Abstract
Purpose
To evaluate the utility of CT perfusion (CTP) for the assessment of superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis in patients with Moyamoya syndrome (MMS).
Subjects and methods
Twenty-four consecutive MMS patients, who underwent unilateral STA-MCA bypass surgery, received CTP before and after surgery. The relative perfusion parameter values of surgical hemispheres before treatment were compared with post-treatment values. All patients underwent CT angiography (CTA) before and after surgery in order to confirm the patency of bypass.
Results
The follow-up CTA after surgery clearly demonstrated 20 (20/24, 83.3 %) bypass arteries, whereas four (16.7 %) bypass arteries were occluded or very small. Postoperative rMTT and rTTP values (P < 0.05) of the surgical side were significantly lower than pre-operation. In patients (n = 20) with bypass patency, postoperative rCBF, rMTT and rTTP values (P < 0.05) of the surgical side were significantly improved. However, the differences of all parameters were not significant (P > 0.05) in the patients (n = 4) without bypass patency after revascularization.
Conclusion
This study demonstrates that CTP can provide a crucial quantitative assessment of cerebral haemodynamic changes in MMS before and after STA-MCA anastomosis.
Key Points
• Twenty-four MMS patients undergoing STA-MCA bypass received CTP pre- and post-surgery
• Cerebral haemodynamics improved on the surgical side post-surgery on CTP maps
• rCBF might have a better correlation with patency of the bypass artery.
• CTP can evaluate cerebral perfusion changes in MMS patients after cerebral revascularization
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Moyamoya syndrome (MMS) refers to the presence of stenosis or occlusion of the terminal portion of the internal carotid artery or proximal portion of the anterior and/or middle cerebral arteries accompanied by an abnormal vascular network at the base of the brain. MMS may be idiopathic or may occur in association with an underlying disease [1, 2]. Broadly speaking, the most common symptoms in patients with MMS are stroke, transient ischaemic attacks (TIAs), seizures that are due to brain ischaemia and haemorrhage, and headache due to the deleterious consequences of the compensatory mechanisms responding to the ischemia [1]. Although the natural history of MMS is variable, it progresses in the majority of patients [1–4].
Surgical revascularization, which aims to improve the cerebral haemodynamics and metabolism, has been reported to be an effective treatment in preventing stroke in MMS patients with haemodynamic compromise [2, 5, 6]. As the haemodynamics of MMS are extraordinarily complex, evaluation of haemodynamic changes is clinically meaningful for assessment of therapeutic effects and prognosis [2]. To date, positron emission tomography (PET), single-photon emission CT (SPECT) and magnetic resonance brain perfusion (MRP) have been applied for evaluating cerebral haemodynamics. However, due to some drawbacks, they are not optimal, such as limited availability, contraindications, the limited potential to monitor critically ill patients and high costs. CT perfusion (CTP) imaging, as a common method, has been relatively widely used in clinical practice for brain perfusion measurement. Thus, the purpose of this study is to evaluate the utility of CTP for the assessment of superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis in patients with MMS.
Subjects and methods
Patients
Twenty-four consecutive patients with MMS confirmed by digital subtraction angiography (DSA) were retrospectively selected between April 2010 and July 2014 for assessment. The diagnostic criteria for MMS was based on the guidelines published in 2012 [2]. We have used the term “moyamoya syndrome (MMS)” in all cases, whether or not there was an associated diagnosis, to describe the characteristic vasculopathy. All the patients underwent a unilateral STA-MCA bypass surgery on either the symptomatic side (bilateral MMS) or the affected side (unilateral MMS).
All patients underwent CTP examination within 2 weeks before surgery. Postoperative CTP images were performed no less than 2 weeks after surgery. All patients received CT angiography (CTA) before and after surgery in order to confirm the patency of bypass. Written informed consent was obtained from all patients or their next of kin.
Imaging protocol and post-processing procedure
The admission studies were obtained with two multidetector spiral CT systems (Somatom Definition and Definition FLASH, Siemens Healthcare, Forchheim, Germany).
Definition of Somatom
-
(1)
Non-contrast enhanced transaxial CT of the whole brain (section thickness 5 mm, no overlap, slices parallel to orbito-meatal line, 120 kV, 350 mAs).
-
(2)
Dynamic CT perfusion imaging: 30 s of constant scanning at the level of basal ganglia, 3 × 9.6 mm slices, 80 kV, 140 mAs, 1.0 s/rotation, 24 × 1.2 mm collimation and 30 images per section. Contrast medium administration (Ultravist 300; Bayer Schering, Berlin, Germany) 50 ml, delay 7 s, bolus 5.0 ml/s.
-
(3)
CTA imaging: Firstly, unenhanced CT was routinely performed at 120 kV, 130 mAs, a collimation of 64 × 0.6 mm, a pitch of 1.2 and a rotation time of 0.5 s. Images were reconstructed with a 0.75-mm section thickness and a 0.5-mm increment with an H10f kernel. Then whole-brain CTA was performed with an additional 50 ml of contrast agent, bolus 4.0 ml/s. Un-enhanced CT was reconstructed with identical parameters to enhanced CT. All reconstructed datasets were sent to a dedicated workstation (syngo.via, VA 20B, Siemens Healthcare) to generate digital subtraction CT angiographic images. Bone subtraction was performed automatically without user interaction using vendor bone subtraction software (Neuro DSA, Siemens Healthcare) based on the enhanced CT and unenhanced CT datasets as described elsewhere[7].
FLASH Somatom Definition
-
(1)
Dynamic CT perfusion imaging was initiated 7 s after the start of the injection at a rate of 5 ml/s with the following acquisition parameters: 80 kV, 200 mAs, 0.75-mm slice thickness, 128 × 0.6-mm collimation, 0.28-s rotation time, total CTP imaging time of 53 s and approximately 10.0-cm scan coverage in z-axis using adaptive spiral scanning technique (‘shuttle mode’). A set of axial images with a slice thickness of 10.0 mm for perfusion analysis was reconstructed without overlap.
-
(2)
CTA images were reconstructed from source data acquired with dynamic scans. Digital subtraction CTA images were generated by selecting two sets of data (the first unenhanced phase and the arterial phase) from the CTP resources to remove bones using the same software as above.
All reconstructed axial CTP images were transferred to a workstation (Syngo MMWP, VE 40C, Siemens Healthcare). Perfusion analysis was performed for all datasets with the vendor given ‘Neuro-VPCT’ software, using the semi-automatic deconvolution algorithm ‘Auto Stroke MTT’. The first artery to reach peak enhancement on the time-attenuation curve was selected as the arterial input function. The venous input region of interest (ROI) was placed in the superior sagittal sinus. Perfusion parameter maps for cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT) and time to peak (TTP) were generated. Two experienced neuroradiologists identified the areas of abnormal perfusion in the cortical gray matter of MCA territory of the surgical side by visual inspection. When the judgements of the two observers conflicted, a discussion was held to reach a consensus. Then, two standardized elliptical mirrored ROIs were drawn manually on the basal ganglia section level of the reference CT image (Fig. 1) over the cortical grey matter of MCA territory independently. An attempt was made to select the same location for each measurement and avoid the infarct location. The relative CTP values in our study were defined as the ratios between absolute CTP values of the surgical site and that of contralateral mirroring areas.
Patients were subsequently assigned to one of two groups: patients with bypass patency (no stenosis, no significant stenosis or minor stenosis) and patients without bypass patency (not visible or significant stenosis). Image analysis was assessed by the same two neuroradiologists on the follow-up CTA data referring to the criteria reported elsewhere [8].
Statistical analysis
For CTP parameters, the mean of the ROI values on each ipsilateral and mirrored contralateral hemisphere was calculated. Our study aimed at evaluating the perfusion changes on the surgical hemisphere, so we selected the ROIs of the surgical side for comparison and statistical analysis. Relative values of the surgical side before and after surgery were compared with a paired-samples t test in all 24 patients and the subgroup patients with bypass patency (n = 20) because data were normally distributed. Differences in patients (n = 4) without bypass patency before and after surgery perfusion CT relative values were assessed with a matched-pairs signed-ranks test because data were not normally distributed. P values P < 0.05 were considered statistically significant. All raw data were analysed using SPSS statistical software (Version 20.0, SPSS, Chicago, IL, USA).
Results
Patient demography and revascularization
The demographics and surgical interventions of the 24 enrolled patients are shown in Table 1. The clinical presentations consisted of three (12.5 %) with prior ischaemic stroke, five (20.8 %) with prior brain haemorrhage, 16 (66.7 %) with TIA, including two with sensory body changes. The mean age of this cohort was 40.0 years (range, 26–56 years); 16 (66.7 %) patients were female. In the clinical course, among the 24 surgeries, a 46-year-old woman undergoing unilateral STA-MCA bypass presented with headache and aphasia on the seventh postoperative day. Other patients with an onset of TIA (16/24) or completed stroke (2/24) obtained disappearance or improvement of ischaemic attack during the follow-up period (range, 3–11 months; mean 4.9 months). Five haemorrhagic-onset patients had no re-haemorrhagia during the follow-up period.
The follow-up (range, 3–11 months; mean 4.9 months) CTA after surgery clearly demonstrated 20 (83.3 %) bypass arteries (Figs. 2 and 4), whereas four (16.7 %) bypass arteries were occluded or very small (Fig. 6).
A 41-year-old male patient who underwent right superficial temporal artery-middle cerebral artery (STA-MCA) bypass due to a history of Moyamoya syndrome. (a) Preoperative right carotid DSA showed marked stenosis of the right middle cerebral artery (MCA) (white arrowhead). (b) Preoperative CT angiography (CTA) also showed marked stenosis of the bilateral MCA (white arrowhead). (c) Postoperative CTA demonstrated that right STA-MCA bypass resulted in a patent artery (white arrow). (d) Enlarged image from (c) showing the detail (white arrow)
Haemodynamic evaluations
The pre- and postoperative rCTP parameters showed that postoperative rMTT and rTTP values from the surgical side were significantly lower than those before surgery (t = 4.04 and t = 4.21, respectively; P < 0.05 for all) for all patients (n = 24). However, no significant differences in rCBF and rCBV (t = −0.99 , t = −0.03 , respectively; P > 0.05 for all) were found after revascularization (Table 2; Figs. 3 4, 5 and 6).
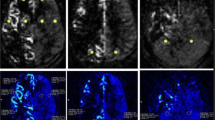
CTP images of the same patient. Images show cerebral haemodynamic changes pre and post superficial temporal artery-middle cerebral artery (STA-MCA) surgery. Axial preoperative perfusion CT images show reduced cerebral blood flow (CBF), reduced cerebral blood volume (CBV), delayed mean transit time (MTT) and time to peak (TTP) in the region of right hemisphere toward the contralateral side. The postoperative perfusion CT images show markedly increased CBF, slightly increased CBV, markedly improved MTT and TTP in the right hemisphere
A 47-year-old female patient who underwent right superficial temporal artery-middle cerebral artery (STA-MCA) bypass due to a history of Moyamoya syndrome. (a) Preoperative right carotid digital subtraction angiography (DSA) showed marked stenosis of the right middle cerebral artery (MCA) (white arrowhead). (b) Preoperative CT angiography (CTA) also showed marked stenosis of the right middle cerebral artery (MCA) (white arrowhead). (c) Postoperative CTA demonstrated that right STA-MCA bypass resulted in a patent artery (white arrow). (d) Enlarged image from (c) showing the detail (white arrow)
CTP images of the same patient. Images show cerebral haemodynamic changes pre and post superficial temporal artery-middle cerebral artery (STA-MCA) surgery. Axial preoperative perfusion CT images show reduced cerebral blood flow (CBF), increased cerebral blood volume (CBV), delayed mean transit time (MTT) and time to peak (TTP) in the region of right hemisphere toward the contralateral side. The postoperative perfusion CT images show increased CBF, decreased CBV, improved MTT and TTP in the right hemisphere
A 31-year-old male patient who underwent right superficial temporal artery-middle cerebral artery (STA-MCA) bypass due to a history of Moyamoya syndrome. (a) Preoperative CT angiography (CTA) showed occlusion of bilateral middle cerebral artery (MCA) (white arrowhead) and patency of bilateral superficial temporal artery (STA) (white arrow). (b) Postoperative CTA showed occlusion of the right STA branches (white arrow) and the bridge artery was not visible
Further assessments were performed in the two subgroups. In the patients (n = 20) with bypass patency, in addition to the significant differences in rMTT and rTTP (t = 3.39 and t = 3.66, respectively; P < 0.05 for all) in the surgical hemisphere, differences in rCBF were detected: rCBF from the surgical side was significantly improved (t = −2.45, P < 0.05). For rCBV, there was still no significant difference detected (t = 0.25, P > 0.05) (Table 3). However, the differences of all parameters were not significant (P > 0.05) in the patients (n = 4) without bypass patency after revascularization (Table 4).
Discussion
Most MMS patients progress to complete occlusion of the internal carotid artery [2]. Treatment strategies are aimed at improving cerebral haemodynamics of the symptomatic hemisphere and preventing recurrent strokes in patients. Although randomized controlled trials (RCTs) have not been performed, there are strong indications from observational studies that neurosurgical intervention, in spite of direct or indirect revascularization techniques, can reduce the risk of ischaemic stroke by improving CBF [9, 10]. However, despite its effectiveness, several postoperative complications, such as cerebral hyperperfusion syndrome (CHS), have been reported with cerebrovascular reconstruction surgery [11–13]. In our study, only one patient (4.2 %) presented with headache and then aphasia at the subacute stage, which was considered to be a result of CHS. The other patients had no perioperative cerebral infarction and showed improved or stable neurological function postoperatively. Therefore, since STA-MCA anastomosis has been used for MMS patients, it has become the cornerstone of direct revascularization with many authors reporting excellent results [14, 15]. So far, different tools including MRP, PET and SPECT have been applied for quantitative haemodynamic analyses. However, CTP is regarded as a more readily accessible method for the evaluation of cerebral perfusion [16–18]. In this setting, we quantitatively analysed haemodynamic changes before and after STA-MCA anastomosis by CTP.
In our study, rMTT and rTTP values were found to be significantly reduced after treatment (P < 0.05), but rCBV and rCBF changed non-significantly in these ROIs. The results indicated that the corresponding cerebral haemodynamics improved after STA-MCA bypass at the surgical site. However, this was a little different from the results of Jun Zhang et al. [19] and Zhengwei Li et al. [20]. Jun Zhang et al. quantitatively evaluated haemodynamic changes before and after STA-MCA bypass surgery combined with encephalo-duro-myo-synangiosis (EDAMS), and then showed that rCBF values from the surgical side in the region of MCA were significantly higher than those before surgery. Zhengwei Li et al. also identified improved rCBF in the temporal lobe by MRP after STA-MCA bypass surgery. After more indepth comparison, we found that all the direct graft patencies were displayed in their studies, which was different from our study in which four bypass arteries were occluded or were very small after revascularization. In order to determine the influence of the patency of bypass artery, further assessments were performed. It is encouraging that, as expected, rCBF revealed significant differences when the analysis was restricted to patients with bypass patency. The reason for these haemodynamic changes can be explained: firstly, TTP and MTT maps have the capability of being quite sensitive to the presence of altered brain perfusion [21, 22]. During this specific phase of haemodynamic improvement, increased cerebral perfusion pressure can result in a reduced MTT and TTP with or without vasodilatation. However, in the absence of cerebral autoregulation caused by chronic cerebral hypoperfusion, the CBF and CBV may remain within the previous range. Secondly, we speculate that rCBF, as a secondary sensitive parameter to altered brain perfusion, might have a better correlation with patency of the bypass artery. And conversely, the rCBF value increasing markedly post-operation might reflect the patency graft indirectly. In this study, no change in rCBV could be detected in all ROIs after treatment. The reason might be that CBV is a complex physiological parameter [21]. It is composed of arterial, capillary and venous compartments, as well as parenchymal and pial components. The vasodilatory response of these different compartments to reduced perfusion pressure is variable.
Our study has several limitations. First, in our study, most examinations (44/48; 91.7 %) were performed by classic CTP, which has a limited spatial coverage of 2.88 cm in the direction of the z-axis. However, subsequently four follow-up CTP examinations were performed on a 128-slice CT system (Somatom Definition FLASH, Siemens Healthcare) in a ‘shuttle mode’, which led to an extension range of 10 cm to almost cover the entire brain. Meanwhile, the CTA images could be reconstructed from source data acquired with dynamic images, which could lower both the radiation exposure and the amount of administered contrast agent compared with before. Secondly, drawing the same level and size of ROI was challenging. Therefore, we measured perfusion values in the symptomatic and contralateral hemisphere, and only the relative perfusion values were compared pre- and post-operation to minimize individual variability. Finally, this study only included a small set of patients. Further research with a larger set of clinical data and more individual CTP imaging analyses is needed to confirm the efficacy of STA-MCA bypass in patients with Moyamoya syndrome.
Conclusion
In summary, this study demonstrates that CTP, as a more convenient and less expensive imaging test than other available options, can provide a crucial quantitative assessment of cerebral perfusion changes in MMS before and after surgery.
Abbreviations
- CTP:
-
CT perfusion
- MMS:
-
Moyamoya syndrome
- STA-MCA:
-
Superficial temporal artery-middle cerebral artery
- CTA:
-
CT angiography
- TIA:
-
Transient ischaemic attack
- PET:
-
Positron emission tomography
- SPECT:
-
Single-photon emission CT
- MRP:
-
Magnetic resonance brain perfusion
- ROI:
-
Region of interest
- CHS:
-
Cerebral hyperperfusion syndrome
- EDAMS:
-
Encephalo-duro-myo-synangiosis
References
Scott RM, Smith ER (2009) Moyamoya disease and moyamoya syndrome. N Engl J Med 360:1226–1237
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases (2012) Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 52:245–266
Imaizumi T, Hayashi K, Saito K, Osawa M, Fukuyama Y (1998) Long-term outcomes of pediatric moyamoya disease monitored to adulthood. Pediatr Neurol 18:321–325
Kim SK, Seol HJ, Cho BK, Hwang YS, Lee DS, Wang KC (2004) Moyamoya disease among young patients: its aggressive clinical course and the role of active surgical treatment. Neurosurgery 54:840–846
Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA (2004) Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg 100:142–149
Fung LW, Thompson D, Ganesan V (2005) Revascularisation surgery for paediatric moyamoya: a review of the literature. Childs Nerv Syst 21:358–364
Lu L, Zhang LJ, Poon CS et al (2012) Digital subtraction CT angiography for detection of intracranial aneurysms: comparison with three-dimensional digital subtraction angiography. Radiology 262:605–612
Kemmling A, Nölte I, Groden C, Diehl S (2011) Dual energy bone subtraction in computed tomography angiography of extracranial-intracranial bypass: feasibility and limitations. Eur Radiol 21:750–756
Roach ES, Golomb MR, Adams R et al (2008) Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 39:2644–2691
Smith ER, Scott RM (2012) Spontaneous occlusion of the circle of Willis in children: pediatric moyamoya summary with proposed evidence-based practice guidelines. A review. J Neurosurg Pediatr 9:353–360
Furuya K, Kawahara N, Morita A, Momose T, Aoki S, Kirino T (2004) Focal hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease. Case report. J Neurosurg 100:128–132
Ogasawara K, Komoribayashi N, Kobayashi M et al (2005) Neural damage caused by cerebral hyperperfusion after arterial bypass surgery in a patient with moyamoya disease: case report. Neurosurgery 56:E1380, discussion E1380
Fujimura M, Kaneta T, Mugikura S, Shimizu H, Tominaga T (2007) Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol 67:273–282
Kawaguchi S, Okuno S, Sakaki T (2000) Effect of direct arterial bypass on the prevention of future stroke in patients with the hemorrhagic variety of moyamoya disease. J Neurosurg 93:397–401
Guzman R, Lee M, Achrol A et al (2009) Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg 111:927–935
Wintermark M, Sesay M, Barbier E et al (2005) Comparative overview of brain perfusion imaging techniques. Stroke 36:e83–e99
Rim NJ, Kim HS, Shin YS, Kim SY (2008) Which CT perfusion parameter best reflects cerebrovascular reserve?: correlation of acetazolamide-challenged CT perfusion with single-photon emission CT in Moyamoya patients. AJNR Am J Neuroradiol 29:1658–1663
Lee M, Zaharchuk G, Guzman R, Achrol A, Bell-Stephens T, Steinberg GK (2009) Quantitative hemodynamic studies in moyamoya disease: a review. Neurosurg Focus 26, E5
Zhang J, Wang J, Geng D, Li Y, Song D, Gu Y (2013) Whole-brain CT perfusion and CT angiography assessment of Moyamoya disease before and after surgical revascularization: preliminary study with 256-slice CT. PLoS One 8, e57595
Li Z, Zhou P, Xiong Z et al (2013) Perfusion-weighted magnetic resonance imaging used in assessing hemodynamics following superficial temporal artery-middle cerebral artery bypass in patients with Moyamoya disease. Cerebrovasc Dis 35:455–460
Derdeyn CP, Videen TO, Yundt KD et al (2002) Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 125:595–607
Wintermark M, Flanders AE, Velthuis B et al (2006) Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 37:979–985
Acknowledgments
The scientific guarantor of this publication is Yueqin Chen. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding from the Shandong Provincial Development Program of Medical Science and Technology (No. 2014WS0518). No complex statistical methods were necessary for this paper. Institutional review board approval was not required because of its retrospective nature. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: retrospective, observational study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Xu, W., Guo, X. et al. CT perfusion assessment of Moyamoya syndrome before and after direct revascularization (superficial temporal artery to middle cerebral artery bypass). Eur Radiol 26, 254–261 (2016). https://doi.org/10.1007/s00330-015-3802-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3802-4