Abstract
Introduction
Flow diversion is increasingly used for treating intracranial aneurysms. This article aims to review the evidence obtained from animal models and summarizes the findings that might be of clinical interest.
Methods
From a systematic review of studies published between 2000 and 2016, we extracted the data on the following questions: What roles do aneurysm dimension, morphology, and vascular geometry have on success of flow diversion? What characteristics of a flow diverter can influence aneurysm occlusion? What are the risk factors for jailed branch occlusion?
Results
Flow diversion has been shown to be less effective in occluding large aneurysms with wide or undefined necks, as compared to smaller aneurysms with narrower necks. Straight sidewall aneurysms were more likely to occlude after flow diversion than curved sidewall aneurysms or bifurcation aneurysms with branches originating from the neck or the fundus. The main characteristics of devices that may impact on the success of flow diversion are porosity and pore-density, but challenging aneurysm models were not better occluded with devices of lower porosity. Porosity is not uniform when devices deform to adapt to local in vivo anatomy when deployed. Neointima formation on devices correlates with low porosity. Branches are rarely occluded when they are jailed, but persistent branch flow may prevent aneurysm occlusion.
Conclusion
Experimental models may help anticipate clinical results of flow diversion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flow diversion is an innovative approach for the treatment of intracranial aneurysms [1]. A systematic review of animal experiments on flow diversion performed between 2000 and 2015 has recently been published [2]. Animal models may serve to anticipate clinical difficulties when using flow diverters (FDs), explain treatment failures, or generate hypotheses for clinical trials [3, 4]. We aimed to review the evidence obtained from animal models that could be pertinent to users of flow diverters when treating clinical aneurysms.
Methods
Search strategy
The systematic review was performed according to the Cochrane guidelines for systematic reviews [5–7]. Search strategies are provided in the Appendix. The interfaces were PubMed for MEDLINE, Ovid for MEDLINE, EMBASE, Cochrane, and EBSCO for CINAHL. We also hand-searched grey literature sources, including System for Information on Grey Literature in Europe (Open Grey), National Guideline Clearing House, National Institutes for Health and Clinical Excellence (NICE), The Grey Literature Report (NYAM), Google, and Google Scholar.
We included all articles that met the following selection criteria: (1) an animal study, (2) at least one flow diverter used, (3) peer-reviewed, (4) original research (review articles, abstracts, editorials and letters excluded), (5) English or French language.
Data extraction
A case report form (available in Appendix online) was created to examine the following: (i) Study characteristics: year of publication, laboratory of origin, funding source (industry, public, or both); (ii) animal model: species, type of aneurysm(s), aneurysm dimensions, presence of at least one jailed branch; (iii) flow diverter characteristics: brand name(s) and dimensions; (iv) method of treatment: single or multiple FDs and anti-platelet medications; (v) results: method of angiography, aneurysm occlusion rates, incidence of parent or jailed branch vessel stenosis or occlusion, length of follow-up, and whether pathology was performed; (vi) factors potentially influencing aneurysm occlusion (aneurysm dimensions, neck size, persistent branch flow, angle of incident blood flow, porosity/pore density, neointima proliferation); and (vii) FD safety (stenosis/occlusion of parent artery, stenosis/occlusion of jailed branches, hemorrhagic complications, embolic complications).
Three clinically relevant research questions were predefined: What role do aneurysm dimensions, morphology, and vascular geometry have on success of flow diversion? Which flow diverter characteristics can impact aneurysm occlusion? What are the risk factors for jailed branch occlusion?
Two authors (RF and JR) reviewed the methodology of each article to categorize preclinical evidence according to two levels inspired by Claude Bernard [8]: Evidence A (or confirmatory evidence) is evidence supported by the results of a direct experiment, where a predefined factor is manipulated and tested against appropriate controls, while evidence B (observational evidence) is obtained through the careful study of relations that were observed, but not manipulated, in the course of an animal study. Efficacy of aneurysm occlusion was assessed using the Kamran classification [9], where a greater score indicated a better result, according to an ordinal scale.
Results
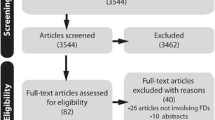
The initial title search yielded 3762 publications (Fig. 1); 3647 articles were excluded after reviewing abstracts, retaining 115 articles for full-text evaluation; and 100 articles did not meet selection criteria (53 articles) or were not relevant to the predefined research questions (47 articles), leaving 15 articles for evaluation.
The main findings of the review are summarized in Tables 1 and 2. Aneurysm-related factors are illustrated in Fig. 2. Neck size was surgically modulated in canine models [10]. The median angiographic occlusion score of aneurysms with small necks was better than for aneurysms with a large neck (level A evidence) [10]. Observations in elastase-induced rabbit models showed a similar effect of neck size on the success of flow diversion (level B evidence) [11, 12].
Aneurysm-related factors influencing results of flow diversion. a Aneurysms of smaller neck size (a) were more likely to be occluded with flow diversion than aneurysms with a wide neck (b). b Aneurysms in a straight lateral wall configuration (a) were more likely to be occluded with flow diversion than curved lateral wall (b) or bifurcation aneurysms (c). c The presence of a jailed branch arising from the neck or the aneurysm was also associated with failures
Small aneurysms were more likely to be occluded with flow diversion than large aneurysms, as suggested by level B evidence in both canines and rabbits [10, 11].
The median angiographic occlusion score of straight sidewall aneurysms was better than scores for curved lateral and bifurcation aneurysms (level A evidence) [13, 14]. There were no conflicting animal reports. The presence of a circulating branch arising from the neck or fundus of the aneurysm was also associated with failure of aneurysm occlusion (level A evidence) (Fig. 2c) [3, 16, 17].
Device-related factors reported to impact aneurysm occlusion rates in animal models are presented in Fig. 3. Low porosity stenting (flow diversion) could successfully occlude lateral wall aneurysms that could not be occluded with a single or two overlapping high porosity stents (level A evidence) [17]. Several animal studies have reported a correlation between porosity (or metallic density) and neointimal coverage of devices (level B evidence) [14, 17]. However, the same low porosity devices capable of occluding lateral wall aneurysms were unsuccessful in challenging curved sidewall and bifurcation aneurysms, and using devices of lower porosity, higher pore density, or overlapping multiple devices still failed to occlude the same challenging aneurysms (level of evidence A) [15].
One study using the rabbit-elastase model compared devices of various porosities and pore densities and found that, at equal porosities, higher aneurysm occlusion rates were obtained with the device with the greatest pore density (level of evidence A) [18].
Deployment technique was also found to influence local porosities and angiographic results: experimental aneurysms treated with axially compacted devices were more likely to become occluded than aneurysms treated with FDs that were simply unsheathed across the aneurysm neck (level of evidence A) [4].
Proper sizing of the FD to the diameter of the recipient vessel was shown in several retrospective animal observations to correlate with better occlusion rates (level of evidence B) [12, 14, 19].
Factors found to favor the occlusion of jailed branches are presented in Table 1. These include an increased amount of metallic coverage from device struts as well as neointimal coverage of the ostium (level B) [20]. Recent studies in swine have suggested that branches with an anastomotic circulation may be more readily occluded than similarly jailed terminal branches (level of evidence A) [21, 22].
Pathologically, residual aneurysms are reproducibly associated with persistent leaks lined with neointima, connecting the arterial with the aneurysmal lumen (level of evidence B) [13, 16, 17].
Discussion
Animal studies have provided evidence that flow diversion may be less effective in occluding large aneurysms with wide or undefined necks, as compared to smaller aneurysms with narrow necks. Straight sidewall aneurysms were more likely to become occluded after flow diversion than curved sidewall or bifurcation aneurysms. Branches originating from the neck or the fundus remain patent and may prevent aneurysm occlusion. Porosity and pore density are important, but challenging aneurysms are difficult to occlude despite the use of very low porosity or telescoping devices. Metallic density may not be uniform as devices adapt to local in vivo conditions.
These laboratory findings have a number of clinical implications. Flow diverters were initially approved for the treatment of unruptured cavernous and ophthalmic carotid aneurysms >10 mm [23]. This makes intuitive sense since large, wide-necked aneurysms are more likely to recur after coiling [24]. Systematic reviews have shown occlusion rates in the range of 75% [1, 25, 26], but these results reflect the frequent use of flow diversion in large aneurysms. The use of flow diversion has been extended beyond initial indications, with many clinical reports detailing use of flow diversion of small [27], bifurcation [28, 29], and even ruptured aneurysms [30]. Animal studies suggest that flow diversion may be particularly effective in small aneurysms with well-defined necks. Since coiling is not only safe but also quite effective in such aneurysms, a large-scale randomized trial is necessary to properly identify which option is best [31].
A survey of expert opinions has previously shown that flow diversion is more readily used in lateral wall than in bifurcation aneurysms [32]. Various teams have published conflicting clinical results in bifurcation aneurysms. While some authors report satisfactory safety and efficacy rates in middle cerebral artery bifurcation aneurysms [33], others are in line with animal studies, and are more pessimistic [28]. Animal findings regarding poor results in aneurysms with a branch originating from the aneurysm neck are also consistent with poor results in clinical aneurysms associated with a fetal-type posterior communicating artery [34, 35].
There has been a growing interest in better understanding, through benchtop or CFD studies, the deployment techniques, local anatomy, and typical deformations of flow diverters, which were first identified in animal models [19, 36, 37]. Problems concerning the more porous “transition zone,” and fish mouthing of device extremities, have been evoked to explain clinical complications [38, 39]. Whether clinicians should modify their technique, for example, by using telescoping devices when confronted with the transition zone problem, remains uncertain [40, 41].
Animal models are efficient methods to explore the use of innovative devices prior to clinical applications. They can permit the intentional manipulation of one variable while keeping all others constant in order to study the effects of that variable on an outcome of interest, such as efficacy. If animal models have helped us better understand factors involved in the success or failure of flow diversion in various aneurysm configurations, they have, in general, been poorly predictive of clinical complications. Several authors have published clinical concerns regarding jailed branch occlusion in patients [1, 42], but branch occlusions have rarely been documented in animal studies [2]. One exception may be the report of Iosif et al. studying the relationship between the type of collateral circulation and the fate of jailed branches; they were capable of reproducing results consistent with clinical series [21, 22].
Other rare but grave clinical complications of flow diversion, such as the rupture of unruptured aneurysms and the occurrence of parenchymal hematomas, have not been anticipated by animal studies [1, 43–46].
We have categorized evidence from animal studies according to two levels inspired from the work of Claude Bernard, often considered the father of modern experimental medicine. “In a word, I consider hospitals only as the entrance to scientific medicine; they are the first field of observation which a physician enters; but the true sanctuary of medical science is a laboratory…. In leaving the hospital, a physician… must go into his laboratory” [8]. Bernard emphasized experimentation, which he favored over observation: “…we give the name observer to a man who applies methods of investigation… to the study of phenomena which he does not vary and which he therefore gathers as nature offers them. We give the name experimenter to the man who applies methods of investigation… so as to make natural phenomena vary… and to make them present themselves in circumstances or conditions in which nature does not show them.” The distinction between observational and experimental evidence is reminiscent of the clinical research distinction between observational studies and clinical trials. If we are sympathetic to Bernard’s emphasis on preclinical experimentation, we do not share the disdain for clinical research that many authors attribute to him [8]. We must, however, remember that he lived before the development of clinical trial methodology. Thus, if important principles pertinent to flow diversion can be tested in the animal laboratory, translation of findings into clinical decisions is always problematic and must, at least in principle, be preceded by proper clinical trials whenever possible.
Conclusion
Factors that may impact aneurysm outcomes after flow diversion can be identified and intentionally manipulated using experiments in animal models. Insights obtained in animals may influence clinical decisions, but extrapolation of laboratory findings to clinical decisions must always be cautious.
References
Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF (2013) Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 44(2):442–447. doi:10.1161/STROKEAHA.112.678151
Fahed R, Raymond J, Ducroux C, Gentric JC, Salazkin I, Ziegler D, Gevry G, Darsaut TE (2016) Testing flow diversion in animal models: a systematic review. Neuroradiology 58(4):375–382. doi:10.1007/s00234-015-1635-0
Fahed R, Gentric JC, Salazkin I, Gevry G, Raymond J, Darsaut TE (2016) Flow diversion of bifurcation aneurysms is more effective when the jailed branch is occluded: an experimental study in a novel canine model. Journal of Neurointerventional Surgery. doi:10.1136/neurintsurg-2015-012240
Gentric JC, Salazkin I, Gevry G, Raymond J, Darsaut T (2015) Compaction of flow diverters improves occlusion of experimental wide-necked aneurysms. Journal of Neurointerventional Surgery. doi:10.1136/neurintsurg-2015-012016
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA: the Journal of the American Medical Association 283(15):2008–2012
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012. doi:10.1016/j.jclinepi.2009.06.005
Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions. Available online at http://www.handbook.cochrane.org/
LaFolette H, Shanks N (1994) Animal experimentation: the legacy of Claude Bernard. Int Stud Philos Sci 28(3):195–210
Kamran M, Yarnold J, Grunwald IQ, Byrne JV (2011) Assessment of angiographic outcomes after flow diversion treatment of intracranial aneurysms: a new grading schema. Neuroradiology 53(7):501–508
Gentric JC, Darsaut TE, Makoyeva A, Salazkin I, Raymond J (2014) The success of flow diversion in large and giant sidewall aneurysms may depend on the size of the defect in the parent artery. AJNR Am J Neuroradiol 35(11):2119–2124. doi:10.3174/ajnr.A4010
Chung B, Mut F, Kadirvel R, Lingineni R, Kallmes DF, Cebral JR (2015) Hemodynamic analysis of fast and slow aneurysm occlusions by flow diversion in rabbits. Journal of Neurointerventional Surgery 7(12):931–935. doi:10.1136/neurintsurg-2014-011412
Hodis S, Ding YH, Dai D, Lingineni R, Mut F, Cebral J, Kallmes D, Kadirvel R (2016) Relationship between aneurysm occlusion and flow diverting device oversizing in a rabbit model. Journal of Neurointerventional Surgery 8(1):94–98. doi:10.1136/neurintsurg-2014-011487
Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J (2012) Flow diverters failing to occlude experimental bifurcation or curved sidewall aneurysms: an in vivo study in canines. J Neurosurg 117(1):37–44. doi:10.3171/2012.4.JNS111916
Raymond J, Darsaut TE, Makoyeva A, Bing F, Salazkin I (2013) Endovascular treatment with flow diverters may fail to occlude experimental bifurcation aneurysms. Neuroradiology 55(11):1355–1363. doi:10.1007/s00234-013-1272-4
Darsaut TE, Bing F, Makoyeva A, Gevry G, Salazkin I, Raymond J (2014) Flow diversion of giant curved sidewall and bifurcation experimental aneurysms with very-low-porosity devices. World Neurosurgery 82(6):1120–1126. doi:10.1016/j.wneu.2013.09.036
Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J (2011) Testing flow diverters in giant fusiform aneurysms: a new experimental model can show leaks responsible for failures. AJNR Am J Neuroradiol 32(11):2175–2179. doi:10.3174/ajnr.A2657
Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J (2012) Flow diverters can occlude aneurysms and preserve arterial branches: a new experimental model. AJNR Am J Neuroradiol 33(10):2004–2009. doi:10.3174/ajnr.A3075
Sadasivan C, Cesar L, Seong J, Rakian A, Hao Q, Tio FO, Wakhloo AK, Lieber BB (2009) An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model. Stroke 40(3):952–958. doi:10.1161/STROKEAHA.108.533760
Bing F, Darsaut TE, Salazkin I, Makoyeva A, Gevry G, Raymond J (2013) Stents and flow diverters in the treatment of aneurysms: device deformation in vivo may alter porosity and impact efficacy. Neuroradiology 55(1):85–92. doi:10.1007/s00234-012-1082-0
Berg P, Iosif C, Ponsonnard S, Yardin C, Janiga G, Mounayer C (2016) Endothelialization of over- and undersized flow-diverter stents at covered vessel side branches: an in vivo and in silico study. J Biomech 49(1):4–12. doi:10.1016/j.jbiomech.2015.10.047
Iosif C, Berg P, Ponsonnard S, Carles P, Saleme S, Ponomarjova S, Pedrolo-Silveira E, Mendes GA, Waihrich E, Trolliard G, Couquet CY, Yardin C, Mounayer C (2016) Role of terminal and anastomotic circulation in the patency of arteries jailed by flow-diverting stents: from hemodynamic changes to ostia surface modifications. J Neurosurg 20:1–12
Iosif C, Berg P, Ponsonnard S, Carles P, Saleme S, Pedrolo-Silveira E, Mendes G, Waihrich E, Trolliard G, Couquet CY, Yardin C, Mounayer C (2016) Role of terminal and anastomotic circulation in the patency of arteries jailed by flow-diverting stents: animal flow model evaluation and preliminary results. J Neurosurg. doi:10.3171/2015.8.JNS151296
Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, Moran CJ, Woo HH, Lopes DK, Berez AL, Cher DJ, Siddiqui AH, Levy EI, Albuquerque FC, Fiorella DJ, Berentei Z, Marosfoi M, Cekirge SH, Nelson PK (2013) Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 267(3):858–868. doi:10.1148/radiol.13120099
Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, Lamoureux J, Chagnon M, Roy D (2003) Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke; a Journal of Cerebral Circulation 34(6):1398–1403. doi:10.1161/01.STR.0000073841.88563.E9
Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M (2013) Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery 73(2):193–199 . doi:10.1227/01.neu.0000430297.17961.f1discussion 199-200
Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, Maiuri F (2015) Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J 28(4):365–375. doi:10.1177/1971400915602803
Puri AS, Massari F, Asai T, Marosfoi M, Kan P, Hou SY, Howk M, Perras M, Brooks C, Clarencon F, Gounis MJ, Wakhloo AK (2016) Safety, efficacy, and short-term follow-up of the use of pipeline embolization device in small (<2.5 mm) cerebral vessels for aneurysm treatment: single institution experience. Neuroradiology 58(3):267–275. doi:10.1007/s00234-015-1630-5
Caroff J, Neki H, Mihalea C, D’Argento F, Abdel Khalek H, Ikka L, Moret J, Spelle L (2016) Flow-diverter stents for the treatment of saccular middle cerebral artery bifurcation aneurysms. AJNR Am J Neuroradiol 37(2):279–284. doi:10.3174/ajnr.A4540
Saleme S, Iosif C, Ponomarjova S, Mendes G, Camilleri Y, Caire F, Boncoeur MP, Mounayer C (2014) Flow-diverting stents for intracranial bifurcation aneurysm treatment. Neurosurgery 75(6):623–631 . doi:10.1227/NEU.0000000000000522quiz 631
Rouchaud A, Brinjikji W, Cloft HJ, Kallmes DF (2015) Endovascular treatment of ruptured blister-like aneurysms: a systematic review and meta-analysis with focus on deconstructive versus reconstructive and flow-diverter treatments. AJNR Am J Neuroradiol 36(12):2331–2339. doi:10.3174/ajnr.A4438
Raymond J, Gentric JC, Darsaut TE, Iancu D, Chagnon M, Weill A, Roy D (2016) Flow diversion in the treatment of aneurysms: a randomized care trial and registry. J Neurosurg November 4:1–9
Darsaut TE, Gentric JC, McDougall CM, Gevry G, Roy D, Weill A, Raymond J (2015) Uncertainty and agreement regarding the role of flow diversion in the management of difficult aneurysms. AJNR Am J Neuroradiol 36(5):930–936. doi:10.3174/ajnr.A4201
Gawlitza M, Januel AC, Tall P, Bonneville F, Cognard C (2016) Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: a single-center series with special emphasis on covered cortical branches and perforating arteries. Journal of Neurointerventional Surgery 8(5):481–487. doi:10.1136/neurintsurg-2015-011682
Kan P, Duckworth E, Puri A, Velat G, Wakhloo A (2016) Treatment failure of fetal posterior communicating artery aneurysms with the pipeline embolization device. Journal of Neurointerventional Surgery 8(9):945–948. doi:10.1136/neurintsurg-2015-011959
Tsang AC, Fung AM, Tsang FC, Leung GK, Lee R, Lui WM (2015) Failure of flow diverter treatment of intracranial aneurysms related to the fetal-type posterior communicating artery. Neurointervention 10(2):60–66. doi:10.5469/neuroint.2015.10.2.60
Shapiro M, Raz E, Becske T, Nelson PK (2014) Variable porosity of the pipeline embolization device in straight and curved vessels: a guide for optimal deployment strategy. AJNR Am J Neuroradiol 35(4):727–733. doi:10.3174/ajnr.A3742
Aurboonyawat T, Blanc R, Schmidt P, Piotin M, Spelle L, Nakib A, Moret J (2011) An in vitro study of silk stent morphology. Neuroradiology 53(9):659–667. doi:10.1007/s00234-010-0784-4
Estrade L, Makoyeva A, Darsaut TE, Ghostine J, Kouznetsov E, Salazkin I, Roy D, Weill A, Raymond J (2013) In vitro reproduction of device deformation leading to thrombotic complications and failure of flow diversion. Interventional Neuroradiology: Journal of Peritherapeutic Neuroradiology, Surgical Procedures and Related Neurosciences 19(4):432–437
Darsaut TE, Rayner-Hartley E, Makoyeva A, Salazkin I, Berthelet F, Raymond J (2013) Aneurysm rupture after endovascular flow diversion: the possible role of persistent flows through the transition zone associated with device deformation. Interventional Neuroradiology: Journal of Peritherapeutic Neuroradiology, Surgical Procedures and Related Neurosciences 19(2):180–185
Makoyeva A, Bing F, Darsaut TE, Salazkin I, Raymond J (2013) The varying porosity of braided self-expanding stents and flow diverters: an experimental study. AJNR Am J Neuroradiol 34(3):596–602. doi:10.3174/ajnr.A3234
Shapiro M, Raz E, Becske T, Nelson PK (2014) Building multidevice pipeline constructs of favorable metal coverage: a practical guide. AJNR Am J Neuroradiol 35(8):1556–1561. doi:10.3174/ajnr.A3902
Lall RR, Crobeddu E, Lanzino G, Cloft HJ, Kallmes DF (2014) Acute branch occlusion after pipeline embolization of intracranial aneurysms. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia 21(4):668–672. doi:10.1016/j.jocn.2013.07.011
Raymond J, Darsaut TE, Kotowski M, Makoyeva A, Gevry G, Berthelet F, Salazkin I (2013) Thrombosis heralding aneurysmal rupture: an exploration of potential mechanisms in a novel giant swine aneurysm model. AJNR Am J Neuroradiol 34(2):346–353. doi:10.3174/ajnr.A3407
Cebral JR, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, Putman CM (2011) Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol 32(1):27–33. doi:10.3174/ajnr.A2398
Kulcsar Z, Houdart E, Bonafe A, Parker G, Millar J, Goddard AJ, Renowden S, Gal G, Turowski B, Mitchell K, Gray F, Rodriguez M, van den Berg R, Gruber A, Desal H, Wanke I, Rufenacht DA (2011) Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 32(1):20–25. doi:10.3174/ajnr.A2370
Rouchaud A, Brinjikji W, Lanzino G, Cloft HJ, Kadirvel R, Kallmes DF (2016) Delayed hemorrhagic complications after flow diversion for intracranial aneurysms: a literature overview. Neuroradiology 58(2):171–177. doi:10.1007/s00234-015-1615-4
Cebral JR, Mut F, Raschi M, Hodis S, Ding YH, Erickson BJ, et al. Analysis of hemodynamics and aneurysm occlusion after flow-diverting treatment in rabbit models. AJNR Am J Neuroradiol. 2014;35:1567–1573
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that this manuscript does not contain clinical studies or patient data.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fahed, R., Darsaut, T.E., Gentric, JC. et al. Flow diversion: what can clinicians learn from animal models?. Neuroradiology 59, 255–261 (2017). https://doi.org/10.1007/s00234-016-1781-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1781-z