Abstract
Objectives
Cerebral hyperperfusion (CH) could be a disastrous outcome causing complication after carotid revascularization if not managed properly and timely. The aim of this study was to investigate the association between preoperative arterial spin labelling (ASL) perfusion features and circle of Willis (CoW) pattern with CH.
Methods
Forty-eight consecutive carotid stenosis patients who underwent carotid endarterectomy (CEA) or carotid artery stenting (CAS) were enrolled. All patients had single post-labelling delay (PLD) ASL, territory-ASL, and 3-dimensional time-of-flight MR angiography (3D TOF MRA) within 2 weeks before surgery and within 3 days after surgery. Spatial coefficient of variation (CoV) of cerebral blood flow (CBF), whole brain, and territory perfusion volume ratio were calculated from ASL and territory-ASL. Postoperative CoW was classified into two groups based on patency of the first segment of the anterior cerebral arteries (A1) and anterior communicating artery (AcomA). ASL perfusion features, type of CoW, and clinical characteristics were analyzed between CH group and non-CH group to identify CH risk factors.
Results
Higher CoV (p = 0.005) of CBF, lower whole brain perfusion volume ratio (p = 0.012), missing any of A1 or AcomA in CoW (p = 0.002 for postoperative MRA and p = 0.004 for preoperative MRA), and large artery stroke history (p = 0.028) were significantly associated with higher risk of CH. Two cases with cerebral hyperperfusion syndrome (CHS) were also discussed, and their perfusion and angiographic features were shown.
Conclusions
Single-PLD ASL and MRA might be useful and non-invasive imaging tools to identify patients with higher risk of CH after carotid revascularization.
Key Points
• Cerebral hyperperfusion is a critical complication after carotid endarterectomy or carotid artery stenting.
• ASL and MRA can be used to identify patients at higher risk of cerebral hyperperfusion
• Pattern of circle of Willis, ASL perfusion features, and whole brain perfusion volume ratio are potential predicting markers for hyperperfusion after carotid revascularization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebral hyperperfusion syndrome (CHS) is an uncommon but critical complication after carotid revascularization procedures, including carotid endarterectomy (CEA) and carotid artery stenting (CAS), which may lead to severe consequences such as intracranial hemorrhage. Almost all reported CHS happened in patients with cerebral hyperperfusion (CH) [1, 2], which is defined as more than 100% CBF increase after carotid revascularization compared with baseline [2]. Reduced preoperative cerebrovascular reserve (CVR) [3,4,5] and poor escape routes (primary collateral circulation) [6] have been reported to be related to CH. Assessing CVR via acetazolamide or CO2 challenge under PET is the golden standard, but it is often impractical to perform it on all preoperative patients due to the invasiveness and potential side effects. It is hence desirable to identify patients with high risk of CH with non-invasive methods prior to carotid revascularization, which may then further proceed to preoperative CVR test.
Arterial spin labelling (ASL) is a MRI method for imaging the brain perfusion, and since magnetically labelled blood rather than contrast agent is used as a tracer, ASL is completely non-invasive. Post-labelling delay (PLD) time reflects the time allowed for the labelled blood to travel from the labelling region to the imaging region, and the recommended PLD for a healthy adult is around 2 s [7]. For patients with steno-occlusive disease, the blood usually takes longer time to arrive at the designated imaging region, i.e., a prolonged arterial transit time (ATT) compared to healthy subjects. When the ATT is longer than PLD, blood will still be contained in the vessels at the time of imaging which would lead to bright vascular signal and hypoperfusion in some brain regions. Based on this phenomenon, the signal heterogeneity in ASL images, expressed as spatial coefficient of variation (CoV), was shown to be strongly correlated with ATT [8]. Moreover, ATT has been reported as a predictor of CVR either in ASL or dynamic susceptibility contrast (DSC) MRI [9, 10].
The assessment of collateral circulation is of clinical interest as it provides information of the compensatory blood flow. Three-dimensional time-of-flight MR angiography (3D TOF MRA) may depict the morphology of the collateral flow but lacks the flow direction information; ASL perfusion may reveal the outcome of compensatory flow but lacks the information of its sources. Territory-ASL is an extension of ASL that further allows the visualization of vessel-specific perfusion territories [11], and thus provides both direction and origin of the collateral circulation. Territory-ASL has been applied in patients with carotid stenosis in perioperative period to study the relationship between changes of cerebral blood flow (CBF) and perfusion territories [12].
The aim of this study was to investigate whether preoperative ASL perfusion features, including CoV of CBF, regional low perfusion in single-PLD ASL, alteration of perfusion territories in territory-ASL, and pattern of circle of Willis (CoW) revealed by pre- and postoperative MRA were associated with cerebral hyperperfusion.
Materials and methods
Patients
This study was approved by the institutional review board of the Peking Union Medical College Hospital, and written informed consents were obtained from all participating patients. Participants were recruited from a carotid stenosis cohort study using ASL perfusion MRI [13]. The inclusion criteria are as follows: (1) underwent CEA or CAS for unilateral or bilateral carotid stenosis (≥ 70% for asymptomatic or ≥ 50% for symptomatic stenosis) and (2) underwent preoperative MRI within 2 weeks before surgery and postoperative MRI within 3 days after surgery (the same protocol). The degree of stenosis was measured on computed tomography angiography (CTA) according to the North American Symptomatic Carotid Endarterectomy within 1 month before surgery. The exclusion criteria were as follows: (1) patients had contralateral CEA or CAS within 3 months; (2) patients with ASL or territory-ASL failure, defined as an extremely low CBF region matches an entire vascular territory in ASL but normal perfusion in the same territory in territory-ASL; or normal perfusion in ASL but low labelling efficiency in territory-ASL; (3) motion artifacts or inferior image quality in ASL and/or territory-ASL images. Figure 1 illustrates the flowchart of patient enrollment. From February 2015 to March 2018, a total 418 patients received CEA or CAS, and 145 patients underwent preoperative MRI, among which 48 patients were finally included on the basis of these criteria. Among the excluded patients, five patients had MRI follow-up 3 months after surgery, and CHS occurred to two of them.
MRI
ASL perfusion MRI was performed on a 3.0-T scanner (Discovery 750, GE Healthcare) with an eight-channel phased array head coil. Standard ASL (pseudo-continuous labelling and a 3D fast spin echo stack of spirals readout with background suppression, without vascular crushing, 8 arms and 3 averages, acquisition time = 4 min 44 s), territory-ASL (super-selective labelling scheme for left and right internal carotid arteries (ICA) respectively, vessel-encoded for vertebrobasilar artery, 4 arms and 2 averages, acquisition time = 4 min 19 s), 3D TOF MRA, 3D T1-weighted inversion recovery prepared fast spoiled gradient recalled echo (BRAVO), and conventional MRI sequences including DWI, T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and susceptibility-weighted imaging were performed. Common parameters for both ASL and territory-ASL were TR/TE 4886/10.5 ms, labelling duration 1450 ms, post-labelling delay 2025 ms, FOV 240 mm × 240 mm, points per arms = 512, slice thickness = 4 mm, and slice gap = 0 mm.
Imaging evaluation
Preoperative MRI including DWI, T1-weighted, T2-weighted, and FLAIR was assessed by one neuroradiologist (F. F., 25 years of experience) to detect previous ischemic stroke, and the type of stroke was defined by SSS-TOAST system [14]. Preoperative and postoperative TOF MRA images were assessed by another neuroradiologist (H. Y., 15 years of experience) blinded to clinical information by reading both source images and maximum intensity projection images at the work station to optimally view the presence of anterior communicating artery (AcomA) and the first segment of the anterior cerebral arteries (A1). The CoW was divided into two types according to the radiological presence of AcomA and A1 segments on MRA: type G, AcomA and bilateral A1 were all visualized and type P, with non-visualized or hypoplastic (< 0.8 mm in diameter) AcomA or any of the A1 [6].
Data processing
The same data preprocessing steps as in [13] were performed, in summary, after co-registration, normalization, segmentation, and rescaling the ASL CBF in SPM12. The normalized standard ASL and territory-ASL CBF maps were masked with gray matter and white matter masks respectively. Threshold of 20 ml/100 g/min (for white matter) and 35 ml/100 g/min (for gray matter) was used to calculate the territory perfusion volume (in territory-ASL) and total perfusion (in standard ASL) in white matter and gray matter respectively [15]. Combining the white matter and gray matter perfusion volumes, the perfusion volume for whole brain in ASL (PV) and territory perfusion volumes for three major arteries, perfusion volume of ipsilateral side of carotid surgery (PVip), perfusion volume of contralateral side (PVco), and perfusion volume of vertebrobasilar artery (PVvba) in territory-ASL, were obtained. Brain volume (BV) in the ASL coverage area (infarction excluded) was also calculated from BRAVO. Then, the following parameters were calculated:
The CoV of CBF as the standard deviation (σ) of ASL CBF divided by the mean CBF (μ) was also derived. CoV was calculated within the whole brain (gray matter and white matter) from non-selective standard ASL.
Cerebral hyperperfusion assessment
Cerebral hyperperfusion [2] was defined as (1) postoperative throbbing frontotemporal or periorbital headache on the ipsilateral side of carotid revascularization, with or without seizure, focal neurological signs, deterioration of consciousness, or intracranial hemorrhage (evaluated on postoperative susceptibility-weighted imaging or head CT); (2) CBF increase of more than 100% compared with baseline CBF, either in the ipsilateral or bilateral hemisphere; and (3) no new ischemic lesion detected on postoperative DWI.
Statistical analysis
Statistical analyses were performed using the SPSS Release for Windows, Version 19.0. Patients’ preoperative characteristics are shown in Table 1. Radiological factors are shown in Table 2. Continuous variables were compared using the Mann-Whitney U test and categorical variables were compared using the Fisher exact test. Group differences were considered significant at values of p < 0.05. Due to the small number of CHS patients, multivariate analysis was not performed.
Results
Out of the 48 patients enrolled, CH occurred in 6 (12.5%) patients, and all cases occurred post-CEA. All these six patients got severe headache and met radiological CBF increase criteria, and the symptoms improved immediately after strict blood pressure control. Patients’ demographic and clinical characteristics that may predispose to CH are summarized in Table 1. Statistically significant difference was observed in whether patients had a large artery atherosclerosis stroke history (odds ratio = 8.5; 95% confidence interval 1.32–54.82, p = 0.028). No statistically significant difference was found in other demographic and clinical characteristics between CH and without CH groups.
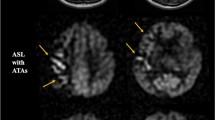
There were two types of hyperperfusion observed in this study. The first type is a significant perfusion signal increase in the surgery side that the absolute CBF value exceeds the value of the contralateral side (a representative case in Fig. 2). The second type is an evident low perfusion area in preoperative ASL, and after carotid surgery, the CBF increase filled up the low perfusion area, but the postoperative absolute CBF was comparable to the contralateral side (a representative case in Fig. 3).
Hyperperfusion type 1. A patient with right-side carotid stenosis. (a–c) Preoperative perfusion territories on territory-ASL. (d) Preoperative CBF of ASL reveals reduced CBF on the right watershed area. e Postoperative CBF of ASL reveals hyperperfusion on perfusion territory of the right internal carotid artery, and signal is much higher than the contralateral side
Hyperperfusion type 2. A patient with right-side carotid stenosis. (a–c) Preoperative perfusion territory on territory-ASL. (d) Preoperative CBF of ASL reveals reduced CBF on the right watershed area, and intravascular high signal was seen on low perfusion area (arrow), indicating long ATT. e Postoperative CBF of ASL reveals regional CBF increase > 100%, but the mean perfusion signal on the right hemisphere is only a little higher than that of the contralateral side
Two patients that had CHS after CAS were excluded due to lack of short-term postoperative MRI. The first case was a 60-year-old male. Preoperative CTA revealed that the patient had right ICA and right middle cerebral artery occlusion, left internal carotid artery severe stenosis, and no A1 of the right anterior cerebral artery (type P CoW). The baseline systolic blood pressure (BP) was 121 mmHg. The patient underwent left CAS. He complained headache at the first night after surgery and at that time, the SBP was 136 mmHg. Symptoms improved after strict blood pressure control, but the patient presented seizure on the seventh postoperative day after the strict blood pressure control was discontinued. Emergency CT showed intracranial hemorrhage on the left side (Fig. 4). The second case was a 59-year-old female who underwent successful right CAS 2 years ago, and this time had left CAS for 90% asymptomatic stenosis in the left ICA (no restenosis in the right side after stenting). The patient presented with headache and difficulty in controlling blood pressure on the first postoperative day and soon developed into deterioration of consciousness and at the same night, the systolic BP fluctuated between 140 and 170 mmHg. CT scan found brain edema on the left hemisphere but no hemorrhage (Fig. 5). The statistical analysis was performed after exclusion of the two CHS patients. If these two patients were included into analysis, the highest systolic BP within 24 h after surgery would become significantly higher in the hyperperfusion group (p = 0.047).
CHS case 1. (a) Preoperative CTA indicates right ICA occlusion and severe stenosis of the left ICA. (b) Preoperative ASL reveals regional reduced CBF and higher signal heterogeneity on the right hemisphere. (c–e) Preoperative territory-ASL shows no perfusion of the right ICA and collateral flow from the left ICA and posterior circulation. Patient underwent left-side CAS and presented with seizure on the 7th postoperative day. (f) CT scan reveals intracranial hemorrhage on the left side. (g) Susceptibility-weighted imaging (2 months after surgery) reveals hypointensity in the sulci of the left hemisphere
CHS case 2. (a) Preoperative MRA reveals right A1 missing. (b–d) Preoperative perfusion territories showed by territory-ASL. The patient complained headache and soon developed into light coma. (e) Emergency CT demonstrated diffused edema of the left hemisphere. (f–h) Postoperative perfusion territories revealed by territory-ASL (3 months after surgery), no identifiable change compared with preoperative territory-ASL
In preoperative MRA, 15 patients (35.7%) in the non-CH group and all 6 patients (100%) in the CH group had type P CoW. The pattern of CoW was not static, and after carotid revascularization, preoperative non-visualized AcomA in two patients and one hypoplastic A1 segment in one patient became visible. Thus, in postoperative MRA, 12 patients (28.6%) in the non-CH group had type P CoW in postoperative MRA. No matter on pre- or postoperative MRA, significantly higher percent of patients had type P CoW with cerebral hyperperfusion than did patients without hyperperfusion (p = 0.002 for postoperative MRA and p = 0.004 for preoperative MRA). Significantly higher CoV of CBF (p = 0.005) and lower RatioPV (p = 0.012) were found in the CH group. No statistical significant differences were found in RatioPVip, RatioPVrest, and AIco/ip (Table 2).
Discussion
The present study demonstrated that previous large artery atherosclerosis stroke and radiological markers including pattern of CoW, CoV of CBF, and whole brain perfusion volume ratio (RatioPV) in ASL may identify patients at risk of cerebral hyperperfusion after carotid revascularization.
Poor preoperative escape route in CoW was reported to be associated with higher CBF increase rates in the operative side after carotid revascularization. However, the presence of bilateral posterior communicating arteries (PcoA) did not affect the postoperative CBF increase difference between the surgery side and the other side [6]; hence, in this study, only the A1 and AcomA were considered. The current study evaluated the CoW both on the preoperative and postoperative MRA while the previous study used preoperative MRA only. We observed that the pattern of the CoW could change after CEA or CAS: invisible AcomA or A1 segment may become visible or the diameter of segments of CoW may increase on MRA after carotid revascularization. This phenomenon may be explained by the increased perfusion pressure from the treated carotid artery after revascularization. Thus, radiological incompleteness or hypoplasia of the CoW before surgery may not be real anatomic absence or hypoplasia, and using the preoperative MRA to identify patients at risk may not be accurate. Although the type P CoW was significantly higher in the CH group in both pre- and postoperative MRA in the present study, we suggested that if conflicts are found between pre- and postoperative MRA, the latter shall be followed when using the pattern of CoW to identify the risk of hyperperfusion.
Reduced CVR has been reported to be an important risk factor for postoperative CH [1, 4, 16, 17]. CVR is the cerebral autoregulation capacity of blood flow coping with decreased perfusion pressure. Impaired CVR also strongly associated with ischemic stroke [18]; our study found that a significant greater percent of CH patients had a history of large artery stroke. Studies have shown that arterial transit time (ATT) or mean transit time (MTT) in perfusion MR imaging methods including multi-delay ASL or dynamic susceptibility contrast (DSC) can predict CVR without the use of ACZ or CO2 [9, 10]. However, multi-delay ASL was not usually performed in clinical practice because of the long scan time and DSC requires injection of a contrast agent. Recently, it was reported that spatial CoV of CBF in single-delay ASL can predict ATT with relatively high precision as an alternative for multiple-delay time acquisition. In the current study, CoV of CBF in single-delay ASL was seen to be significantly associated with cerebral hyperperfusion after carotid revascularization, which could be caused by reduced CVR. This study also demonstrated that patients with some regions of low perfusion signal in a single-delay ASL (low RatioPV) also had higher risk of hyperperfusion. This was also in line with a previous report that CVR impairment combined with severe reduction of CBF was significantly associated with post-CEA hyperperfusion [19]. In addition, the relatively low perfusion signal in non-infarction regions might be an underestimate of CBF due to the long ATT.
In this study, all postoperative patients underwent BP monitoring after CEA or CAS in the administrating hospital, and in most cases, the symptoms disappeared after strict BP control. This may partially explain the results that the systolic BP in the CH group was slightly higher than that of the non-CH group, but without statistical significance. However, patients developed CHS tended to have a labile BP even under strict BP control or BP increased considerably after the strict BP control was discontinued. This may indicate the duration of BP monitor needs further investigation with a larger cohort.
In a previous study, greater CBF increase was associated with reduced territory perfusion volume before surgery [12]. In the current study, there was a trend that ipsilateral perfusion volume ratio revealed by territory-ASL in the CH group being smaller than that of the non-CH group, but the difference was not statistically significant. It should be noted that the previous study excluded patients with aplastic or hypoplastic A1 segment, whereas such exclusion was not made in this study. Individuals with aplastic A1 have smaller but normal internal carotid artery perfusion territory; thus, the type of CoW may become a confounding factor. However, due to the relatively small sample size, CoW was only classified by type G (good collateral) and type P (poor collateral) in this study.
There are several limitations in the current study. First, the sample size was relatively small, and due to the low incidence of hyperperfusion, the number of CH-positive cases was too small for a multivariate analysis. However, the relatively small sample size is not uncommon in perfusion MRI-based studies. Second, only patients met radiological hyperperfusion criteria were included because the two CHS patients’ unstable conditions were not allowed for postoperative perfusion MRI. The risk factors of the CHS cases were considered.
In conclusion, this study reveals that preoperative ASL perfusion features and post/preoperative CoW pattern may be potential markers to identify patients at risk of hyperperfusion after carotid revascularization. Thus, single-delay ASL and MRA could be useful non-invasive screening tools for post-carotid revascularization cerebral hyperperfusion in clinical practice.
Abbreviations
- 3D TOF MRA:
-
3-dimensional time-of-flight MR angiography
- A1:
-
First segment of the anterior cerebral artery
- AcomA:
-
Anterior communicating artery
- AI:
-
Asymmetry index
- ATT:
-
Arterial transit time
- BP:
-
Blood pressure
- BRAVO:
-
3-dimensional T1-weighted inversion-prepared fast spoiled gradient echo
- BV:
-
Brain volume
- CAS:
-
Carotid artery stenting
- CBF:
-
Cerebral blood flow
- CEA:
-
Carotid endarterectomy
- CH:
-
Cerebral hyperperfusion
- CHS:
-
Cerebral hyperperfusion syndrome
- CoV:
-
Spatial coefficient of variation
- CoW:
-
Circle of Willis
- CVR:
-
Cerebrovascular reserve
- DSC:
-
Dynamic susceptibility contrast
- FLAIR:
-
Fluid-attenuated inversion recovery
- ICA:
-
Internal carotid artery
- PLD:
-
Post-labelling delay
- PV:
-
Perfusion volume
References
Ogasawara K, Yukawa H, Kobayashi M et al (2003) Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg 99:504–510
van Mook WN, Rennenberg RJ, Schurink GW et al (2005) Cerebral hyperperfusion syndrome. Lancet Neurol 4:877–888
Iwata T, Mori T, Tajiri H, Nakazaki M (2011) Predictors of hyperperfusion syndrome before and immediately after carotid artery stenting in single-photon emission computed tomography and transcranial color-coded real-time sonography studies. Neurosurgery 68:649–655 discussion 655–646
Komoribayashi N, Ogasawara K, Kobayashi M et al (2006) Cerebral hyperperfusion after carotid endarterectomy is associated with preoperative hemodynamic impairment and intraoperative cerebral ischemia. J Cereb Blood Flow Metab 26:878–884
Fukuda T, Ogasawara K, Kobayashi M et al (2007) Prediction of cerebral hyperperfusion after carotid endarterectomy using cerebral blood volume measured by perfusion-weighted MR imaging compared with single-photon emission CT. AJNR Am J Neuroradiol 28:737–742
Katano H, Mase M, Sakurai K, Miyachi S, Yamada K (2012) Revaluation of collateral pathways as escape routes from hyperemia/hyperperfusion following surgical treatment for carotid stenosis. Acta Neurochir (Wien) 154:2139–2148 discussion 2148–2139
Alsop DC, Detre JA, Golay X et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116
Mutsaerts HJ, Petr J, Václavů L et al (2017) The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. J Cereb Blood Flow Metab 37:3184–3192
Choi HJ, Sohn CH, You SH et al (2018) Can arterial spin-labeling with multiple postlabeling delays predict cerebrovascular reserve? AJNR Am J Neuroradiol 39:84–90
Kawano T, Ohmori Y, Kaku Y et al (2016) Prolonged mean transit time detected by dynamic susceptibility contrast magnetic resonance imaging predicts cerebrovascular reserve impairment in patients with moyamoya disease. Cerebrovasc Dis 42:131–138
van Laar PJ, van der Grond J, Hendrikse J (2008) Brain perfusion territory imaging: methods and clinical applications of selective arterial spin-labeling MR imaging. Radiology 246:354–364
Yamamoto D, Hosoda K, Uchihashi Y et al (2017) Perioperative changes in cerebral perfusion territories assessed by arterial spin labeling magnetic resonance imaging are associated with postoperative increases in cerebral blood flow in patients with carotid stenosis. World Neurosurg 102:477–486
Lin T, Lai Z, Lv Y et al (2018) Effective collateral circulation may indicate improved perfusion territory restoration after carotid endarterectomy. Eur Radiol 28:727–735
Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ (2005) An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 58:688–697
Donahue MJ, Achten E, Cogswell PM et al (2017) Consensus statement on current and emerging methods for the diagnosis and evaluation of cerebrovascular disease. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678x17721830
Kaku Y, Yoshimura S, Kokuzawa J (2004) Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol 25:1403–1408
Sfyroeras GS, Karkos CD, Arsos G et al (2009) Cerebral hyperperfusion after carotid stenting: a transcranial doppler and SPECT study. Vasc Endovasc Surg 43:150–156
Gupta A, Chazen JL, Hartman M et al (2012) Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 43:2884–2891
Hosoda K, Kawaguchi T, Ishii K et al (2003) Prediction of hyperperfusion after carotid endarterectomy by brain SPECT analysis with semiquantitative statistical mapping method. Stroke 34:1187–1193
Funding
This work was supported in part by the Ministry of Science and Technology of China grant (2015CB351701) and the National Nature Science Foundation of China grant (31730039). This work also received funding from Peking Union Medical College.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Feng Feng.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in “Effective collateral circulation may indicate improved perfusion territory restoration after carotid endarterectomy. 2018. Eur Radiol 28:727–735.”, and as a poster “Preoperative predictors of hyperperfusion after CEA: a study using vessel selective ASL” in ISMRM 2018.
Methodology
• prospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Lin, T., Lai, Z., Zuo, Z. et al. ASL perfusion features and type of circle of Willis as imaging markers for cerebral hyperperfusion after carotid revascularization: a preliminary study. Eur Radiol 29, 2651–2658 (2019). https://doi.org/10.1007/s00330-018-5816-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5816-1