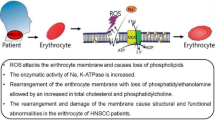
Abstract
Changes in metabolism are mechanisms that are largely implicated in the development, progression, and metastasis of head and neck squamous cell carcinoma (HNSCC) and also in resistance to different anticancer therapies. Identification of biomarkers for differentiation between cancerous and normal epithelium, treatment design and prognosis remain a vital issue in the field of head and neck cancer. The present study analyzed the main biochemical changes that occur in HNSCC tumors by through mechanisms involving oxidative stress. The release of substances reactive to thiobarbituric acid was significantly lower in HNSCC tumor tissue as compared to healthy tissue. The assays related to the lipid profile assays showed changes in membrane biophysics of tumor cells due to an increase in total phospholipids and total cholesterol, as well as an increased activity and expression of the α1 subunit of Na, K-ATPase, which is fundamental in the process of carcinogenesis. The modulation of the antioxidant system was also affected, with a decrease in the catalytic activity of the enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx), as well as a reduction of glutathione (GSH) content and an increase in H2O2 content. A reduction in catalase (CAT) activity was observed. The data presented here are in accordance with important findings described by us in a previous study, involving the same individuals, but with a focus on the damage generated in red blood cells, resulting from tumor installation. Therefore, it was possible to conclude that the biochemical alterations found in HNSCC cells are fundamental for transformation and maintenance of the tumor cell and once it is installed, it is also capable of generating injuries in the patients' red blood cells. Our data demonstrate that this could be a promising biomarker for HNSCC.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is considered to be a highly heterogeneous disease encompassing tumors formed in different anatomical sites of the upper air-digestive tract, such as the hypopharynx, oropharynx, lip, oral cavity, nasopharynx or larynx (Döbrossy 2005). Owing to this heterogeneity, HNSCC has different epidemiologies, etiologies, and therapies, which makes its appropriate clinical management a great challenge (Gupta et al. 2009; Lo Nigro et al. 2017). The combined use of alcohol and tobacco has been described as the main factor for development of HNSCC (Hashibe et al. 2009). In addition, studies have shown that human papilloma virus (HPV) infection is closely related to the pathogenesis of HNSCC originating in the oropharynx (Maxwell et al. 2016; Weinberger et al. 2006). Despite several improvements in treatment of these tumors in the last few decades, overall survival rates have only slowly improved, primarily due to diagnosis in an advanced clinical stage and high rates of treatment failure (Arantes et al. 2018).
Currently, increasing efforts have been directed towards the development of molecular therapies and the elucidation of the main biochemical changes that occur in the metabolism and functioning of these tumor cells, to identify biological markers that can be useful both in predicting treatment and in selecting patients for specific molecular therapies (Ang et al. 2002; Leemans et al. 2018; Quon et al. 2001). Regarding HNSCC, an increasing number of studies have sought to elucidate the implications of using circulating biomarkers for the detection and planning of specific therapies. However, although these markers provide a wide range of possible clinical applications, no validated circulating biomarkers have been integrated into clinical practice for head and neck cancer because they are, for the most part, expensive and difficult for large scale use because of the heterogeneity of the tumors (Nonaka and Wong 2018). The aforementioned supports the need to search for more accessible markers that are easier to sample and more economically viable. A promising approach may be the association of tumors with other tissue markers in blood that are affected by tumor initiation.
Na, K-ATPase (NKA) is the enzyme responsible for maintaining internal ionic homeostasis in cells, enabling high K+ and low Na+ concentrations in the intracellular environment, and also regulates the membrane potential fundamental for cellular growth, differentiation and survival (Sunol et al. 2011). This enzyme is also able to modulate migration and adhesion between cells (Li et al. 2017). Na, K-ATPase not only participates in normal cellular events, but may play key roles in carcinogenesis. Increased expression and activity of the enzyme’s subunits have been implicated in the development and progression of different aggressive cancers such as gastric (Lee et al. 2002), bladder (Espineda et al. 2003), breast (Kometiani et al. 2005), glioblastoma (Lefranc and Kiss 2008), melanoma (Mathieu et al. 2009) and head and neck cancer (Chaves et al. 2014). The enzyme was also identified as a potential biomarker for liver (Baker Bechmann et al. 2016) and breast (Wang et al. 2018) cancers.
Reactive-oxygen species (ROS) are produced naturally during aerobic respiration and their production is very important to the cell, since they are secondary messengers in signal transduction pathways responsible for regulation of multiple physiological processes (Lopez-Lazaro 2007). However, high concentrations of ROS can initiate toxic reactions capable of destabilizing fundamental structures necessary for cell integrity and survival. ROS are also associated with lipid peroxidation and alterations in the membrane microenvironment of important integral proteins, such as NKA (Shah et al. 2016). Oxidative stress plays an essential role in processes that are fundamental to carcinogenesis, such as increased cell proliferation, apoptosis resistance, cellular immortalization, increased angiogenesis, tissue invasion, and metastasis (Klaunig and Kamendulis 2004; Lopez-Lazaro 2007). There is a strong link between the development of head and neck cancer and the use of alcohol and tobacco, because they can increase the production of ROS (Lodovici and Bigagli 2009) and the organism’s antioxidant defense mechanism is not always able to reverse the damage caused by these agents.
One of the main barriers cells have against ROS attack on the cell membrane is reduced glutathione (GSH) and the antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR) (Pisoschi and Pop 2015). Upon failure of the antioxidant system, the consequently high production of ROS by the tumor can lead to secretion of these species from the tumor tissue into the blood, as shown by us in a previous study, causing red blood cell damage and increasing the activity of the NKA in the red blood cells (De Souza Gonçalves et al. 2020). Although the changes in cell membrane integrity and NKA activity in erythrocytes of HNSCC patients was described, there is no evidence of these parameters in tumor tissue. Thus, the elucidation of the biochemical behavior of HNSCC and the effects of its secretions in the blood can be used as a new approach for the noninvasive diagnosis of this type of cancer.
Thus, the present study examined changes that occurred in the control group (safety margin) when compared to the tumor group (tumor tissue) formed by patients with HNSCC. Our results reveal that NKA is associated with the development of HNSCC and may be a promising biomarker for HNSCC when associated with changes in tumor antioxidant balance and erythrocyte levels.
Materials and Methods
Patients
The study population consisted of twenty HNSCC patients without any type of treatment (17 men and 3 women), diagnosed at the oncology unit of the São João de Deus Hospital/Geraldo Correia Foundation (HSJD/FGC), in Divinópolis, MG (Table 1). For the control, normal tissue around the tumor called the safety margin, was used. It is worth mentioning that a free and informed consent form was applied to all those involved in the research and that this study was approved by the Campus Centro-Oeste Dona Lindu Ethics Committee (CEUA no. 07004713.9.0000.5545). None of those involved in the study had undergone chemotherapy or radiotherapy before sample collection. Sixteen of these patients were smokers (15 men and 1 women), all of whom reported heavy smoking for at least 36 years. Eighteen patients reported being alcoholics (16 men and 2 woman), of whom three reported drinking socially and the others reported drinking every day and in large quantities. Most patients had both habits, except for one male patient who reported no smoking. The average number of years of smoking was 36 and ranged from 16 to 70 years. Regarding alcoholism, the average number of years was approximately 34 years, ranging from 19 to 60 years. In regard to the histopathological results, eight patients had well-differentiated HNSCC, eleven patients had moderately differentiated tumors and one received a diagnosis of poorly differentiated tumor. Only three main sites were utilized in the study, namely oral mucosa, tongue, and larynx.
Biological Samples
The samples were collected during the surgical process to remove the tumor in São João de Deus Hospital. The oncological procedure was performed in a standard way, with cervical lymphadenectomy in cases where indicated, followed by resection of the primary tumor with a safety margin of about 1 cm into normal mucosa. As soon as the primary tumor was removed, samples from its center were removed carefully so as not to collect areas with necrosis and also as far as possible from the mucosal surface to reduce contamination by bacterial flora of the upper digestive airway. The margins were collected using a new, sterile material that was opened at the surgical table and samples of the remaining mucosa were cut and collected. Once the tumor samples and margins were collected, they were put in bottles, frozen in liquid nitrogen, and stored in a − 80 °C freezer at the Laboratory of Cell Biochemistry of the Federal University of São João Del Rei, Campus Centro-Oeste, Dona Lindu.
Membrane Homogenate Preparation
Samples were homogenized in preparation buffer (10 mM Tris–HCl pH 7.4, 0.32 M sucrose, 0.5 mM EDTA, and 1 mM MgCl2) using a potter homogenizer. The buffer volume/wet mass ratio used was 4:1 (mL/g). The homogenate was centrifuged for 20 min at 1000×g, 4 °C. The supernatants were then ultracentrifuged at 100,000×g for 1 h, at 4 °C. The new supernatants obtained corresponded to the soluble fraction and were used to evaluate oxidative stress biomarker enzymes. The pellet was resuspended in 1 mL of preparation buffer and corresponded to the membrane fraction of samples from the HNSCC patients.
Thiobarbituric Acid Reactive Substances (TBARS) Determination
For the TBARS determination, 25 μL of tissue homogenate was mixed in 175 μL of a 50 mM sodium phosphate buffer pH 7.4, to which 200 μL of a 12% trichloroacetic acid (TCA) solution and then 400 μL of a 1% thiobarbituric acid solution were added. The mixture was incubated at 95 °C for 30 min. Thereafter, it was placed in an ice bath for 10 min. After this time, the absorbance was read in a spectrophotometer at 532 nm (Buege and Aust 1978).
Lipid Extraction
Tissue membrane lipid extraction was performed based on the method described by Folch et al. (1957). An 100 μL aliquot of tissue fractions sample was mixed with 20 volumes of chloroform/methanol (2:1 v/v). After one hour at rest, the samples were centrifuged at 300×g in a nonrefrigerated clinical centrifuge for 20 min. Then, distilled water was added at 1/5 the volume. The samples were carefully mixed and allowed to rest again for 1 h, and the centrifuged. The samples were left for 24 h to allow separation of the aqueous and organic phases. Thereafter, the organic phase was concentrated in a rotary evaporator, resuspended in 1 mL of chloroform and finally used for membrane lipids analysis and identification.
Total Phospholipids Determination
Total phospholipids quantification was performed using the method described by Chen et al. (1956). A 50 μL aliquot of a lipid extract sample was dried using nitrogen and digested with 0.5 mL of a 65% (v/v) nitric acid solution at about 120 °C. Then, 0.5 mL of distilled water and 1.0 mL of Chen's reagent were added to the sample that was kept for 20 min in a 45 °C water bath. The samples were analyzed with a spectrophotometer at an 820 nm wavelength.
Total Cholesterol Determination
Cholesterol content determination was performed by the method described by Higgins and Fieldsend (1987), based on the ferric chloride complexation (FeCl3·6H2O) with cholesterol in an acidic medium. A 0.375 mL volume of acetic acid and 0.25 mL of ferric chloride reagent (2.5% w/v FeCl3 in 85% w/v H3PO4) diluted in concentrated H2SO4, 8% (v/v) were added to each sample. A pinkish color appeared after 20 min, and the absorbance was read in a spectrophotometer at 550 nm.
Total Protein Determination
In order to standardize the amount of proteins used in the next evaluations, the membrane protein content of the samples was determined by the Bradford method (Bradford 1976) using bovine serum albumin (BSA).
Na, K-ATPase Activity Determination
The tissue membrane preparation (20 μg of protein) was used to measure NKA activity. The determination was made at 37 °C, measuring the inorganic phosphate (Pi) released from the reaction in buffer (50 mM Tris–HCl, pH 7.5, 120 mM NaCl, 20 mM KCl, 4 mM MgCl2, 3 mM ATP, and 20 μg of protein in a final volume of 0.25 μL). The experiments were carried out in the presence and absence of ouabain (1 mM) to specifically determine Na, K-ATPase activity. Absorbances obtained when ouabain was used were subtracted from those obtained in the absence of ouabain to discount the Pi released by other ATPases present in the membrane fraction. The reaction was started by adding the samples to HEPES buffer and stopped with 100 μL of 1% SDS after exactly 60 min. The inorganic phosphate released through ATP hydrolysis was determined by spectrophotometry, according to Fiske and Subbarow method (Fiske and Subbarow 1925) at 660 nm.
Analysis of Protein Expression by Western Blotting
Tissue membrane proteins (30 µg of protein) were subjected to 10% SDS-PAGE in a 90 V Bio-Rad mini-Protean III (Bio-Rad, Hercules, CA, USA) apparatus. Proteins were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) and incubated with α1-Na, K-ATPase specific antibodies (sc-21712, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Ponceau red staining was used to ensure equal protein loading. The proteins recognized by the antibodies were revealed by a mixture of equimolar solution (Solution 1: 100 mM Tris [pH 8.5], 2.5 mM Luminol, and 0.396 mM p-coumaric acid; Solution 2: 100 mM Tris [pH 8.5] and 0.06% H2O2). Immunoblots were quantified using ImageJ software.
Superoxide Dismutase (SOD) Enzymatic Activity Determination
SOD activity was determined using the methodology described by Misra and Fridovich (1972). The method is based on the ability of SOD to inhibit the autooxidation of epinephrine to adenocarbon. 50 mM glycine buffer (pH 10.0), cell extract, and 60 mM epinephrine (pH 2.0) were used for the assay. SOD activity was measured indirectly by the variation of absorbance of epinephrine oxidized product, at 480 nm.
Catalase (CAT) Enzymatic Activity Determination
The catalytic activity of catalase was determined as described by Maehly and Chance (1954). H2O2 substrate decomposition was monitored using a spectrophotometer at 240 nm. Catalase activity was calculated as H2O2 nmol consumed/min/protein μg.
Hydrogen Peroxide (H2O2) Content Determination
Hydrogen peroxide content determination was carried out by the Zhen-Yue method (Jiang et al. 1990). This method consists in the reaction of H2O2 with ferrous sulfate (FeSO4) leading to the formation of Fe3+, which under acidic conditions reacts with xylenol orange to form a complex of yellowish/purple coloration. An 100 μL aliquot of the samples was added to 900 μL of reaction media (0.5 mM ammoniacal ferrous sulfate, 0.2 mM xylenol orange in 25 mM H2SO4). The purple color produced was quantified in a spectrophotometer at 580 nm.
Glutathione Peroxidase (GPx) Content Determination
GPx activity was evaluated by NADP(H) oxidation based on the Flohe method (Flohe et al. 1973). The reaction medium contained 250 μL of 50 mM phosphate buffer (pH 7.4), 40 μL of 4 mM EDTA, 12.5 μL of 1.25 mM sodium, 250 μL of 1 mM GSH, 16 μL of 0.16 mM NADP(H), 50 μL of 0.25 mM H2O2, 331.5 μL of distilled water, and 50 μL of total homogenate soluble fraction. Sample absorbances were read in a spectrophotometer at 340 nm.
Reduced Glutathione Content (GSH) Determination
GSH content was estimated according to the Beutler method (Beutler et al. 1963). The method consists in the reaction of the sulfhydryl group of GSH with the reagent DTNB (5,5′-dithio-bis-2-nitrobenzoic acid) forming TNB (5-thio-2-nitrobenzoic acid) with yellow coloration. A 50 μL aliquot of the samples was added to 225 μL of reaction medium (0.1 M phosphate buffer pH 8.0 and 1 mM EDTA), then 10 mM DNTB was added. The yellow color produced was quantified in a spectrophotometer at 412 nm.
Statistical Analysis
Kolmogorov–Smirnov and Shapiro–Wilk normality tests were performed to verify the probability distributions of the data. Student's t test was used to compare two groups at a 5% level of significance (p value < 0.05). All analyzes and graphs were made with the program GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
Results
Lipid Peroxidation
Owing to the intense metabolism and production of free radicals in tumor cells, damage to the membrane was initially assessed using the lipid peroxidation index of the tissues under study, quantifying the MDA, a by-product released from the direct oxidation of membrane lipids. The tumor tissue group had a significantly lower peroxidation levels than the control group (Fig. 1).
Total Phospholipid and Cholesterol Content Determination in Membrane Tissues
A significant increase in lipid extract phospholipid concentrations was observed in the tumor tissue group when compared with the control group (Fig. 2a). For the total cholesterol content (Fig. 2b), an increase in the tumor tissue group was also found in comparison to the control group.
Na, K-ATPase Enzyme Activity and Expression
Figure 3 shows the analysis of the Na, K-ATPase activity profile, an enzyme that has been shown to be involved in progression and metastasis processes in certain types of aggressive tumors. Thus, it was observed that the enzymatic activity had a significant increase in the tumor tissue group in comparison to the control group.
Na, K-ATPase activity can be altered by modulating the lipid environment or by increasing the α1 subunit expression in the plasma membrane. Therefore, to evaluate whether the expression levels of this Na, K-ATPase subunit would be increased, western blotting experiments were performed. A significant increase in α1 subunit expression was found in the tumor tissue group when compared with the control group (Fig. 4).
Na, K-ATPase α1 subunit expression in membrane preparation of healthy and tumor tissues. Representative Western blotting of 3 patient samples collected from the total of 12 and ponceau staining as loading control **Significant difference relative to the control group (**p < 0.01); n = 12. All experiments were performed in triplicate and the error bars represent the SEM
Superoxide Dismutase (SOD), Catalase (CAT) Enzymatic Activity and Hydrogen Peroxide Content (H2O2) Determination
Regarding the redox system, a reduction in superoxide dismutase catalytic activity occurred (Fig. 5a). In contrast, there was a significant increase in H2O2 production (Fig. 5b) and catalase activity (Fig. 5c) in the tumor tissue group as compared to the control group.
Oxidative stress measurement in healthy and tumor tissues homogenate. a Superoxide dismutase catalytic activity. b H2O2 levels. c Catalase catalytic activity. *Significant difference relative to the control group (*p < 0.05); n = 20. All experiments were performed in triplicate and the error bars represent the SEM
Glutathione Peroxidase (GPx) and Reduced Glutathione Content (GSH) Determination
Glutathione peroxidase activity was considerably decreased in the tumor tissue group when compared with the healthy group (Fig. 6a). Regarding GSH levels (Fig. 6b), a reduction in its concentration was also found in the tumor tissue group relative to the control group.
Discussion
Tumor cells are under intense oxidative stress caused by a constant imbalance between the production of free radicals and an inefficient antioxidant system. This intrinsic ROS increase is due to changes in several factors, including oncogenic stimulation, intense cellular metabolism and mitochondrial malfunction (Ziech et al. 2011). Our previous study revealed higher rates of lipid peroxidation in erythrocyte membranes of HNSCC patients when compared with healthy controls (De Souza Gonçalves et al. 2020). This shows that the onset of the disease may be related to the establishment of an oxidative stress situation related to the increase in the production of ROS by the tumor. Thus, it was expected that, with the overproduction of ROS, the tumor cell membranes would be under intense attack and consequently would suffer great damage. However, when we evaluated the lipid peroxidation index (Fig. 1), our results demonstrated a significant reduction of damage in tumor cells when compared to the control. This decrease may be related to the ability of cancer cells to promote increased membrane saturation, modulating their biophysics by activating the de novo lipogenesis pathway (Rysman et al. 2010). In general, increased lipid synthesis and changes in membrane composition—increased in phosphatidylcholines and saturated phospholipids content—are typical in the cancer phenotype. In addition, the increased number of lipid rafts (enriched in cholesterol and sphingolipids) involved in cell signaling is observed in cancer cells (Bednarczyk et al. 2019). Because saturated lipids are less susceptible to ROS molecules attacks, this change protects tumor cells against cell death mediated by high levels of lipid peroxidation (Hilvo et al. 2011).
Another important factor in the carcinogenesis process is the lipid profile, since lipids have multiple biochemical functions during the development of cancer besides the production of new membranes (Li et al. 2006). The significant increase in total phospholipid synthesis observed in HNSCC tumors (Fig. 2a), was also found in other cancers, such as prostate (Rysman et al. 2010) and breast (Hilvo et al. 2011). These studies also demonstrated an increase in the phosphatidylcholine content, a lipid responsible for promoting less fluidity in tumor cells membranes. Lladó et al. (2014) reported lipid composition changes in different tumors. One of the changes is in the ceramide content. Ceramide is a pro-apoptotic lipid whose concentration is inversely associated with tumor progression. Ceramide’s low concentration is also related to high ganglioside expression due to immunosuppressive activity and angiogenesis promotion.
The cholesterol accumulation shown in Fig. 2b is closely related with the increase in cell cycle progression and tumor growth (Llado et al. 2014). Li et al. (2006) evidenced a primary role of cholesterol in the lipid raft stabilization in epithelial tumors, and when these were removed, lipid modulation resulted in inhibition of the AKT signaling pathway culminating in reduced cell proliferation that prevented tumor progression. Thus, the modulation found in tumoral membranes caused by the increase in saturated lipids leading to lower fluidity may suggest that phosphatidylcholine and cholesterol are factors responsible for affecting sensitivity to chemotherapy and the increase in tumor aggressiveness (Hilvo et al. 2011). Such changes observed in the lipid composition of tumor membranes in relation to the control have already been pointed out as possible biomarkers for oral cancer (Bednarczyk et al. 2019).
Our previous study found a significant decrease in erythrocyte total phospholipid content in patients with head and neck squamous cell carcinoma (0.06 nmol Pi/μL) as compared to erythrocytes in healthy subjects (0.065 nmol Pi/μL). There was also a significant decrease in the erythrocyte cholesterol concentration of cancer patients (0.04528 μg/μL) as compared to the control group (0.093 μg/μL) (De Souza Gonçalves et al. 2020).
These results help to elucidate the effects and damages in a patient’s red blood cells caused by the onset of this type of cancer. The decrease in both the content of total phospholipids and cholesterol in red blood cell membranes of patients affected by HNSCC was observed. This modulation, different from that observed in tumor tissue, can be explained by the loss of phospholipids and cholesterol from the membrane due to the attack by free radicals, with the high metabolism of cancer cells generating the increase in these species. Their increase leads to the breakdown of these molecules leading to their depletion from the membrane, indicating that tumor initiation is related to secretions that are harmful to tissues other than those affected by the tumor.
Na, K-ATPase, a multifunctional protein, plays different roles in cell viability, such as maintenance of intracellular homeostasis, cell junctions, adhesion, motility, and signal transduction (Li et al. 2017; Sunol et al. 2011). Studies report a large increase in activity and overexpression of the α1 subunit of this enzyme in lung cancer (Mijatovic et al. 2007), renal cell carcinoma (Seligson et al. 2008) and glioblastoma (Lefranc et al. 2008). Regarding HNSCC, Chaves et al. (2014) revealed that 28% of oral squamous cell carcinoma (OSCC) patients had diffuse α1 subunit expression and 55% were β1 positive. It was also seen that when SCC-25 cells (oral squamous cell carcinoma cells) were treated with 1 μM digoxin (a cardiotonic steroid inhibitor of Na, K-ATPase) cell shrinkage, normal nuclear loss and decreased cell proliferation occurred. At a dose of 250 nM of the same drug, a decrease in cell viability was observed showing that this enzyme may be important for cell maintenance. The increases in enzymatic kinetic activity and in membrane expression levels (Fig. 3) in HNSCC tumor (Fig. 4) found in this study were also reported in other studies. Using the microarray technique, Zhuang et al. (2015) evidenced the effects of inhibiting α1 subunit expression in several genes associated with the cell cycle and metabolism of hepatocellular carcinoma cells. This promotes great oxidative stress caused by intracellular accumulation of ROS, which inhibits cell growth due to DNA damage. This whole process leads to the induction of apoptosis or cell cycle stoppage by activating the checkpoints. Mijatovic et al. (2007) also reported a remarkable reduction in lung cancer cell proliferation and migration when treated with the semisynthetic cardiotonic steroid UNBS1450 that has high affinity for the α1 subunit of Na, K-ATPase. This promotes cytoskeleton actin fiber disorganization. Therefore, the α1-subunit can be considered a promising target in anticancer therapy, given its increased expression and performance in several intramolecular targets observed in different types of human cancers. Also, the increase in the expression of α1-subunit may be associate with HNSCC development.
Tumor cells exhibit different sensitivities to oxidative stress compared to normal cells. In the carcinogenesis process there is a change in antioxidant enzymes benefitting the progression of the tumor. SOD activity alteration in tumor development is not fully elucidated, but its decrease shown in Fig. 5a has also been reported in other tumor types, such as laryngeal cancer (Seidman et al. 1999). Burdon (1995) described that the superoxide anion at high concentrations in tumor cells can act as a second messenger by interacting with specific receptors or redox signal transduction molecules that are related to cell proliferation or metastasis, such as, protein kinases, phosphatases, transcription factors or inhibitors of the transcription factor.
Some studies suggest that the increase in intracellular H2O2 concentration as was shown in Fig. 5b plays a key role in the development of the tumor cell characteristics (Lopez-Lazaro 2007; Polytarchou et al. 2005). H2O2 is involved in DNA damage mediated by the hydroxyl radical produced by Fenton's reaction (Park et al. 2005), mutations and genetic instability (Hunt et al. 1998; Pericone et al. 2002), cell proliferation (Polytarchou et al. 2005), resistance to apoptosis (Brown et al. 1999), increased angiogenesis (Qian et al. 2003) and invasion and metastasis (Polytarchou et al. 2005). Klaunig et al. (2004) demonstrated that, although ROS are essential in tumor cell development, their excessive production can be harmful and lead to cell death, so the increase in catalase activity (Fig. 5c) can be considered as a mechanism of H2O2 level regulation, preventing intense damage to the cell and activation of apoptotic pathways.
Dequanter et al. (2017) evidenced that in several types of HNSCC there is a reduction in GPx expression levels. Walshe et al. (2007) found that reduction in GPx activity and expression in squamous cell cancer is due to the high H2O2 concentration. Therefore, the results presented in Fig. 6a corroborate these findings. High levels of H2O2, may lead to momentary GPx activity inactivation that is responsible for its degradation, confirming the role of H2O2 as an intermediary in the process of carcinogenesis. On the other hand, the increase in GSH metabolism (Fig. 6b) has been correlated with high tumor aggressiveness, since in conjunction with catalase activity, they are responsible for maintaining the ROS levels under conditions that are favorable for development and progression of tumor cells.
The consumption of alcohol and tobacco (Boffetta and Hashibe 2006; Hashibe et al. 2009) both alone or combined is closely associated with the development of HNSCC (Hashibe et al. 2009). In addition to the oxidative stress in this type of cancer caused by the prolonged use of alcohol and tobacco (Catalá 2009), chemotherapy and radiotherapy are also involved in the production of reactive-oxygen species (ROS), which also occurs in other types of cancer (Huang and Pan 2020).
Our findings are important because they reveal a pattern of oxidative stress that is not related to chemotherapy or radiation therapy. Different levels of ROS and their actions on tumor tissue and safety margin were observed, revealing behavior restricted to cancer. Because the tumor and safety margin samples compared in this study were obtained from the same patient, smoking and alcoholism have the same impact on both regions collected, making it possible to hypothesize that the observed oxidative state is largely a result of tumor development. These results reveal an inefficiency of the antioxidant system in combatting the action of ROS in tumor tissue. This, associated with the high formation of hydrogen peroxide, shows an undeniably close connection between damages suffered by the erythrocytes of patients affected with HNSCC (De Souza Gonçalves et al. 2020) and tumor initiation (Fig. 7).
Conclusion
The findings in this study demonstrate that for the development and progression of HNSCC tumors, complex biochemical alterations are necessary. Tumor cells alter the dynamics and biophysics of their membranes and the activity and expression of several proteins through ROS that provide significant advantages against therapeutic intervention. These results together with our previous findings of alterations that occur in erythrocyte membrane of patients with HNSCC, more efficiently illustrate the effects of the onset of HNSCC in the tissue and blood of the patients, since the subjects were the same in both studies. Therefore, it is possible to consider that the changes observed in patients, can be used as a possible biomarker for diagnosis and development of new therapies for HNSCC.
References
Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L (2002) Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 62:7350–7356
Arantes L, De Carvalho AC, Melendez ME, Lopes Carvalho A (2018) Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev Mol Diagn 18:85–112
Baker Bechmann M, Rotoli D, Morales M, Maeso Mdel C, García Mdel P, Ávila J, Mobasheri A, Martín-Vasallo P (2016) Na, K-ATPase isozymes in colorectal cancer and liver metastases. Front Physiol 7:9
Bednarczyk K, Gawin M, Chekan M, Kurczyk A, Mrukwa G, Pietrowska M, Polanska J, Widlak P (2019) Discrimination of normal oral mucosa from oral cancer by mass spectrometry imaging of proteins and lipids. J Mol Histol 50:1–10
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Boffetta P, Hashibe M (2006) Alcohol and cancer. Lancet Oncol 7:149–156
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown MR, Miller FJ Jr, Li WG, Ellingson AN, Mozena JD, Chatterjee P, Engelhardt JF, Zwacka RM, Oberley LW, Fang X, Spector AA, Weintraub NL (1999) Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res 85:524–533
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Burdon RH (1995) Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18:775–794
Catalá A (2009) Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids 157:1–11
Chaves AL, De Lima PO, Soares JM, Dos Santos HB, Silva AG, Muniz LV, Dos Santos FV, Pereira MC, De Azambuja Ribeiro RI, Barbosa LA (2014) Effects of digoxin and Na, K-ATPase immunoexpression on human oral squamous carcinomas. Anticancer Res 34:5397–5403
Chen P, Toribara Tt, Warner H (1956) Microdetermination of phosphorus. Anal Chem 28:1756–1758
De Souza Gonçalves B, Toledo MM, Colodette NM, Chaves ALF, Muniz LV, Ribeiro RIMDA, Dos Santos HB, Cortes VF, Soares JMA, Barbosa LA, de Lima Santos H (2020) Evaluation of the erythrocyte membrane in head and neck cancer patients. J Membr Biol 253:617–629
Dequanter D, Dok R, Koolen L, Vander Poorten V, Nuyts S (2017) Prognostic significance of glutathione peroxidase levels (GPx1) in head and neck cancers. Front Oncol 7:84
Döbrossy L (2005) Epidemiology of head and neck cancer: magnitude of the problem. Cancer Metastasis Rev 24:9–17
Espineda C, Seligson DB, James Ball W Jr, Rao J, Palotie A, Horvath S, Huang Y, Shi T, Rajasekaran AK (2003) Analysis of the Na, K-ATPase alpha- and beta-subunit expression profiles of bladder cancer using tissue microarrays. Cancer 97:1859–1868
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Flohe L, Gunzler WA, Schock HH (1973) Glutathione peroxidase: a selenoenzyme. FEBS Lett 32:132–134
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Gupta A, Bhatt ML, Misra MK (2009) Lipid peroxidation and antioxidant status in head and neck squamous cell carcinoma patients. Oxid Med Cell Longev 2:68–72
Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wünsch-Filho V, Franceschi S, Hayes RB, Herrero R, Kelsey K, Koifman S, La Vecchia C, Lazarus P, Levi F, Lence JJ, Mates D, Matos E, Menezes A, McClean MD, Muscat J, Eluf-Neto J, Olshan AF, Purdue M, Rudnai P, Schwartz SM, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Talamini R, Wei Q, Winn DM, Shangina O, Pilarska A, Zhang ZF, Ferro G, Berthiller J, Boffetta P (2009) Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomark Prev 18:541–550
Higgins JA, Fieldsend JK (1987) Phosphatidylcholine synthesis for incorporation into membranes or for secretion as plasma lipoproteins by Golgi membranes of rat liver. J Lipid Res 28:268–278
Hilvo M, Denkert C, Lehtinen L, Muller B, Brockmoller S, Seppanen-Laakso T, Budczies J, Bucher E, Yetukuri L, Castillo S, Berg E, Nygren H, Sysi-Aho M, Griffin JL, Fiehn O, Loibl S, Richter-Ehrenstein C, Radke C, Hyotylainen T, Kallioniemi O, Iljin K, Oresic M (2011) Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res 71:3236–3245
Huang G, Pan S-T (2020) ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid Med Cell Longev 2020:5047987
Hunt CR, Sim JE, Sullivan SJ, Featherstone T, Golden W, Von Kapp-Herr C, Hock RA, Gomez RA, Parsian AJ, Spitz DR (1998) Genomic instability and catalase gene amplification induced by chronic exposure to oxidative stress. Cancer Res 58:3986–3992
Jiang ZY, Woollard AC, Wolff SP (1990) Hydrogen peroxide production during experimental protein glycation. FEBS Lett 268:69–71
Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–267
Kometiani P, Liu L, Askari A (2005) Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol 67:929–936
Lee S, Baek M, Yang H, Bang YJ, Kim WH, Ha JH, Kim DK, Jeoung DI (2002) Identification of genes differentially expressed between gastric cancers and normal gastric mucosa with cDNA microarrays. Cancer Lett 184:197–206
Leemans CR, Snijders PJF, Brakenhoff RH (2018) The molecular landscape of head and neck cancer. Nat Rev Cancer 18:269–282
Lefranc F, Kiss R (2008) The sodium pump α(1) subunit as a potential target to combat apoptosis-resistant glioblastomas. Neoplasia (new York, NY) 10:198–206
Lefranc F, Mijatovic T, Kondo Y, Sauvage S, Roland I, Debeir O, Krstic D, Vasic V, Gailly P, Kondo S, Blanco G, Kiss R (2008) Targeting the alpha 1 subunit of the sodium pump to combat glioblastoma cells. Neurosurgery 62:211–221; discussion 221-222
Li YC, Park MJ, Ye SK, Kim CW, Kim YN (2006) Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol 168:1107–1118; quiz 1404–1405
Li L, Feng R, Xu Q, Zhang F, Liu T, Cao J, Fei S (2017) Expression of the β3 subunit of Na(+)/K(+)-ATPase is increased in gastric cancer and regulates gastric cancer cell progression and prognosis via the PI3/AKT pathway. Oncotarget 8:84285–84299
Llado V, Lopez DJ, Ibarguren M, Alonso M, Soriano JB, Escriba PV, Busquets X (2014) Regulation of the cancer cell membrane lipid composition by NaCHOleate: effects on cell signaling and therapeutical relevance in glioma. Biochim Biophys Acta 1838:1619–1627
Lo Nigro C, Denaro N, Merlotti A, Merlano M (2017) Head and neck cancer: improving outcomes with a multidisciplinary approach. Cancer Manag Res 9:363–371
Lodovici M, Bigagli E (2009) Biomarkers of induced active and passive smoking damage. Int J Environ Res Public Health 6:874–888
Lopez-Lazaro M (2007) Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett 252:1–8
Maehly AC, Chance B (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1:357–424
Mathieu V, Pirker C, Martin de Lassalle E, Vernier M, Mijatovic T, DeNeve N, Gaussin JF, Dehoux M, Lefranc F, Berger W, Kiss R (2009) The sodium pump alpha1 sub-unit: a disease progression-related target for metastatic melanoma treatment. J Cell Mol Med 13:3960–3972
Maxwell JH, Grandis JR, Ferris RL (2016) HPV-associated head and neck cancer: unique features of epidemiology and clinical management. Annu Rev Med 67:91–101
Mijatovic T, Roland I, Van Quaquebeke E, Nilsson B, Mathieu A, Van Vynckt F, Darro F, Blanco G, Facchini V, Kiss R (2007) The alpha1 subunit of the sodium pump could represent a novel target to combat non-small cell lung cancers. J Pathol 212:170–179
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Nonaka T, Wong DTW (2018) Liquid biopsy in head and neck cancer: promises and challenges. J Dent Res 97:701–708
Park S, You X, Imlay JA (2005) Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx-mutants of Escherichia coli. Proc Natl Acad Sci USA 102:9317–9322
Pericone CD, Bae D, Shchepetov M, McCool T, Weiser JN (2002) Short-sequence tandem and nontandem DNA repeats and endogenous hydrogen peroxide production contribute to genetic instability of Streptococcus pneumoniae. J Bacteriol 184:4392–4399
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74
Polytarchou C, Hatziapostolou M, Papadimitriou E (2005) Hydrogen peroxide stimulates proliferation and migration of human prostate cancer cells through activation of activator protein-1 and up-regulation of the heparin affin regulatory peptide gene. J Biol Chem 280:40428–40435
Qian Y, Luo J, Leonard SS, Harris GK, Millecchia L, Flynn DC, Shi X (2003) Hydrogen peroxide formation and actin filament reorganization by Cdc42 are essential for ethanol-induced in vitro angiogenesis. J Biol Chem 278:16189–16197
Quon H, Liu FF, Cummings BJ (2001) Potential molecular prognostic markers in head and neck squamous cell carcinomas. Head Neck 23:147–159
Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniels VW, Machiels J, Vanderhoydonc F, Smans K, Waelkens E, Verhoeven G, Swinnen JV (2010) De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res 70:8117–8126
Seidman MD, Quirk WS, Shirwany NA (1999) Reactive oxygen metabolites, antioxidants and head and neck cancer. Head Neck 21:467–479
Seligson DB, Rajasekaran SA, Yu H, Liu X, Eeva M, Tze S, Ball W Jr, Horvath S, deKernion JB, Rajasekaran AK (2008) Na, K-adenosine triphosphatase alpha1-subunit predicts survival of renal clear cell carcinoma. J Urol 179:338–345
Shah PT, Martin R, Yan Y, Shapiro JI, Liu J (2016) Carbonylation modification regulates Na/K-ATPase signaling and salt sensitivity: a review and a hypothesis. Front Physiol 7:256
Sunol M, Cusi V, Cruz O, Kiss R, Lefranc F (2011) Immunohistochemical analyses of alpha1 and alpha3 Na+/K+-ATPase subunit expression in medulloblastomas. Anticancer Res 31:953–958
Walshe J, Serewko-Auret MM, Teakle N, Cameron S, Minto K, Smith L, Burcham PC, Russell T, Strutton G, Griffin A, Chu FF, Esworthy S, Reeve V, Saunders NA (2007) Inactivation of glutathione peroxidase activity contributes to UV-induced squamous cell carcinoma formation. Cancer Res 67:4751–4758
Wang Q, Li SB, Zhao YY, Dai DN, Du H, Lin YZ, Ye JC, Zhao J, Xiao W, Mei Y, Xiao YT, Liu SC, Li Y, Xia YF, Song EW, Tang GH, Zhang WG, Li ZJ, Zheng XB, Cao DH, Li MZ, Zhong Q, Chen ZP, Qian CN, Fan W, Feng GK, Zeng MS (2018) Identification of a sodium pump Na(+)/K(+) ATPase α1-targeted peptide for PET imaging of breast cancer. J Control Release 281:178–188
Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A (2006) Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 24:736–747
Zhuang L, Xu L, Wang P, Jiang Y, Yong P, Zhang C, Zhang H, Meng Z, Yang P (2015) Na+/K+-ATPase α1 subunit, a novel therapeutic target for hepatocellular carcinoma. Oncotarget 6:28183–28193
Ziech D, Franco R, Pappa A, Panayiotidis MI (2011) Reactive oxygen species (ROS)–induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res 711:167–173
Acknowledgements
This work was funded by FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais) PPM-00307-18, APQ-00855-19; CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) Finance Code 01, and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) 305173/2018-9, 409436/2016-0. We would like to thank all the patients and volunteers for their contribution to this study.
Author information
Authors and Affiliations
Contributions
MNT, BDSG, and NMC wrote the main manuscript text. ALFC, LVM and JMAS performed the collection of samples and managed patient inclusion in this research. RIMDAR, HBDS prepared Figs. 1 and 2 and MMT, BDSG, and NMC prepared Figs. 3, 4, 5, and 6. VFC, HdLS, and LAB reviewed all the experiments, mentored the students, and reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest.
Ethical Approval
All procedures were performed in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toledo, M.M., De Souza Gonçalves, B., Colodette, N.M. et al. Tumor Tissue Oxidative Stress Changes and Na, K-ATPase Evaluation in Head and Neck Squamous Cell Carcinoma. J Membrane Biol 254, 475–486 (2021). https://doi.org/10.1007/s00232-021-00185-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-021-00185-y