Abstract
Shroom is a family of related proteins linked to the actin cytoskeleton, and one of them, xShroom1, is constitutively expressed in Xenopus laevis oocytes which is required for the expression of the epithelial sodium channel (ENaC). On the other hand, ENaC and the cystic fibrosis transmembrane regulator (CFTR) are co-expressed in many types of cells with a negative or positive interaction depending on the studied tissues. Here, we measured the amiloride-sensitive ENaC currents (INaamil) and CFTR currents (ICFTR) with voltage clamp techniques in oocytes co-injected with ENaC and/or CFTR and xShroom1 antisense oligonucleotides. The objective was to study the mechanism of regulation of ENaC by CFTR when xShroom1 was suppressed and the endocytic traffic of CFTR was blocked. CFTR activation had a measurable negative effect on ENaC and this activation resulted in a greater inhibition of INaamil than with xShroom1 antisense alone. Our results with Dynasore, a drug that acts as an inhibitor of endocytic pathways, suggest that the changes in INaamil by xShroom1 downregulation were probably due to an increment in channel endocytosis. An opposite effect was observed when ICFTR was measured. Thus, when xShroom1 was downregulated, the ICFTR was larger than in the control experiments and this effect is not observed with Dynasore. A speculative explanation could be that xShroom1 exerts a dual effect on the endocytic traffic of ENaC and CFTR and these actions were canceled with Dynasore. In the presence of Dynasore, no difference in either INaamil or ICFTR was observed when xShroom1 was downregulated.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two main pieces of information are associated with the objective of this investigation: the regulation of function and the interaction of the epithelial sodium channel (ENaC) with the cystic fibrosis transmembrane regulator (CFTR) channel. ENaC is a member of the ENaC/degenerin ion channel family composed of three homologous subunits (α, β, and γ). It mediates entry of Na+ from the luminal fluid into the cells in many reabsorbing epithelia, it is blocked by the diuretic amiloride, it is sensitive to many hormones such as aldosterone, cytosolic, and extracellular pH (Collier and Snyder 2009; Kashlan et al. 2015; Reddy et al. 2008), and it is activated by proteases which cleave specific sites in the extracellular loops of the α, γ subunits but not the β subunit (Gentzsch et al. 2010; Kashlan et al. 2011; Zachar et al. 2015). Shroom is a family of four different proteins (Shroom1 to Shroom4) involved in the regulation of cytoskeletal architecture by binding to actin, morphogenesis of embryonic epithelial tissues, and neuronal growth (Hagens et al. 2006; Hildebrand et al. 2021). Of particular interest for us is xShroom1 (APX), a large protein constitutively expressed in Xenopus laevis oocytes and initially identified as a molecule required in ENaC activity in X. laevis epithelial cells (Staub et al. 1992). It has been well described that Shroom family proteins influence both microtubules and actin cytoskeletons. xShroom1 is associated with α-spectrin, a cytoskeletal protein known to shape the plasma membranes of cells (Zuckerman et al. 1999), and ectopic expression of xShroom1 causes accumulation of γ-tubulin, a microtubule nucleating protein, at the apical surface of epithelial cells (Lee et al. 2007). In addition, Shroom family genes are expressed in many thickened epithelial sheets. xShroom1 and xShroom2 are expressed in the deep layer of neuroepithelium and control apicobasal cell elongation (Lee et al. 2009). It has been shown that xShroom1 has a dual action on the expression and level of activity of ENaC and CFTR. Suppression of xShroom1 resulted in a decrement of ENaC function (Assef et al. 2011; Prat et al. 1996; Zuckerman et al. 1999), whereas the opposite was found on CFTR in X. laevis (Palma et al. 2016).
Second, ENaC and CFTR are co-expressed at the apical surface of epithelia and other tissues. CFTR is a cAMP activated, ATP-dependent Cl− channel, which transports Cl− and also HCO3− ions from the intracellular to luminal space in several tissues. In addition to these functions, both ENaC and CFTR channels are involved in cell migration and proliferation (Liao et al. 2018; del Mónaco et al. 2009; Marino and Kotsias 2014; Schiller et al. 2010; Sun et al. 2011). CFTR is also a regulator of other channels, and the modulation of ENaC function serves as a prime example of the regulatory function of CFTR in tissues. It is also an extracellular chloride sensor (Broadbent et al. 2015). The functional positive or negative interplay between ENaC and CFTR is complex and incompletely understood (see “Discussion”).
In a past publication (Palma et al. 2016), we reported an increment in CFTR currents and CFTR cell-surface expression in oocytes co-injected with xShroom1 antisense oligonucleotides, and we suggested a number of factors controlling the expression or activity of CFTR including membrane insertion, degradation, channel synthesis, intracellular channel trafficking, and open probability. In this investigation, we further pursued these experiments using oligonucleotides against xShroom1 and Dynasore to block the dynamin-dependent endocytosis in oocytes expressing the wild-type mouse ENaC and human CFTR. Our results suggest that xShroom1 downregulation decreases CFTR endocytosis, and in this way, CFTR caused a greater inhibition of the amiloride-sensitive Na+ currents (INaamil) than would be predicted by the downregulation of xShroom1 alone. In addition, our results also confirm that heterologous expression in X. laevis oocytes is a suitable system for the study of this interaction.
Material and Methods
Xenopus laevis Oocytes
Adult female Xenopus laevis frogs were anesthetized with 0.3% tricaine (MS-222), and the oocytes were surgically removed from the abdominal incision. Oocytes were defolliculated by incubation with 1 mg/ml type IV collagenase for 40 min. The oocytes were placed in ND96 medium containing (in mM) NaCl 96, KCl 2, CaCl2 1.8, and HEPES 5 (pH 7.4) supplemented with 1 μg/ml gentamicin. We synthesized complementary RNAs (cRNAs) for human wild-type CFTR using the T7 mMessagemMachine kit (Ambion, Austin, TX), and for α, β, and γ mouse wild-type ENaC subunits using the T3 mMessage mMachine kit (Ambion, Austin, TX). We used synthetic oligodeoxynucleotides complementary to nucleotides + 455 to + 479 of xShroom1 (Zuckerman et al. 1999) (sense, 5′-GCA TTA AGC AGA ATC GCC CTA ACC AC-3′; antisense, 5′-GTG GTT AGG GCG ATT CTG CTT ATG C-3′, Integrated DNA Technologies, Biodynamics SRL). Oocytes were injected with a Drummond injector (Drummond, Broomall, PA) with 4 ng of CFTR cRNA, 2 ng of α, β, and γ ENaC cRNA and/or 25 ng of xShroom1 sense or antisense oligonucleotides (total volume 50 nl).
Reagents
The reagents used were amiloride 10 μM (Alomone Labs, Jerusalem, Israel), forskolin 10 μM (Alomone Labs, Jerusalem, Israel), IBMX 1 mM (Sigma-Aldrich, St. Louis, USA), and dynasore 80 μM (Sigma-Aldrich, St. Louis, USA).
Electrophysiology
A standard two-electrode voltage clamp was performed using a Warner Oocyte Clamp OC 725C (Warner Instruments, Hamden, CT) with a bath probe circuit. We acquired data through Clampex 8.0 (Axon Instruments, Union City, CA) using a DigiData 1220A interface at 1 kHz. Micropipettes had resistances of 1–3 MΩ when filled with 3 M KCl. We clamped the bath with two chloride silver wires through 3% agar bridges in 3 M KCl and positioned close to the oocyte. In the well with the oocyte, we estimated the bath-fluid resistance as the resistance between both electrodes (about 100–200 Ω). Without the bath probe, this value is increased by a factor of 10 or 20. Thus, all the experiments were done using the bath probe circuit to keep this resistance in series with the membrane and between electrodes as low as possible. We perfused the oocyte chamber (0.6 ml/min) with a peristaltic pump (Dynamax RP-1; Rainin Instruments, Woburn, MA) and the solution ejected by a needle placed on top of the well containing the oocyte. Following the insertion of both microelectrodes, we waited for 5 min before starting the experiment. We ran two sets of records with a delay of 5 min to be sure that the currents were stable. Then we applied amiloride and we recorded the currents at 3 and 5 min, enough time to have a stable blocking effect. For activation of CFTR, we applied 10 μM of forskolin + 1 mM of IBMX, and the currents were recorded at 15 min of incubation, enough time to have a stable channel activation effect. For the current–voltage (I–V) relationships, we applied a series of 500 ms voltage steps from − 140 to + 60 mV in 20 mV increments. The currents were measured after 400 ms at a clamp potential of 0 mV. ENaC-mediated Na+ currents were defined as the current difference measured in the absence versus the presence of 10 μM amiloride in the bath solution. CFTR-mediated Cl− currents were defined as the current difference measured in the absence versus the presence of forskolin + IBMX in the bath solution (Kunzelmann 2011; Qadri et al. 2011).
Statistical Analysis
Data were expressed as mean values ± standard error (SE) (n = number of cells and repetitions). Statistical analysis for differences between experimental groups was performed using Graphpad Prism software, applying unpaired Student’s t test. Differences were considered statistically significant when p < 0.05.
Results
Basic ENaC Currents in Oocytes
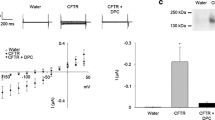
The first experiments were done to determine the expression of ENaC currents in X. laevis oocytes as the basis for subsequent experiments. We recorded ENaC currents in oocytes co-injected with human CFTR cRNAs and mouse ENaC cRNAs and the results are shown in Fig. 1 when ENaC was inhibited with amiloride. Under these conditions, only ENaC currents are present because CFTR is inactive (Bachhuber et al. 2005; Drumm et al. 1991, see below). The left panel shows the currents in response to negative or positive pulses in the control solution (ND96) and after the inhibition with amiloride. After the subtraction of the blocked component, we obtained amiloride-sensitive sodium currents (INaamil) and the average results obtained from 8 experiments are shown in the right panel with the I–V curves. With a pulse of − 100 mV amiloride significantly reduced the currents from − 1.96 ± 0.44 to − 0.60 ± 0.23 µA (p < 0.05, n = 8).
Left panel. Records of ENaC currents in oocytes co-injected with human CFTR cRNAs and mouse ENaC cRNAs and the inhibition of these currents when 10 µM amiloride was added to the bath. The potential of the cell was held at 0 mV and switched to values of between − 160 and + 40 mV for 500 ms. Under our experimental conditions, only ENaC currents are present because CFTR is inactive. The right panel shows the I-V plot with the average results of INaamil obtained from oocytes after subtracting the current remnant in amiloride from the control values
Activation of CFTR Inhibits Amiloride-sensitive Sodium Currents
The next experiments were done to evaluate INaamil when the CFTR channels were active. To do so, we incubated CFTR and ENaC co-injected oocytes with 10 μM of forskolin and 1 mM of IBMX, both drugs known to stimulate CFTR (Bachhuber et al. 2005; Drumm et al. 1991), and the results are shown in Fig. 2. As it can be seen, the activation of CFTR clearly diminished the INaamil obtained with the same protocol as in Fig. 1. With a − 100 mV pulse, the INaamil was − 1.38 ± 0.40 μA vs. − 0.67 ± 0.40 μA (n = 8, p < 0.01).
I-V plot showing the reduction in INaamil when the CFTR channels were activated. In these experiments, the CFTR and ENaC co-injected oocytes were incubated in 10 μM of forskolin and 1 mM of IBMX to stimulate the CFTR channels. The INaamil was obtained with the same protocol as in Fig. 1
xShroom1 Downregulation Enhances Amiloride-Sensitive Sodium Current Inhibition by CFTR
To determine if xShroom1 protein is involved in the CFTR and ENaC regulation, we studied oocytes expressing both channels and co-injected with xShroom1 antisense oligonucleotides. In Fig. 3a, the I–V curves show that xShroom1 downregulation reduced the INaamil in every pulse applied. Thus, the INaamil with a − 100 mV pulse was about a quarter (− 0.26 ± 0.11 µA, n = 5) with respect to control oocytes co-injected with xShroom1 sense oligonucleotides (− 1.97 ± 0.62 µA, n = 6, p < 0.05).
Panels a and b show the INaamil in oocytes expressing ENaC and CFTR. a I-V curve with the average values when xShroom1 was downregulated with antisense oligonucleotides in comparison with the control ones (sense). b INaamil when CFTR was activated with Forskolin plus IBMX in oocytes with xShroom1 downregulated. c Comparison in the reduction of INaamil (pulse − 100 mV) when CFTR was activated in oocytes injected with sense or antisense oligonucleotides against xShroom1. It is evident that the INaamil inhibition by activation of CFTR was higher when xShroom1 was downregulated
Figure 3b shows the I-V curve of INaamil in oocytes injected with xShroom1 antisense and incubated with forskolin and IBMX to induce CFTR activity. The incubation was for 15 min, enough time to stably activate the channel (Palma et al. 2016). Under these conditions, i.e., downregulation of CFTR by xShroom1, we observed an additional reduction in amiloride-sensitive ENaC current beyond that observed with xShroom1 alone (100 mV pulse: − 0.07 ± 0.05 µA, n = 8, p < 0.05). Figure 3c shows a summary of the inhibition of INaamil (− 100 mV pulse) by activation of CFTR in oocytes injected with sense or antisense oligonucleotides against xShroom1.
xShroom1 Downregulation Effects Upon INaamil and ICFTR in the Presence of Dynasore
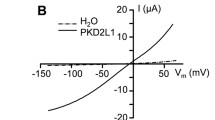
The next experiments were done to compare the effect of downregulating xShroom1 on the INaamil and ICFTR and also to see the effect of blocking the endocytic traffic of these channels by means of Dynasore. ICFTR was recorded in oocytes injected with antisense oligonucleotides for xShroom1 and incubated for 24 h in the absence or presence of 80 μM of Dynasore, a concentration enough to inhibit dynamin, a protein necessary for the formation of clathrin-coated vesicles and used in endocytic-trafficking studies of ion channels (Pergel et al. 2021; Wesch et al. 2012; Young et al. 2009). The left panels of Fig. 4a and b show the effect of downregulation of xShroom1 on the ICFTR and INaamil (− 100 mV pulse) in the absence of Dynasore. When xShroom1 was downregulated, the currents through IBMX/forskolin-activated CFTR were increased by a factor of 5 over the control. Regarding the effect of xShroom1 on ENaC, the comparison between Fig. 4a and b (Control) shows a dual action of xShroom1 on the level of activity of ENaC and CFTR. Suppression of xShroom1 resulted in an increment in CFTR function whereas the opposite was found for ENaC, although this negative effect of xShroom1 antisense on ENaC was not observed in the presence of Dynasore. In addition, our results also confirm that heterologous expression in X. laevis oocytes is a suitable system for the study of this interaction.
ICFTR and INaamil in oocytes injected with xShroom1 antisense or sense oligonucleotides in the absence (control) and presence of 80 µM Dynasore for 24 h. When xShroom1 was downregulated, the ICFTR was increased by a factor of 5 over the control and the opposite result was obtained in INaamil (left panels). In the presence of Dynasore, no difference in these currents was recorded in oocytes with xShroom1 downregulated (right panels)
Dynasore Effect of CFTR Activation on the INaamil
Next, we measured the effect of Dynasore upon the reduction in INaamil once CFTR was activated. Figure 5a, b shows that the reduction in INaamil when CFTR was activated was not changed with the xShroom1 antisense. Thus, the negative effect of CFTR activation on the INaamil is not dependent on xShroom1.
In these sets of panels, the INaamil (− 100 mV pulse) was recorded when CFTR was activated in the presence of Dynasore in oocytes with xShroom1 expressed (a) and when it was downregulated with antisense oligonucleotides (b). In both conditions, the activation of CFTR with forskolin plus IBMX reduced the INaamil (see Fig. 3c for comparison)
Discussion
In this work, we analyzed the regulation of the epithelial sodium channel (ENaC) by proteins of the Shroom family involved in the cytoskeletal function (see “Introduction”) and the interaction of ENaC with the cystic fibrosis transmembrane regulator (CFTR) channel. First, we will discuss the role of xShroom1 protein on the activity of ENaC and CFTR, second, the interaction between these two proteins, then the role of endocytic trafficking in the mentioned effects and finally the comparison between different species of these channels.
It is evident from our previous results (Assef et al. 2011; Palma et al. 2014, 2016) and the comparison between Fig. 4a and b (Control) that there is a dual action of xShroom1 on the level of activity of ENaC and CFTR in oocytes from X. laevis. Much to our surprise, suppression of xShroom1 resulted in an increment in CFTR function whereas the opposite was found for ENaC. Many of the functions of ENaC and CFTR are through interactions with actin, actin-binding proteins, or scaffolding proteins (Karpushev et al. 2010; Santos et al. 2020). Thus, several ENaC-regulatory proteins function within a multiprotein complex which controls the channel expression and activity. This is the case of CNK3, a scaffold protein which has a PDZ domain and serves as a stimulatory factor for ENaC (Soundararajan et al. 2012). On the other hand, Boucherot et al. (2001) found that a CFTR mutant, which lacks the last six amino acids encoding the PDZ-binding domain, resulted in a larger current than wild-type CFTR.
CFTR activation had a measurable negative effect on ENaC as it is shown in Fig. 3, and this activation resulted in a greater inhibition of INaamil than the one obtained with the xShroom1 antisense alone. The interplay between CFTR and ENaC is complex and incompletely understood. Tissues and species differences may account for the discrepant findings reported in the literature. For example, activation of ENaC requires CFTR function in sweat ducts (Reddy et al. 1999; Reddy and Quinton 2005) and in human alveolar type II cell (Bove et al. 2010), whereas Na+ absorption is elevated in defective airways in cystic fibrosis (see Collawn et al. 2012; Strandvik 2021, for references). There are reports showing that CFTR activation by cAMP caused an inhibition of INaamil in oocytes co-expressing rat α, β, and γ ENaC and CFTR (Bachhuber et al. 2005; Briel et al. 1998; Chabot et al. 1999), and similar results were obtained with mouse α,β,γ ENaC co-expressed with CFTR (Yan et al. 2004) but not in human α,β,γ ENaC when co-expressed with CFTR (Nagel et al. 2005; Yan et al. 2004; see references in Rauh et al. 2017). In addition, we showed that the inhibition of ENaC by the activation of CFTR is greater when xShroom1 is blocked with the antisense oligonucleotides, and this is also in agreement with the results of Boucherot et al. (2001), showing that inhibition of ENaC was linked to Cl− currents generated by CFTR and was observed in the presence of Cl−, I−, or Br− but not gluconate, although Suaud et al. (2007) found that chloride transport is not necessary for inhibition of ENaC.
Third, we will discuss the effects of Dynasore upon the interplay between ENaC and CFTR. Dynasore acts as a potent inhibitor of endocytic pathways known to depend on dynamin, essential for clathrin-dependent coated vesicle formation, by rapidly blocking coated vesicle formation (Macia et al. 2006). It has been previously used (at the same concentration as in our experiments) to probe the role of dynamin in the endocytic trafficking of CFTR and ENaC by Young et al. and Wesch et al. with similar results. Our experiments support the idea that CFTR and ENaC undergo endocytosis, at least in part, through the classically described dynamin-dependent, clathrin-mediated endocytosis. The results presented in Figs. 4 and 5 with Dynasore suggest that the changes in INaamil by xShroom1 downregulation were probably due to an increment in the endocytosis of the channels. In other words, xShroom1 impairs in some manner the endocytic traffic of ENaC, and this effect is antagonized with Dynasore. Unexpectedly, an opposite effect was observed when ICFTR was measured. Thus, when xShroom1 was downregulated, the ICFTR was larger than in the control experiments and this effect is not observed with Dynasore (Fig. 4a).
Finally, the use in the present study of two channels from different species to analyze the interaction between them will be discussed.
Although there is a possibility that the relationship between CFTR and ENaC channels from same species may be different to the found in our investigation, it has been well described that αβγ-ENaC subunits are highly conserved between different species, including rat and human ENaC (Hanukoglu and Hanukoglu 2017; Voilley et al. 1994). Additionally, β and γ rat ENaC share sequence identity to human ENaC in the proline-rich P2 regions (Staub et al. 1996), which have been shown to bind the E3 ubiquitin ligases Nedd4 suppressing ENaC activity by decreasing its cell-surface stability (Lu et al. 2007).
Moreover, the literature shows that mouse ENaC has several characteristics in common with human ENaC that could suggest that the relationship between human CFTR and mouse ENaC could be the same as the relationship between human CFTR and human ENaC.
In previous studies, it was reported that the relationship between CFTR and ENaC is inhibitory in human cells, as the results presented here. In human primary culture from airways, it was shown that CFTR impedes the proteolytic processing of ENaC, regulating the channel negatively (Gentzsch et al. 2010). Besides, Mall et al. (1999) found that the amiloride-sensitive sodium currents are inhibited by CFTR activation in normal human colon biopsies but not in tissue biopsies from cystic fibrosis patients.
In addition, both human and mouse ENaC are endocyted from the plasma membrane through clathrin-mediated endocytosis; thus, we would expect the same results from human and mouse ENaC. Evidence from oocytes studies demonstrate that the process is dynamin dependent, consistent with a role for clathrin-mediated endocytosis. With the use of two different tools, Pitstop-2, an inhibitor of the clathrin-mediated endocytosis or mutating clathrin adaptor protein 2 (AP-2) recognition motifs in the C-termini of β- and/or γ-ENaC, it was shown that ENaC endocytosis is clathrin mediated in X. laevis oocytes injected with human ENaC cRNA (Ilyaskin et al. 2021). Moreover, Shimkets et al. (1997) found that rat ENaC channels are also removed from the plasma membrane through clathrin-mediated endocytosis in X. laevis oocytes. Wang et al. (2006) demonstrated that ENaC is present in clathrin-coated vesicles in mouse mpkCCDc14 cells and is efficiently endocytosed. They also showed in X. laevis oocytes that the co-expression of mouse ENaC and epsin, a clathrin adaptor protein, resulted in the downregulation of the channel activity.
Furthermore, several studies demonstrated that the endocytosis inhibitor used in the present study, Dynasore, blocked both human and mouse ENaC endocytosis, as would be expected for a channel whose surface expression is regulated by clathrin-mediated endocytosis. ENaC endocytosis was inhibited with dynasore in X. laevis oocytes injected with rat ENaC cRNAs and in mouse M1 cortical-collecting duct cells (Almaça et al. 2009). In addition, Wesch et al. (2012) showed a significant increase of amiloride-sensitive sodium current due to blocked endocytosis of the channel in the presence of dynasore, in X. laevis oocytes injected with human ENaC cRNAs.
The number of channels at the cell surface is determined by the balance between insertion of new channels into the plasma membrane and the endocytosis and degradation of channels from the membrane, whereas other additional factors influence the amount, stability, half-life, and activity of CFTR and ENaC at the surface membrane (see Butterworth 2010; Farinha et al. 2013 for references). In this context, a speculative explanation could be that xShroom1 exerts a dual effect on the endocytic traffic of ENaC and CFTR, a negative action upon ENaC and a positive one with CFTR, and both of these actions were canceled with Dynasore. In its presence no difference in either INaamil or ICFTR was observed when xShroom1 was downregulated.
For the maintenance of cellular homeostasis a coordinated interaction between ENaC and CFTR is necessary. The importance of a correct balance of these channels' activity is demonstrated in several pathologies. Therefore, it is essential to understand the mechanism of regulation of ENaC by CFTR. Our data show an interaction between CFTR and ENaC and suggest that xShroom1 regulates both channels, indicating that xShroom1 could have a role in the channels' deregulation in several pathologies.
References
Almaça J, Kongsuphol P, Hieke B, Ousingsawat J, Viollet B, Schreiber R, Amaral MD, Kunzelmann K (2009) AMPK controls epithelial Na+ channels through Nedd4-2 and causes an epithelial phenotype when mutated. Pflugers Arch - Eur J Physiol 458:713–721. https://doi.org/10.1007/s00424-009-0660-4
Assef YA, Ozu M, Marino GI, Galizia L, Kotsias BA (2011) ENaC channels in oocytes from Xenopus laevis and their regulation by xShroom1 protein. Cell Physiol Biochem 28:259–266
Bachhuber T, König J, Voelcker T, Mürle B, Schreiber R, Kunzelmann K (2005) Cl- Interference with the Epithelial Na+ Channel ENaC. J Biol Chem 280:31587–31594. https://doi.org/10.1074/jbc.M504347200
Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, O’Neal WK, Boucher RC (2010) Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem 285:34939–34949
Briel M, Greger R, Kunzelmann K (1998) Cl transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol 508:825–836
Boucherot A, Schreiber R, Kunzelmann K (2001) Role of CFTR’s PDZ1-binding domain, NBF1 and Cl- conductance in inhibition of epithelial Na+ channels in Xenopus oocytes. BB Acta 1515:64–71
Broadbent SD, Ramjeesingh M, Bear CE, Argent BE, Linsdell P, Gray MA (2015) The cystic fibrosis transmembrane conductance regulator is an extracellular chloride sensor. Pflugers Arch 467:1783–1794
Butterworth MB (2010) Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Biochim Biophys Acta 1802:1166–1177
Chabot H, Vives MF, Dagenais A, Grygorczyk C, Berthiaume Y, Grygorczyk R (1999) Downregulation of epithelial sodium channel (ENaC) by CFTR co-expressed in Xenopus oocytes is independent of Cl conductance. J Membr Biol 169:175–188
Collawn JF, Lazrak A, Bebok Z, Matalon S (2012) The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302:L1141–L1146
Collier DM, Snyder PM (2009) Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 284:792–798. https://doi.org/10.1074/jbc.M806954200
del Mónaco SM, Marino GI, Assef YA, Damiano AE, Kotsias BA (2009) Cell migration in BeWo cells and the role of epithelial sodium channels. J Membr Biol 232:1–13
Drumm ML, Wilkinson DJ, Smit LS, Worrell RT, Strong TV, Frizzell RA, Dawson DC, Collins FS (1991) Chloride conductance expressed by AF508 and other mutant CFTRs in Xenopus oocytes. Science 254:1797–1799
Farinha CM, Matos P, Amaral MD (2013) Control of cystic fibrosis transmembrane conductance regulator membrane trafficking: not just from the endoplasmic reticulum to the Golgi. FEBS J 280:4396–4406
Gentzsch M, Dang H, Dang Y, Garcia-Caballero A, Suchindran H, Boucher RC, Stutts MJ (2010) The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na channel. J Biol Chem 285:32227–32232
Hagens O, Ballabio A, Kalscheuer V, Kraehenbuhl JP, Schiaffino MV, Smith P, Staub O, Hildebrand J, Wallingford JB (2006) A new standard nomenclature for proteins related to Apx and Shroom. BMC Cell Biol 7:18. https://doi.org/10.1186/1471-2121-7-18
Hanukoglu I, Hanukoglu A (2017) Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 579:95–132. https://doi.org/10.1016/j.gene.2015.12.061
Hildebrand JD, Leventry AD, Aideyman OP et al (2021) A modifier screen identifies regulators of cytoskeletal architecture as mediators of Shroom-dependent changes in tissue morphology. Biol Open 10:055640. https://doi.org/10.1242/bio.055640
Ilyaskin AV, Korbmacher C, Diakov A (2021) Inhibition of the epithelial sodium channel (ENaC) by connexin 30 involves stimulation of clathrin-mediated endocytosis. J Biol Chem 296:100404. https://doi.org/10.1016/j.jbc.2021.100404
Karpushev AV, Ilatovskaya DV, Pavlov TS, Negulyaev YA, Staruschenko A (2010) Intact cytoskeleton is required for small G protein dependent activation of the epithelial Na+ channel. PLoS ONE 5:e8827. https://doi.org/10.1371/journal.pone.0008827
Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M (2011) Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel α subunit reveals a mechanism of channel activation by proteases. J Biol Chem 286:649–660. https://doi.org/10.1074/jbc.M110.167098
Kashlan OB, Blobner BM, Zuzek Z, Tolino M, Kleyman TR (2015) Na+ inhibits the epithelial Na+ channel by binding to a site in an extracellular acidic cleft. J Biol Chem 290:568–576. https://doi.org/10.1074/jbc.M114.606152
Kunzelmann K (2011) Introduction to section V: assessment of CFTR function. Methods Mol Biol 741:407–418
Lee C, Scherr HM, Wallingford JB (2007) Shroom family proteins regulate-tubulin distribution and microtubule architecture during epithelial cell shape change. Development 134:1431–1441. https://doi.org/10.1242/dev.02828
Lee C, Le M-P, Wallingford JB (2009) The shroom family proteins play broad roles in the morphogenesis of thickened epithelial sheets. Dev Dyn 238:1480–1491
Liao H, Chen Y, Li Y et al (2018) CFTR is required for the migration of primordial germ cells during zebrafish early embryogenesis. Reproduction 156:261–268. https://doi.org/10.1530/REP-17-0681
Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D (2007) The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic 8:1246–1264. https://doi.org/10.1111/j.1600-0854.2007.00602.x
Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10:839–850
Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K (1999) CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am J Physiol 277:G709–G716
Marino GI, Kotsias BA (2014) Cystic fibrosis transmembrane regulator (CFTR) in human trophoblast BeWo cells and its relation to cell migration. Placenta 35:92–98
Nagel G, Barbry P, Chabot H, Brochiero E, Hartung K, Grygorczyk R (2005) CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes. J Physiol 564:671–682
Palma AG, Galizia L, Kotsias BA et al (2016) CFTR channel in oocytes from Xenopus laevis and its regulation by xShroom1 protein. Pflugers Arch Eur J Physiol 468:871–880
Palma AG, Kotsias BA, Marino GI (2014) CFTR and ENaC functions in cystic fibrosis. Medicina (b Aires) 74:133–139
Pergel E, Veres I, Csigi GI, Czirják G (2021) Translocation of TMEM175 lysosomal potassium channel to the plasma membrane by dynasore compounds. Int J Mol Sci 22:10515. https://doi.org/10.3390/ijms221910515
Prat AG, Holtzman EJ, Brown D, Cunningham CC, Reisin IL, Kleyman TR, McLaughlin M, Jackson GR Jr, Lydon J, Cantiello HF (1996) Renal epithelial protein (Apx) is an actin cytoskeleton-regulated Na+ channel. J Biol Chem 271:18045–18053
Qadri YJ, Cormet-Boyaka E, Benos DJ, Berdiev BK (2011) CFTR regulation of epithelial sodium channel. Methods Mol Biol 742:35–50
Rauh R, Hoerner C, Korbmacher C (2017) δβγ-ENaC is inhibited by CFTR but stimulated by cAMP in Xenopus laevis oocytes. Am J Physiol Lung Cell Mol Physiol 312:L277–L287
Reddy MM, Light MJ, Quinton PM (1999) Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl- channel function. Nature 402:301–304
Reddy MM, Quinton PM (2005) ENaC activity requires CFTR channel function independently of phosphorylation in sweat duct. J Membr Biol 207:23–33. https://doi.org/10.1007/s00232-005-0798-8
Reddy MM, Wang XF, Quinton PM (2008) Effect of cytosolic pH on epithelial Na+ channel in normal and cystic fibrosis sweat ducts. J Membr Biol 225:1–11. https://doi.org/10.1007/s00232-008-9126-4
Santos JD, Pinto FR, Ferreira JF, Amaral MD, Zaccolo M, Farinha CM (2020) Cytoskeleton regulators CAPZA2 and INF2 associate with CFTR to control its plasma membrane levels under EPAC1 activation. Biochem J 477:2561–2580. https://doi.org/10.1042/BCJ20200287
Schiller KR, Maniak PJ, O’Grady SM (2010) Cystic fibrosis transmembrane conductance regulator is involved in airway epithelial wound repair. Am J Physiol Cell Physiol 299:C912–C921
Shimkets RA, Lifton RP, Canessa CM (1997) The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem 272:25537–25541
Soundararajan R, Ziera T, Koo E, Ling K, Wang J, Borden SA, Pearce D (2012) Scaffold protein connector enhancer of kinase suppressor of Ras isoform 3 (CNK3) coordinates assembly of a multiprotein epithelial sodium channel (ENaC)-regulatory complex. J Biol Chem 287:33014–33025
Staub O, Verrey F, Kleyman TR, Benos DJ, Rossier BC, Kraehenbuhl J-P (1992) Primary structure of an apical protein from Xenopus laevis that participates in amiloride-sensitive sodium channel activity. J Cell Biol 119:1497–1506
Staub O, Dho S, Henry PC, Correa J, Ishikawa T, McGlade J, Rotin D (1996) WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J 15:2371–2380
Strandvik B (2021) Is the ENaC dysregulation in CF an effect of protein-lipid interaction in the membranes? Int J Mol Sci 22:2739. https://doi.org/10.3390/ijms22052739
Suaud L, Yan W, Carattino MD, Robay A, Kleyman TR, Rubenstein RC (2007) Regulatory interactions of N1303K-CFTR and ENaC in Xenopus oocytes: evidence that chloride transport is not necessary for inhibition of ENaC. Am J Physiol Cell Physiol 292:C1553-1561
Sun YH, Reid B, Fontaine JH, Miller LA, Hyde DM, Mogilner A et al (2011) Airway epithelial wounds in rhesus monkey generate ionic currents that guide cell migration to promote healing. J Appl Physiol 111:1031–1041
Yan W, Samaha FF, Ramkumar M, Kleyman TR, Rubenstein RC (2004) Cystic fibrosis transmembrane conductance regulator differentially regulates human and mouse epithelial sodium channels in Xenopus oocytes. J Biol Chem 279:23183–23192
Young A, Gentzsch M, Abban CY, Jia Y, Meneses PI, Bridges RJ, Bradbury NA (2009) Dynasore inhibits removal of wild-type and DeltaF508 cystic fibrosis transmembrane conductance regulator (CFTR) from the plasma membrane. Biochem J 421:377–385
Voilley N, Lingueglia E, Champigny G, Mattéi M-G, Waldmann R, Lazdunski M, Barbry P (1994) The lung amiloride-sensitive Na+ channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning. PNAS 91:247–251. https://doi.org/10.1073/pnas.91.1.247
Wang H et al (2006) Clathrin-mediated endocytosis of the epithelial sodium channel. J Biol Chem 281:14129–14135
Wesch D, Althaus M, Miranda P, Cruz-Muros I, Fronius M, González-Hernández T, Clauss WG, Alvarez de la Rosa D, Giraldez T (2012) Differential N termini in epithelial Na+ channel δ-subunit isoforms modulate channel trafficking to the membrane. Am J Physiol Cell Physiol 302:C868–C879
Zachar RM, Skjødt K, Marcussen N et al (2015) The epithelial sodium channel γ-subunit is processed proteolytically in human kidney. J Am Soc Nephrol 26:95–106. https://doi.org/10.1681/ASN.2013111173
Zuckerman JB, Chen X, Jacobs JD, Hu B, Kleyman TR, Smith PR (1999) Association of the epithelial sodium channel with Apx and alpha-spectrin in A6 renal epithelial cells. J Biol Chem 274:23286–23295
Acknowledgements
Many thanks to Dr CM Fuller from the University of Alabama at Birmingham, AL and GI Marino and L Galizia from the University of Buenos Aires for their help.
Funding
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PRESTAMO BID PICT 2010-1861).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AGP. The first draft of the manuscript was written by BAK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palma, A.G., Kotsias, B.A. The Effect of Dynasore Upon the Negative Interaction Between ENaC and CFTR Channels in Xenopus laevis Oocytes. J Membrane Biol 255, 61–69 (2022). https://doi.org/10.1007/s00232-021-00212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-021-00212-y