Abstract
Purpose
Cerebral hyperperfusion syndrome (CHPS) can result after anastomotic surgery as the reperfusion is established in chronically ischemic cerebral territories in patients of moyamoya disease (MMD). In this study, we have evaluated the feasibility of arterial spin labelling (ASL) perfusion MRI to predict cerebral hyperperfusion syndrome based on changes of cerebral blood flow (CBF) after revascularisation surgery in patients of MMD.
Methods
Our prospective study included 25 patients with MMD who underwent superficial temporal artery-middle cerebral artery (STA-MCA) bypass with or without dural/muscle synangiosis. ASL MRI was performed before and 1–7 days after surgery. On the side planned for operation, 5-mm ROI circle was drawn on the predetermined regions in frontal lobe, temporal lobe, parietal lobe and basal ganglia in proximal and distal territories of MCA to calculate ipsilateral CBF values (CBFi). An attempt was made to select the same location on contralateral side (non-operative) (CBFc) for each measurement for calculation of hemispheric normalised CBF (nCBFh) ratios. To adjust for inter individual variation among MR imagers and CBF, additional regions of interest were drawn within the cerebellum (CBFcbl) for cerebellar CBF normalised ratios (nCBFCbl).
Results
Of the 25 patients (26 operated hemispheres), 5 patients showed significant immediate postoperative symptoms suggestive of CHPS. Based on our findings, sensitivity and specificity of ASL perfusion to detect CHPS were evaluated. ASL was found to have 47–100% sensitivity and 45–88% specificity to detect CHPS. We have tried to calculate the prevalence of CHPS in postoperative patients of moyamoya disease, which in our study ranged from 6.83 to 40.70%.
Conclusion
Based on our results, we concluded that ASL perfusion is an appropriate alternative to standard nuclear medicine studies to monitor the changes in perfusion after STA-MCA bypass surgery in moyamoya patients. ASL MR perfusion can be used to identify changes in cerebral blood flow (CBF) for early detection of cerebral hyperperfusion syndrome in patients with otherwise normal conventional MRI sequences with very high sensitivity but moderate specificity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Initially described in 1957, moyamoya ‘disease’ (MMD) is a progressive, occlusive vasculopathy involving intracranial vessels, especially anterior circulation [1], predisposing the affected individuals to stroke, either ischemic or haemorrhagic. Progressive stenosis of anterior intracranial vasculature stimulates the development and compensation of circulation through multiple small collateral vessels, from posterior circulation and external carotid artery branches, at the base of the brain, on the cortical surface and leptomeninges. Anastomotic bypass surgery is the mainstay of treatment. After revascularisation surgery, blood flow in the chronically ischemic areas can lead to cerebral hyperperfusion syndrome (CHPS); the incidence of which has been reported between 15.0 and 38.2% [2, 3]. This shows the importance of evaluating postoperative changes in CBF after the anastomosis in chronic ischemic territories. MR imaging with ASL perfusion has been successfully used to assess CBF qualitatively and quantitatively avoiding radiation exposure and contrast agents. This is particularly useful in paediatric patients [4]. The aim of the present study is to prospectively assess changes in cerebral haemodynamics in the patients of moyamoya disease before and after STA-MCA bypass using 3D pseudocontinuous ASL (3DPCASL) MR imaging to diagnose CHPS on the basis of the degree of increased normalised CBF in the early postoperative period in symptomatic or asymptomatic patients. Sensitivity, specificity and accuracy of ASL for detection of cerebral perfusion per territory before and after revascularisation surgery were also calculated. We hypothesise that non-contrast radiation-free 3DPCASL MRI-based CBF measurement in the initial stages after revascularisation surgery is an effective tool for early diagnosis and optimal management of cerebral hyperperfusion syndrome.
Materials and methods
Study design
This prospective study was conducted on patients diagnosed with moyamoya disease and admitted for surgical revascularisation from May 2017 up to December2018.
Inclusion criteria
Patients of all age groups with moyamoya disease admitted for surgical treatment.
Exclusion criteria
-
Associated any other neurosurgical disease
-
Any contraindication for MR imaging
-
Postoperative general status of patient where MRI was not feasible
Methodology
A total of 25 patients who were operated for moyamoya disease from May 2017 to November 2018 were included in the study. All the patients with clinical symptoms of occlusive vascular disease were diagnosed as MMD based on imaging findings. These patients were admitted for revascularisation surgery. All the patients underwent routine preoperative MRI with ASL perfusion sequence to assess baseline CBF status within a week before surgery.
MR imaging protocol
MRI was performed on a 1.5-T MR system (Magnetom Aera; Siemens Healthineers, Germany) by using an 8-channel head-neck-spine coil. Brain MR imaging protocol included a 3D T1-weighted gradient echo sequence (MPRAGE), 3D time-of-flight head and neck MR Angiography, Axial FLAIR, Axial T2 TSE, Diffusion and Susceptibility weighted sequences. Non-contrast perfusion using background suppressed 3D pseudocontinuousASL MRI (40 axial partitions of 3-mm thickness; FOV 192 mm; TE 36 ms; TR 4000 ms; labelling duration 1500 ms; post-labelling delay 1500 ms; flip angle 180°; acquisition time 4 min 17 s). No contrast injection was used. After scanning, the raw perfusion-weighted images were transferred to the workstation (syngo software, Siemens Healthineers) for further processing. Colour-coded maps were generated from raw images and were analysed at appropriate window settings in viewing a task card. On the side planned for operation, 5-mm ROI circle was drawn on the predetermined regions in the frontal lobe, temporal lobe, parietal lobe and basal ganglia in proximal and distal territories of MCA to calculate ipsilateral CBF values (CBFi) (Fig. 1). A similar location was selected on a non-operative side (CBFc) for each measurement for calculation of hemispheric normalised CBF (nCBFh) ratios. To adjust for the inter-individual variation among MR imagers and CBF, additional regions of interest were drawn within the cerebellum (CBFcbl) for cerebellar CBF normalised ratios (nCBFCbl), based on the fact that the cerebellum is spared in MMD. Thus, normalised CBF ratios adjusted to cerebellum (nCBFCbl = CBFc/CBFCbl) and normalised hemispheric CBF ratios adjusted to contralateral side (nCBFh = CBFi/CBFc) were calculated to minimise an inhomogeneous perfusion effect. Sensitivity, specificity and accuracy of ASL for detection of cerebral perfusion per territory were calculated from Chi-squared tables of contingency with clinical assessment for CHPS serving as the standard of reference. Routine MR Sequences were also analysed to look for cerebral infarcts, cerebral haemorrhage, etc. MRA images were analysed for Suzuki staging of moyamoya disease to assess collateral circulation.
Follow-up imaging
After revascularisation surgery, the patients were assessed based on their clinical status for at least seven postoperative days. Patients who developed new neurological deficits with suspicion of cerebral ischemic attacks or intracranial haemorrhage underwent a follow-up MRI with ASL perfusion on the same day of symptom onset to look for cerebral hyperperfusion syndrome. CBF values were recorded in a similar manner as described above. Asymptomatic patients underwent a follow-up MRI on the seventh postoperative day to look for changes in perfusion after revascularisation. All patients also underwent non-contrast TOF MR angiography after surgery in order to confirm the bypass patency.
Statistical analysis
All data management and analyses were conducted using SPSS statistics (version 20.0; IBM Corporation, Armonk, NY, USA). Changes in CBF were compared by using the one-way analysis of variance with Bonferroni correction for multiple comparisons. Patency of STA-MCA anastomosis was assessed and graded using a ‘bright vessel artefact’ compared between pre- and postoperative ASL images. A McNemar two-tailed test was conducted to compare the “bright vessel appearance” on ASL with visual assessment of anastomotic site patency on time of flight (TOF) MR angiogram images. P value < 0.05 was kept as statistically significant. A paired t test was used for CBF comparison in preoperative and immediate postoperative MR scans. Data was normalised using the Kolmogorov-Smirnov test.
Results
Out of 25 patients, 20 were paediatric (< 18 years) and 5 were adult patients with 13 (52%) males and 12 (48%) females (male to female ratio of 1:0.76). The classical bimodal age distribution described in the literature [3] was not seen among these patients, probably because of the relatively small sample size. Motor weakness was seen in 19 patients (89.1%), seizures in 7 patients (47.8%), ICH in 3 adult patients, headache in 8 patients and symptoms of transient ischemic attacks (TIA) in 3 patients. The clinical features at presentation are detailed in Table 1. All the patients had Glasgow coma scale of 15 at presentation, no family history of moyamoya disease or any associated disorder. Pre- and postoperative MRI scans with ASL perfusion were done in all our patients and were statistically analysed. Non-contrast TOF MR angiogram was done to assess the status of cervical carotids, intracranial vasculature, leptomeningeal cortical and basal collaterals, Suzuki grading of moyamoya vasculature and anatomic variants, if any, in intracranial vasculature. The supraclinoid ICA or ICA terminus demonstrated occlusion in all the patients in preoperative imaging with varying degrees of occlusion of the A1 ACA and M1 MCA vessels. This is in agreement with the criteria for the diagnosis of moyamoya disease. The Suzuki classification was used to grade the occlusion and presence of moyamoya vessels in preoperative patients. Among 50 hemispheres analysed, 7 were grade II, 17 were grade III, 11 were grade IV, and 14 were grade V. No moyamoya changes were seen in one cerebral hemispheric vasculature. Persistent trigeminal artery was seen in one patient.
Out of the 25 patients (26 hemispheres) operated upon, unilateral revascularisation was done in 24 patients and bilateral in 1 patient. Eleven patients underwent surgery on the right hemisphere and 13 patients on the left side. A combined procedure (STA-MCA bypass with EDAMS) was the most commonly performed surgery in our series (22/26 hemispheres). Only the STA-MCA bypass was done in 4 patients. The most common reason for combined procedure in such patients was inadequate calibre of the donor (STA) or recipient artery (MCA branch) on that side. No surgery related complications were encountered in any patient. Clinically cerebral hyperperfusion syndrome (CHPS) was suspected based on acute onset of symptoms like moderate to severe headache, seizures or focal neurological deficits in the postoperative period. Five patients had moderate to severe headache that started mostly on the first/second postoperative days and subsided within 3–4 days; 4 had a mild headache, 3 patients had a single episode of seizures, and 2 patients had a mild fever for which only symptomatic management was done (Table 2). Careful vital monitoring with blood pressure control to prevent postoperative bleed secondary to anastomosis in chronically ischemic territory. No mortality occurred during the prospective study period. Postoperative MRI with TOF MR angiography and ASL perfusion was done in all 25 patients to look for postoperative bleed, acute infarct and patency of anastomosis. One patient who was operated on the left side and had an episode of seizures post-surgery was found to have right high parietal infarct. Three patients had a small area of diffusion restriction at the anastomotic side. Apart from the postoperative changes, no postoperative bleed was seen in any of the patients. There was a statistically significant increase (P < 0.05) in postoperative nCBFh values in the frontal, temporal and parietal regions but not in the basal ganglia (Table 3).On imaging, a normal increase in perfusion was defined as an increase in nCBF values to 30–100% as compared to the pre-operative levels without any clinical deficit. The possibility of cerebral hyperperfusion was kept whenever the percentage increase in nCBF values were more than 100% of preoperative nCBFh or nCBFcbl values on the operated site, either in the fronto-temporo-parietal region or basal ganglia in the absence of obvious infarcts or postoperative collections. ASL showed more than 100% increase in nCBFh and nCBFcbl in 11 patients, but only 5 patients had significant postoperative symptoms. The remaining 6 patients where ASL showed raised nCBFh or nCBFcbl values had only mild symptoms like mild headache and low grade fever. No seizures or focal neurological deficits were seen in these patients. These patients were managed conservatively with appropriate control of blood pressure and frequent clinical examinations. None of these had any deficits on follow-up after 3 months.
Based on the above findings, sensitivity and specificity of ASL perfusion for detecting CHPS were evaluated taking clinical criteria as a gold standard as described below. ASL was found to have 47–100% (95 CI) sensitivity and 45–88% (95% CI) specificity (Table 4). Based on the above data, we have tried to calculate incidence of CHPS in postoperative patients of moyamoya disease, which in our study ranged from 6.83 to 40.70% (20%; 95% confidence interval). Using Mann Whitney U test, a statistically significant increase in post-revascularisation nCBFh was noted in frontal and temporal locations (Table 5).
Case 1
A 6-year-old female patient presented with right lower limb weakness. Left-sided EDAMS and STA-MCA bypass were done. The patient had moderate headache with seizures on the second postoperative day and was clinically suspected as hyperperfusion syndrome (Figs. 2 and 3).
Preoperative T2W axial image (a) shows chronic infarct in left temporo-occipital region. Images (b) and (c) represent postoperative T2WI and DWI, respectively. There is a hyperintense gyral swelling showing diffusion restriction at the operative site in the left lateral temporal region. Pre- and postoperative TOF MRA (e and f) shows a patent left STA-MCA bypass with good flow-related enhancement in the left MCA branches
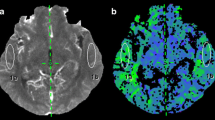
Grey scale ASL perfusion images: First image (1) represents the preoperative ASL images, and second image (2) represents the comparative postoperative ASL images. There was significantly raised cortical perfusion, particularly in the left temporal region (yellow circles) suggestive of the cerebral hyperperfusion
Case 2
A 4-year-old male patient presented with right sided hemiparesis. The patient had STA-MCA bypass on the left side. On the second postoperative day, he had moderate to severe headache with seizures and was clinically suspected as hyperperfusion syndrome (Figs. 4 and 5).
Preoperative T2W axial image (a) shows a chronic infarct in the right temporal region. Images (b), (c) and (d) represent the postoperative T2WI, DWI and ADC images respectively in the same patient. There is a small focal area of diffusion restriction at the operative site in THE left lateral temporal region. No significant gyro swelling is, however, noted. Pre- and postoperative TOF MRA (e and f) shows a patent left STA-MCA bypass with a good flow-related enhancement in left MCA branches
Colour-coded ASL perfusion images with ROIs placed at predetermined places in the parietal and occipital regions. The first row represents preoperative images, and the second row represents postoperative ASL images. There was a significantly raised perfusion (more than 100% increase in CBF values) in the left parietal and occipital regions (yellow arrows). This case shows that the ASL changes can occur prior to parenchymal changes
Case 3
A 5-year-old female patient presented with seizures and right hemiparesis. The patient had combined EDAMS and STA-MCA bypass procedure on the left side. On the second postoperative day, she had moderate to severe headache and was clinically suspected as hyperperfusion syndrome. ASL perfusion images showed increased perfusion in the temporal and parietal regions (Fig. 6.).
Grey scale ASL perfusion images: Preoperative ASL images (a and b) represent cortical perfusion deficits in the left temporal region (yellow and blue arrows in (a) and (b)). Images (d) and (e) are postoperative ASL images showing an increased cortical perfusion in the left temporal region (yellow and blue arrows), resulting in CHPS. Pre-operative TOF MRA (c) shows a patent previously done in the right STA-MCA bypass. Left STA-MCA bypass is not well visualised (blue arrow) on postoperative TOF MRA (f)
Discussion
Our study assessed whether ASL MR imaging can be used to identify changes in CBF after revascularisation surgery in patients with moyamoya disease for early detection and management of CHPS to decrease postoperative morbidity in these patients. Our results demonstrated that 3DPCASL MRI can be used with high sensitivity and moderate specificity to detect perfusion changes in patients with clinical suspicion of CHPS.
Cerebral hyperperfusion syndrome is well known in other cervico-cranial vascular reconstructive surgeries like carotid endarterectomy or extracranial to intracranial anastomotic bypass in patients with atherosclerotic steno-occlusive disease [5].There is a literature evidence that cerebral hyperperfusion syndrome after direct STA-MCA anastomosis is more common in moyamoya disease patients as compared to the patients of steno-occlusive atherosclerotic cervico-cranial vascular disease [6].Cerebral hyperperfusion syndrome usually presents with symptoms that includes unilateral or bilateral moderate to severe headache, seizures or focal sensorimotor symptoms secondary to cerebral vasogenic oedema or intracerebral bleed [3]. Conventionally, SPECT and PET imaging was used frequently to assess the perfusion changes in patients of moyamoya disease. Zaharchuk et al. [4] reported application of ASL perfusion in MMD in 2011 and compared MRI ASL and DSA perfusion. He found a statistically significant agreement between the two perfusion techniques for assessment of transosseous collaterals. Calculation of quantitative CBF values using ASL MR imaging and comparison of these values with those calculated using 123I-iodoamphetamine SPECT in MMD patients showed that ASL is superior as it could identify a decrease in CBF values of lesser amplitude than the decrease measured by SPECT studies. However, the use of nuclear medicine is limited due to rare availability of PET/SPECT and significant radiation doses, especially in paediatric patients. MRI ASL perfusion has an advantage as it is radiation-free and non-invasive without the need of contrast use. Sugino et al. [7] compared ASL and 123 I-IMP SPECT for detection of cerebral hyperperfusion during the early postoperative period after STA-MCA bypass surgery and founded a strong correlation between the two.
To define cerebral hyperperfusion radiologically, we followed the criteria described by Lee et al. [8] of more than 100% increase in postoperative ASL CBF value compared with preoperative CBF value. ASL showed more than 100% increase in CBF in 11 patients, predominantly in frontal and temporal MCA territories. Only 5 of these 11 patients had significant postoperative symptoms. Vigilant vital monitoring and adequate blood pressure management were done in these patients to prevent postoperative bleed secondary to anastomosis in chronically ischemic territory. No mortality occurred during the prospective study period. We found that ASL has around 47–100% sensitivity (99%; 95% confidence interval) for detecting CHPS while specificity is moderate (45–88%) (70%; 95% confidence interval). None of the patients, who were clinically suspected to have CHPS, had normal ASL perfusion. Based on the above data, we also calculated the incidence of CHPS in postoperative patients of moyamoya disease, which in our study ranges from 6.83 to 40.70% (20%; 95% confidence interval).
According to Goetti et al. [9], ASL MRI is comparable with dynamic susceptibility perfusion MR images for detection of microvascular perfusion changes after revascularisation changes in children with moyamoya disease. Lee et al. [8] also found that the changes in quantitative CBF after revascularisation surgery measured using ASL were comparable with DSA findings. However, there are some studies [8, 10, 15] that suggest the disadvantage of a single post-label delay ASL perfusion in quantification of CBF due to delay in arterial transit time of labelled blood through the affected vascular territories. Lee et al. [7] purposed a serial analysis of patients by using ASL to look for improvement and stabilisation of CBF after revascularisation. Fan et al. [10] concluded that the long delay ASL of ≥ 4 s with or without multilevel delay provides more accurate ASL CBF measurements in MMD. According to Wang et al. [11], multi-delay ASL can improve CBF quantification in MMD with improved correlation with CT perfusion. Schmid et al. [12] compared velocity (VS-ASL) and acceleration selective ASL with [15O]H2O positron emission tomography(PET) for detection of CBF and found similar qualitative and quantitative CBF correlation between VS ASL and [15O]H2O PET. Domenico et al. [13] used a spatial coefficient of variation (sCoV) of ASL CBF to quantitatively express ASL signal heterogeneity due to arterial transit time in MMD with good accuracy. The authors concluded that ASL sCoV is a sensitive measure to detect perfusion changes after revascularisation surgery which can overcome limitations of absolute ASL CBF quantification. However, recent studies suggest that CBF calculation using single post-label delay ASL in patients with MMD have negligible overestimation effects on calculated CBF values [14].
Preoperative and immediate postoperative change in the status of moyamoya collaterals was also evaluated. No statistically significant difference was seen in the status of moyamoya collaterals in immediate postoperative period. From this, it can be concluded that probably a certain time period is required for moyamoya vessels to regress in cases of patent anastomosis/EDAMS as stated by other authors in the literature [7, 8].
Our prospective study did have some limitations like inter-observer variability in ROI selection in the MCA territory and selection of window width levels for calculation of CBF values. We tried to overcome this limitation by choosing ROI over the predetermined regions with the window width settings same in the pre-operative and post-operative ASL scans. We did not compare our ASL perfusion results with the conventionally considered gold standard tests like SPECT/PET or CT perfusion. Low resolution of ASL images to distinguish between true parenchymal hyperperfusion from arterial transit artefacts was also a minor limitation. For ASL perfusion calculation, we used a standard single post-labelling delay; however, there is a suggestion in literature (as discussed above) for the use of multi-delay ASL, long label delay ASL and ASL sCoV acquisition which corresponded much better with PET imaging for CBF quantification. However, we conclude that single post-label delay ASL can be used to compare preoperative and postoperative normalised CBF perfusion to detect CHPS with high sensitivity.
Conclusion
Based on our results, we concluded that ASL perfusion is an appropriate alternative to standard nuclear medicine studies to monitor the changes in perfusion after STA-MCA bypass surgery in moyamoya patients. ASL MR perfusion can be used to identify changes in cerebral blood flow (CBF) for early detection of cerebral hyperperfusion syndrome in patients with otherwise normal conventional MRI sequences with very high sensitivity but moderate specificity.
References
Janda PH, Bellew JG, Veerappan V (2009) Moyamoya disease: case report and literature review. J Am Osteopath Assoc 109(10):547–553
Kaku Y, Iihara K, Nakajima N (2012) Cerebral blood flow and metabolism of hyperperfusion after cerebral revascularization in patients with moyamoya disease. J Cereb Blood Flow Metab 32:2066–2075
Uchino H, Kuroda S, Hirata K (2012) Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke 43:2610–2616
Zaharchuk G, Do HM, Marks MP (2011) Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with Moyamoya disease. Stroke 42:2485–2491
Hayashi K, Horie N, Suyama K, Nagata I (2012) Incidence and clinical features of symptomatic cerebral hyperperfusion syndrome after vascular reconstruction. World Neurosurg 78:447–454
Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM, O'Fallon WM (1981) Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc 56:533–543
Sugino T, Mikami T, Miyata K, Suzuki K, Houkin K, Mikuni N (2013) Arterial spin-labeling magnetic resonance imaging after revascularization of moyamoya disease. J Stroke Cerebrovasc Dis 22(6):811–816
Lee S, Yun TJ, Yoo RE (2018) Monitoring cerebral perfusion changes after revascularization in patients with moyamoya disease by using arterial spin-labeling MR imaging. Radiology 288(2):565–572
Goetti R, O’Gorman R, Khan N, Kellenberger CJ, Scheer I (2013) Arterial spin label- ling MRI for assessment of cerebral perfusion in children with moyamoya disease: comparison with dynamic susceptibility contrast MRI. Neuroradiology 55(5):639–647
Fan AP, Guo J, Khalighi MM, Gulaka PK, Shen B (2017) Long-delay arterial spin labeling provides more accurate cerebral blood flow measurements in moyamoya patients. Stroke 28(9):2441–2449
Wang R, Yu S, Alger JR (2014) Multi-delay arterial spin labeling perfusion MRI in moyamoya disease--comparison with CT perfusion imaging. Eur Radiol 24(5):1135–1144
Schmid S, Heijtel DF, Mutsaerts HJ (2015) Comparison of velocity- and acceleration-selective arterial spin labeling with [15O]H2O positron emission tomography. J Cereb Blood Flow Metab 35(8):1296–1303
Tortora D, Scavetta C, Rebella G (2020) Spatial coefficient of variation applied to arterial spin labeling MRI may contribute to predict surgical revascularization outcomes in pediatric moyamoya vasculopathy. Neuroradiology 62(8):1003–1015
Fahlström M, Lewén A, Enblad P, Larsson EM, Wikström J (2020) High intravascular signal arterial transit time artifacts have negligible effects on cerebral blood flow and cerebrovascular reserve capacity measurement using single post label delay arterial spin-labeling in patients with moyamoya disease. AJNR Am J Neuroradiol 41(3):430–436
Ukai R, Mikami T, Nagahama H, Wanibuchi M, Akiyama Y, Miyata K (2020) Arterial transit artifacts observed by arterial spin labeling in moyamoya disease. J Stroke Cerebrovasc Dis 29(9):105058
Author information
Authors and Affiliations
Contributions
Dr. Vivek Agarwal made a substantial contribution to the concept or design of the work, or acquisition, analysis or interpretation of data; drafted the article or revised it critically for important intellectual content; and approved the version to be published. Dr. Paramjeet Singh made a substantial contribution to the concept or design of the work, or acquisition, analysis or interpretation of data; drafted the article or revised it critically for important intellectual content; and approved the version to be published. Dr. Chirag K Ahuja made a substantial contribution to the concept or design of the work, or acquisition, analysis or interpretation of data, and drafted the article or revised it critically for important intellectual content. Dr. Sunil Kumar Gupta made a substantial contribution to the concept or design of the work, or acquisition, analysis or interpretation of data, and drafted the article or revised it critically for important intellectual content. Dr. Ashish Aggarwal made a substantial contribution to the concept or design of the work, or acquisition, analysis or interpretation of data; drafted the article or revised it critically for important intellectual content; and approved the version to be published. Dr. Rajashekhar Narayanan made a substantial contribution to the concept or design of the work, or acquisition, analysis or interpretation of data, and drafted the article or revised it critically for important intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agarwal, V., Singh, P., Ahuja, C.K. et al. Non-invasive assessment of cerebral microvascular changes for predicting postoperative cerebral hyperperfusion after surgical revascularisation for moyamoya disease: an arterial spin labelling MRI study. Neuroradiology 63, 563–572 (2021). https://doi.org/10.1007/s00234-020-02583-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02583-w