that good clinical results and scar quality might be reached by this treatment technique in severe wounds.
The overall complication rate was 25% in the current study. In 15% of the cases, a surgical revision had to be performed. The number of patients with documented adverse events (33%) or necessary revision surgery (21%) was higher in the group treated with STSG and additional MatriDerm® application than in the comparison group. In addition, the mean number of hospitalization days was higher in this group. This might reflect that the more complex wounds and more patients with concomitant diseases were treated using this method (Fig. 2, Table 1), but on the other hand, also the treatment regime itself may have been a reason for revision surgery. Furthermore, we observed that open fractures were associated with more complicated wounds. Therefore, more often, treatment with MatriDerm® was chosen since MatriDerm® facilitates the bridging of exposed soft tissues, such as tendons, ligaments, and vessels, and was then used instead. In contrast, due to the frequent use of NPWT in burns, the dermis quite often remained intact so that treatment with MatriDerm® was not necessary [19].
Another aspect to be discussed is that in the current study, a longer duration of treatment in hospital and more surgical revisions were found in the group with additional MatriDerm® use. Although this is actually disadvantageous compared to the standard therapy with STSG coverage alone, it must be taken into account that treatment with MatriDerm® may avoid plastic flap coverage, joint disarticulation, or limb amputation in extreme cases. In this regard, it is an established salvage procedure for the treatment of highly problematic wounds in our setting.
The limitations of this study include the variety of age of patients as well as the sequential nature of the two treatment groups. However, the most important outcome assessment of this study was to evaluate the healing rate of the soft tissue defect using the surgical technique as described above. The decision to use MatriDerm® was dependent on the intra-operative assessment and the discretion of the treating surgeon, and was not subject to a randomized or blinded protocol. Since the treatment group with STSG and MatriDerm® included some cases with a two-stage treatment concept in addition to those with a one-stage approach, a bias could not be excluded with certainty here. STSG and STSG plus MatriDerm® both were used with or without NPWT postoperatively. As the use of NPWT may have an impact on the take rate of STSG, this might be a confounder imparing the statistical power of the study resulting in a potential bias. Nevertheless, the power of the retrospective study includes the large number of patients managed according to clear inclusion and exclusion criteria, with a consistent treatment concept in the same hospital by the same group of experienced surgeons.
Conclusion
Based on the good clinical results of this study, surgical treatment with STSG and additional MatriDerm® application can be recommended as satisfactory alternative for dermis replacement in patients with severe skin defects, independent of age. The additional MatriDerm® use allows for bridging of exposed ligaments, tendons, vessels or bones. Relevant differences in cosmetical outcome were not observed.
Availability of data and materials
The datasets analyzed during the current work are available from the corresponding author upon reasonable request.
References
Bertolli E, Campagnari M, Molina AS, Macedo MP, Pinto CA, Cunha IW, Duprat Neto JP. Artificial dermis (Matriderm) followed by skin graft as an option in dermatofibrosarcoma protuberans with complete circumferential and peripheral deep margin assessment. Int Wound J. 2015;12(5):545–7. https://doi.org/10.1111/iwj.12157.
Lempert M, Halvachizadeh S, Salfelder CC, Neuhaus V, Pape HC, Jukema GN. Long-term experience with a collagen-elastin scaffold in combination with split-thickness skin grafts for the treatment of full-thickness soft tissue defects: improvements in outcome - a retrospective cohort study and case report. Langenbecks Arch Surg. 2021. https://doi.org/10.1007/s00423-021-02224-7.
Jeon H, Kim J, Yeo H, Jeong H, Son D, Han K. Treatment of diabetic foot ulcer using matriderm in comparison with a skin graft. Arch Plast Surg. 2013;40(4):403–8. https://doi.org/10.5999/aps.2013.40.4.403.
Lempert M, Pape HC, Jukema GN. Salvage of a mangled limb with Matriderm() augmented split-skin grafting and maggot biodebridement. Clin Case Rep. 2021;9(9):e04676. https://doi.org/10.1002/ccr3.4676.
Niedermueller B, Singer G, Pickl P, Jesacher M. Necrotizing fasciitis of the hand and forearm: acute surgical treatment and defect reconstruction with MatriDerm and split-thickness skin graft. Unfallchirurg. 2018;121(3):256–60. https://doi.org/10.1007/s00113-017-0451-x.
Almeida IR, Gonçalves AC, Corrêa FB, Castro JCD, Guirro ECO, Junior JAF, Coltro PS. Evaluation of clinical and biomechanical features of scars resulting from the treatment of burn contractures comparing acellular dermal matrices: a randomized clinical trial. Ann Surg. 2022. https://doi.org/10.1097/SLA.0000000000005371.
Gümbel D, Ackerl M, Napp M, Daeschlein G, Spranger N, Stope MB, Ekkernkamp A, Matthes G. Retrospective analysis of 56 soft tissue defects treated with one-stage reconstruction using dermal skin substitutes. Dtsch Dermatol Ges. 2016;14(6):595–601. https://doi.org/10.1111/ddg.12874.
Choi JY, Kim SH, Oh GJ, Roh SG, Lee NH, Yang KM. Management of defects on lower extremities with the use of matriderm and skin graft. Arch Plast Surg. 2014;41(4):337–43. https://doi.org/10.5999/aps.2014.41.4.337.
Min JH, Yun IS, Lew DH, Roh TS, Lee WJ. The use of matriderm and autologous skin graft in the treatment of full thickness skin defects. Arch Plast Surg. 2014;41(4):330–6. https://doi.org/10.5999/aps.2014.41.4.330.
Goutos I, Ghosh SJ. Gauze-based negative pressure wound therapy as an adjunct to collagen-elastin dermal template resurfacing. J Wound Care. 2011;20(2):55–6. https://doi.org/10.12968/jowc.2011.20.2.55.
Sullivan T, Smith J, Kermode J, McIver E, Courtemanche DJ. Rating the burn scar. J Burn Care Rehabil. 1990;11(3):256–60. https://doi.org/10.1097/00004630-199005000-00014.
Holavanahalli RK, Helm PA, Kowalske KJ. Long-term outcomes in patients surviving large burns: the skin. J Burn Care Res. 2010;31(4):631–9.
Petersen W, Rahmanian-Schwarz A, Werner JO, Schiefer J, Rothenberger J, Hübner G, Schaller HE, Held M. The use of collagen-based matrices in the treatment of full-thickness wounds. Burns. 2016;42(6):1257–64. https://doi.org/10.1016/j.burns.2016.03.017.
Halim AS, Khoo TL, Yussof SJM. Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg. 2010;43(Suppl):S23–8.
Haslik W, Lumenta DB, Kamolz LP, Frey M. The use of a collagen– elastin matrix as dermal regeneration template for the treatment of full-thickness skin defects. Adv Wound Care. 2010;1:438–44.
Coulie J, Gerdom A, Chrelias T, Lengelé B, Coyette M. The use of MATRIDERM(R) as a single stage salvage procedure to cover exposed dura Mater. JPRAS Open. 2020;27:53–7. https://doi.org/10.1016/j.jpra.2020.09.009.
Ryssel H, Andreas RC, Germann G, Otte M, Gazyakan E. Single-stage MatriDerm and skin grafting as an alternative reconstruction in high-voltage injuries. Int Wound J. 2010;7(5):385–92.
Ryssel H, Germann G, Czermak C, Kloeters O, Gazyakan E, Riedel K. MatriDerm® in depth-adjusted reconstruction of necrotising fasciitis defects. Burns. 2010;36(7):1107–11.
Jiang ZY, Yu XT, Liao XC, Liu MZ, Fu ZH, Min DH, Guo GH. Negative-pressure wound therapy in skin grafts: a systematic review and meta-analysis of randomized controlled trials. Burns. 2021;47(4):747–55. https://doi.org/10.1016/j.burns.2021.02.012.
Funding
Although none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non-profit organization with which one or more of the authors are associated.
Author information
Authors and Affiliations
Contributions
Study conception and design: BW, MÖ. Acquisition, analysis and/or interpretation of data: BW, MÖ, CvR. Drafting/revision of the work for intellectual content and context: BW, CvR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Britta Wallner and Markus Öhlbauer report institutional provision of materials (paid to BG Unfallklinik Murnau) from MedSkin Solutions Dr. Suwelack AG, Billerbeck, Germany. Christian von Rüden declares that he has no conflict of interest related to this study.
Ethical approval
The project was conducted in compliance with the project plan, the quality standards of the institution (ISO 9001; 2015) and the International Council for Harmonization Good Clinical Practice (ICH‐GCP) Guidelines. Ethics Committee approval is not required for this retrospective collection of de-identified data according to the Ethics Committee of the Bavarian State Medical Association.
Consent for publication
The research posed negligible risk to privacy. Multiple steps were taken to ensure subject privacy, including the protection of the Personal Health Identifiers and the removal of subject identifiers from the study data.
Informed consent
Obtaining informed consent is not required for an anonymous data collection according to Data Protection Law.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wallner, B., Öhlbauer, M. & von Rüden, C. Long-term results of split-thickness skin grafting with and without additional dermal matrix in severe traumatic soft tissue defects of the lower limb. Eur J Trauma Emerg Surg 49, 551–557 (2023). https://doi.org/10.1007/s00068-022-02107-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-022-02107-6