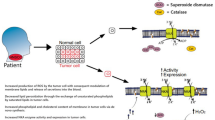
Abstract
Head and neck squamous cell carcinoma (HNSCC) is a heterogeneous and complex disease, both from a clinical and molecular point of view. The prolonged use of alcohol and tobacco, along with the release of tumor secretions can modulate blood cells, such as erythrocytes. Here, this study was conducted with 24 patients diagnosed with HNSCC and an equal number of healthy individuals are matched by age and gender. The levels of lipid peroxidation were measured using the individual plasma, while for lipid concentrations, identification and quantification Na, K-ATPase activity and osmotic fragility, the red blood cell concentrate were used. The release of TBARS was significantly higher in patients with HNSCC. The lipid profile assays demonstrated a rearrangement of the erythrocyte membrane due to a decrease in total phospholipids and phosphatidylethanolamine followed by an increase in total cholesterol and phosphatidylcholine. Na, K-ATPase activity also increased. Erythrocytes were more fragile in patients with HNSCC than in health individuals. Therefore, the membrane of erythrocytes were rearranged and Na, K-ATPase function altered in the HNSCC patients. Our findings suggests that the alcohol, tobacco and tumor secretion modulate in a specific manner that the erythrocytes membranes of these patients making this system a potential tool for HNSCC biomarker of tumor progression.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, about 600,000 cases of head and neck squamous cell carcinoma (HNSCC) are diagnosed each year, occupying the sixth position among the various types of tumors and accounting for 4% of all cancer deaths in the world (Ferlay et al. 2015). The main risk factors associated with HNSCC are alcohol and tobacco consumption (Boffetta and Hashibe 2006; Hashibe et al. 2007) and the combined use of these factors has great relevance in the developing of this malignant neoplasia (Hashibe et al. 2009). In the context of HNSCC beyond the oxidative stress provoked by the prolonged used of alcohol and tobacco (Catalá 2009), it is important to mention that a wide variety of different cancers, chemotherapy and radiotherapy are also involved with reactive oxygen species (ROS) production (Huang and Pan 2020). The high concentration of these molecules is extremely damaging to the cell, since ROS can interact with different molecular targets, such as membrane lipids, specifically those with unsaturated fatty acids, causing lipid peroxidation (Valko et al. 2006). The products of lipid peroxidation are responsible for causing large structural and functional changes in the cell membrane, such as decreased fluidity, increased of the permeability and inactivation of the membrane integrated enzymes together with the loss of essential fatty acids (van Ginkel and Sevanian 1994).
Fluidity of biological membranes is mainly determined by their lipid composition. Cholesterol plays a key role in maintaining the lipid bilayer in an intermediate fluid state, regulating the mobility of phospholipids. However, the molar ratio of cholesterol/phospholipids, is not the only factor responsible for maintaining fluidity; the composition of the phospholipids, the length and degree of unsaturation in the phospholipid chains are also part of this complex system of membrane stability (Kolanjiappan et al. 2002).
ROS attacks increased the permeability by lipid peroxidation or oxidation of proteins, such as Na, K-ATPase (Maturu et al. 2013). Currently, the intracellular sodium concentration has been demonstrated to promotes cellular invasion and migration. Thereby, alterations in the Na, K-ATPase by oxidative stress can interfere in the intracellular sodium, making this enzyme other interesting target for metastasis biomarker (Poku et al. 2020).
The majority of cases of HNSCC are diagnosed at an advanced stage, when treatment is more aggressive and multimodal. Due to its complexity, HNSCC should be studied beyond the genetic and common signal pathways. Having said that, there is an urgent demand for the development of fast, accurate and non-invasive tools for cancer screening, early detection, diagnosis, staging and prognosis.
It was demonstrated that breast cancer can send a paracrine signal to stroma cells causing an epigenetic regulation of LINE-1 classified as an important metastasis cancer biomarker (Puttipanyalears et al. 2016). Recently, Arayataweegool et al. (2020) showed that HNSCC cells secretions alter the peripheral blood mononuclear cells (PBMCs) mRNA expression and supported the hypothesis of cancer secretion can affect and modified other tissues as blood cells.
Therefore, we hypothesize that the secretions and the oxidative stress generated by HNSCC can change the erythrocyte membrane altering the lipid pattern and enzymes function, such as Na, K-ATPase. The role of erythrocytes membranes as a target of the damage caused by ROS and other secretion produced by tumors has become a promising way to develop a method of assessing tumor progression.
Materials and Methods
Subjects
Twenty-four newly diagnosed patients (21 men and 3 women) with biopsy-proven head and neck squamous cell carcinoma, registered at the Department of Oncology at the São João de Deus Hospital/Geraldo Correa Foundation (HSJD/FGC) located in Divinópolis, state of Minas Gerais, were recruited for this study. Seventeen of these patients were smokers (15 men and 2 women), all of whom reported heavy smoking for at least 16 years. Eighteen patients reported being alcoholics (17 men and 1 woman), of whom three reported drinking socially and the others reported drinking every day and in large quantities. All the patients were smokers and/or alcoholics, except for one male patient who reported no smoking. The average amount in years for smoking was 36 years, ranging from 16 to 70 years cultivating the habit. With regard to alcoholism, the average in years for the habit was approximately 36 years, ranging from 19 to 64 years. Regarding the histopathological results, eight patients had well-differentiated squamous cell carcinoma (SCC), fifteen patients with moderately differentiated tumors and one received a diagnosis of poorly differentiated tumor. Only three main sites were found during the study, namely oral mucosa, tongue and larynx.
Twenty-four healthy individuals free of any hematological disease were included in the control group, seventeen men and eight women with an average age of 50.5 years ranging from thirty-two to seventy years. None of them reported being a smoker, however, eight of them (5 men and 3 women) said they drink socially with a maximum frequency of twice a month. Individuals were recruited from within the university, including students and campus staff. It is noteworthy that a free and informed consent term was applied to all involved in the research (Ethics Committee Approval 07004713.9.0000.5545). The data cited above are described in Table 1.
Collection of Samples
Patients’ blood samples were taken at the time of tumor removal surgery. The volume collected was ten milliliters (10 mL) of venous blood in EDTA directly into properly identified Vacutainer system tubes (Becton Dickinson). These were stored in thermal boxes at 2 to 6 °C and sent for testing at the Laboratory of Cellular Biochemistry of the Federal University of Sao Joao del Rei, West Campus, Dona Lindu. The samples were centrifuged at 3000 rpm to separate the plasma from the red blood cells. For control, blood samples from healthy individuals of the same sex and age of the patients were used. Biochemical estimations were carried out immediately. All the estimations in cancer and control samples were performed in concurrently. None of the subjects included in the study were on any medical treatment. It is noteworthy that a free and informed consent term was applied to all involved in the research (Ethics Committee Approval 07004713.9.0000.5545).
Thiobarbituric Acid Reactive Substances (TBARS) Quantification
200 μL of plasma were mixed with 2 mL of a solution containing 15% trichloroacetic acid, 0.38% thiobarbituric acid and 0.25 N hydrochloric acid. The mixture was incubated at 100 °C for 30 min and after a 10 min centrifugation at 3000 rpm, the absorbance was measured at 535 nm in a spectrophotometer according to the method described by Costa et al. (2006) and is expressed as µmol of TBARS/L.
Lipid Extraction
Lipid extraction was based on the methods described by Rose and Oklander (1965) and Vokurková et al. (2005). Initially, 1 mL of the red blood cells (control and patient) were placed in separate tubes and washed with 0.9% NaCl. After washing, the supernatant was discarded and 10 mL of distilled water was added, the contents were stirred for 10 s, then left at rest for 30 min. Subsequently, 20 mL of isopropanol was added under high stirring and then allowed to stand under occasional stirring for one hour. Finally, 30 mL of chloroform was added under stirring, then kept at rest for one hour in an ice bath and centrifuged at 4 °C for 15 min at 800×g. The organic phase was collected, dried on an evaporator and the samples were dissolved in 1 mL of chloroform and stored at − 20 °C.
Quantification of Total Phospholipids
Total phospholipids quantification was performed using the method described by Chen et al. (1956). A 50 μL aliquot of the lipid extract samples was dried under nitrogen and hydrolysed with 0.5 mL of nitric acid 65% (v/v) at about 120 °C. Following, 0.5 mL of distilled water and 1.0 mL of Chen's reagent were added to the sample, that was maintained in a 45 °C water bath for 20 min and then subjected to spectrophotometric analysis at a 820 nm wavelength.
Determination of Cholesterol Content
Cholesterol content determination was performed according to Maia et al. (2014), based on the complexation of ferric chloride (FeCl3·6H2O) with cholesterol in an acidic medium. A volume of the 0.375 mL of acetic acid and 0.25 mL of ferric chloride reagent (2.5% w/v FeCl3 in 85% w/v H3PO4 in H2SO4, 8% v/v) were mixed with the samples. A pink coloration appeared after 20 min, and quantified at 550 nm in a spectrophotometer.
Membrane Lipids Analysis and Identification
Lipid analysis and identification was performed by thin layer chromatography (TLC), with silica gel as the stationary phase. Two standards were used to confirm the presence of the respective phospholipids in the extract, PC and PE. Chloroform/methanol/ammonium hydroxide 65:35:5 (v/v), was the mobile phase using for the TLC. Subsequently, the plate was dried and developed with iodine vapors and photographed for quantification. Phospholipids quantification was done using the ImageJ program, in which the measurement of the area and the intensity of the stain obtained through chromatography was converted to the concentration of Pi nmols through a standard curve of PC and PE of known concentrations (Dyńska-Kukulska and Ciesielski 2012).
Hemoglobin-Free Erythrocyte Membrane Preparation (Ghost)
Human plasma erythrocyte membrane preparation (Ghost) was performed by the method described by Sousa et al. (2015). Red blood cells were isolated from whole blood by centrifugation at 6000×g for 17 min at 4 °C. Then they were resuspended in buffer (20 mM Tris–HCl, pH 7.4, 130 mM KCl and protease inhibitor PMSF 0.6 mg/mL) and centrifuged at 6000×g for 17 min at 4 °C. The pellet was rapidly frozen in liquid nitrogen, then thawed at room temperature and resuspended in buffer (5 mM Hepes, pH 7.4, 1 mM EDTA and 0.6 mg/mL PMSF) and centrifuged again at 8000×g for 17 min at 4 °C. This step was repeated four times, the last wash being done in the absence of PMSF and EDTA. After each buffer wash, the samples were homogenized in a Potter type tissue homogenizer and after each centrifugation the supernatant was discarded. The pellet obtained was resuspended in buffer (Hepes pH 7.4) and centrifuged at 8000×g for 17 min at 4 °C. The supernatant was discarded again and the pellet resuspended in buffer (10 mM Tris–HCl, pH 7.4, 130 mM KCl, 0.5 mM MgCl2 and 50 μM CaCl2) and centrifuged at 8000×g for 17 min at 4 °C.
Total Protein Determination
The Bradford method was using for quantified the protein content of the membrane samples (Bradford 1976).
Na, K-ATPase Activity Determination
Erythrocyte plasma membranes (20 μg of protein) were added to ATPase Buffer [120 mM NaCl, 2 mM MgCl2, 20 mM KCl, 50 mM HEPES, (pH 7.5)] and incubated at 37 °C for 1 h with 3 mM ATPNa2 to start the reaction. Specific Na, K-ATPase activity was calculated from the difference in the amount of inorganic phosphate released by the enzyme in the absence and presence of 1 mM ouabain. After 60 min, 100 μL of 1% SDS was used to stop the reaction. The hydrolysis of ATP caused by the activity of the enzyme, produced the inorganic phosphate measured at 660 nm, as described by Fiske and Subbarow (1925).
Osmotic Fragility Determination
The test for osmotic fragility in erythrocytes was adapted from the methodology of Parpart et al. (1947). This test is based on hemolysis resistance measurement of cells in hypotonic solutions of NaCl. Initially, a 2.475 mL NaCl solution at concentrations of 0.1–0.9% was added in test tubes and then 25 μL of the red blood cell concentrate were also added. The tubes were mixed and placed in a water bath at 37 °C for 30 min. These were then centrifuged for 15 min at 1500 rpm, the supernatant was removed and spectrophotometric reading was done at 540 nm.
Statistical Analysis
Kolmogorov–Smirnov and Shapiro Wilk normality tests were performed to attest the probability distributions of the data. Student's t-test was used to compare the two groups, at a 5% level of significance (p-value < 0.05). All analyses and graphs were performed with the program GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
Results
Lipid Peroxidation
TO study the effects of tumors on the morphological alterations of patients' erythrocytes, we first analyzed the membrane damage caused by the production of free radicals through lipid peroxidation. The method consists in the quantification of TBARS produced as a byproduct of this reaction using the study participant’s plasma. Figure 1 shows the levels of plasma TBARS in healthy individuals and patients with HNSCC. The extent of lipid peroxidation as evidenced by the significantly increased in the patients with HNSCC (0.1994 ± 0.01129 µmol TBARS/L) when compared to the healthy individuals (0.1378 ± 0.006396 µmol TBARS/L), indicating that the erythrocytes of these patients were suffering membrane damage.
Lipid peroxidation determination in healthy individuals and in patients with HNSCC. Determination was performed by the quantification of TBARS produced in the patients’ plasma. ***Significant difference in relation to the control group (p < 0.0001); n = 24. All experiments were performed in triplicate and the line in bars represented the mean and the error bar denotes SEM
Erythrocyte Membrane Total Phospholipid and Cholesterol Content Determination
Figure 2 shows the concentrations of total phospholipid and cholesterol in the erythrocyte membrane of healthy subjects and patients with HNSCC. The phospholipids content observed in Fig. 2a, was measured from the cell lipid extract organic phase quantification the phosphate amount released after acid hydrolysis of lipids. A reduction in total phospholipid content in the patients with HNSCC (0.7037 ± 0.05901 nmol Pi/µL) was found when compared to the healthy individuals (1.304 ± 0.06530 nmol Pi/µL). For the total cholesterol content (Fig. 2b), a contrary profile was observed, a significant increase in HNSCC group (1.248 ± 0.08072 µg/µL) in relation to the control group (0.9543 ± 0.07867 µg/µL).
Lipid content determination in erythrocyte membranes of healthy individuals and patients with HNSCC. a Total phospholipids. b Total cholesterol. *,***Significant difference in relation to the control group (*p < 0.05; ***p < 0.001); n = 24. All experiments were performed in triplicate and the line in bars represented the mean and the error bar denotes SEM
PC and PE Quantification and Identification
Distribution in the erythrocyte bilayer occurs in a very organized way. The extracellular portion of the membrane has predominantly a higher content of sphingomyelin and PC while in the intracellular portion the presence of phosphatidylserine and PE is higher (Dyńska-Kukulska and Ciesielski 2012). The analysis and identification of the main phospholipids constituting the erythrocyte membranes were performed using two phospholipids as standards, PC and PE. When analyzing the content of PC (Fig. 3a) in the lipid extracts there is a significant increase in the HNSCC group (45.01 ± 1.404%) when compared to the control group (40.83 ± 0.9029%), as for PE (Fig. 3b) the opposite is found, there is a significant reduction in its concentration in the HNSCC group (20.43 ± 1.034%) when compared to the control group (24.00 ± 0.9635%).
Phosphatidylcholine (a) and phosphatidylethanolamine (b) lipid contents in the erythrocyte membranes of healthy individuals and patients with HNSCC is presented as percentage of total phospholipids. *Significant difference in relation to the control group (p < 0.05); n = 15. All experiments were performed in triplicate and the line in bars represented the mean and the error bar denotes SEM
Na, K-ATPase Activity
After verifying the erythrocyte membrane damage caused by lipid peroxidation, we analyzed the Na, K-ATPase activity, one of the main enzymes related to the maintenance of erythrocyte homeostasis (Khoshbin et al. 2015). Figure 4 shows a significant increase in the enzyme activity in the group with HNSCC (4.189 ± 0.4860 nmol Pi/min/µg protein) in comparison to the control group (2.591 ± 0.3432 nmol Pi/min/µg protein).
Na, K-ATPase activity in erythrocyte membrane preparations of healthy individuals and patients with HNSCC. **Significant difference in relation to the control group (p < 0.01); n = 20. All experiments were performed in triplicate and the line in bars represented the mean and the error bar denotes SEM
Osmotic Fragility Evaluation
Figure 5 shows the red blood cell osmotic fragility curves in patients with HNSCCC and healthy individuals. It was possible to observe that in the HNSCC group (51.9279 ± 6.778%) were increased osmotic fragility when compared to the control group (1.187 ± 0.5192%), indicating greater sensitivity of these cells in hypotonic medium. This increase in fragility was significant in the saline solution at 0.5%, indicating a greater lysis of red blood cells.
Osmotic fragility curves of healthy individuals and patients with HNSCC. The degree of hemolysis was calculated by comparison with 0.1% NaCl solution which represented 100% lysed. *,***Significant difference in relation to the control group (*p < 0.05; ***p < 0.001); n = 24. All experiments were performed in triplicate and the line in bars represented the mean and the error bar denotes SEM
Discussion
At this time, it is extremely important to seek not only new therapies for the treatment of HNSCC, but also to reinforce the search for biomarkers that assist in monitoring patients. Currently, there are no validated biomarkers for diagnosis of new, persistent or recurrent HNSCC, and the evaluation of patients' blood can be an excellent alternative, since the sample is accessible and non-invasive, avoiding the need for biopsies. It has recently been shown that changes in phospholipids in the erythrocyte membrane and changes in rheological properties during the development of cancer can serve as biomarkers in the diagnosis of this pathology (de Castro et al. 2008; Sánchez-Rodríguez et al. 2015). Thus, in our study, we sought to investigate changes in the lipid and enzymatic profile that occur in erythrocytes in patients with HNSCC that can act as a new strategy for the non-invasive diagnosis of this type of cancer.
It is increasingly evident that potentially important cytotoxic agents in the etiology of cancer are generated primarily by the interaction of ROS. Thus, currently the antioxidant system and lipid peroxidation products are being increasingly studied (Frijhoff et al. 2015). ROS have the ability to diffuse from where they are produced in tumor cells and their effects can be quantified in blood (Abou-Seif et al. 2000). HNSCC patients have an aggravating factor, in addition to the free radicals produced by the tumor, it is already described that prolonged exposure to alcohol in conjunction with tobacco (Table 1), is directly associated with overproduction of ROS and reactive nitrogen species (RNS) potentiating oxidative stress in the organism. Lipid peroxidation has the ability to cause major cellular changes, such as depolarization of the lipid bilayer, modification in the structural organization of the membrane lipids, reflecting on the fluidity of the membrane, and consequently, on the physiological functions of erythrocytes (Catalá 2009). Therefore, due to its great deleterious potential, our group evaluated the lipid peroxidation indices in erythrocytes of patients with HNSCC. The increase in the lipid peroxidation indexes found in patients with HNSCC (Fig. 1) is directly associated with the content of membrane lipids, since larger amounts of polyunsaturated fatty acids are found (Eritsland 2000), which favors ROS attack. Our data corroborate with Pretorius et al. (2013) which reported increased erythrocyte lipid peroxidation in smokers, Maturu et al. (2010) in chronic alcoholics, in addition to the data published by Abou-Seif et al. (2000) showing increased erythrocyte lipid peroxidation in patients with lymphoma and Kolanjiappan et al. (2002) also found this increase, however in patients with cervical cancer.
Lipids play multiple roles in biological membranes, promoting the ideal spatial arrangement necessary for enzymatic activity and membrane-bound proteins and maintaining polarity, essential for various cellular events (Bulle et al. 2017). It has already been described that the physicochemical properties of plasma membranes are partially determined by concentration and composition of phospholipids, phospholipid fatty acid pattern, cholesterol content and the presence of other neutral lipids (Knapp et al. 1994; Padmavathi et al. 2010). Rodrigo et al. (2007b) demonstrated that human erythrocytes are mainly composed of monounsaturated and polyunsaturated fatty acids, which make them vulnerable to ROS attacks due to the susceptibility of unsaturated fatty acids to lipid peroxidation. Our findings support the idea that the decrease in total phospholipids (Fig. 2a) found in patients with HNSCC is due to oxidative stress generated by the consumption of alcohol and tobacco, potentiated by the presence of the tumor, culminating in attacks on erythrocyte membranes and consequently in lipid losses, compromising membrane functionality.
The increase in total cholesterol in red blood cells (Fig. 2b) was also reported in some studies (Bulle et al. 2017; Cooper 1977; Gut et al. 1985; Padmavathi et al. 2010) indicating that this increase results certain adaptive alterations cause in loss of membrane fluidity of these cells. Manoharan et al. (2004), demonstrated that patients with uterine cervix cancer had accumulated cholesterol in erythrocyte membranes, causing a change in the surface area of these cells that led to a distortion in their form. Thus, changes in cholesterol and total phospholipid contents observed in the results shown, lead to a modulation of membrane fluidity and may influence ion transport, the activity of membrane integrated enzymes, altering cellular homeostasis and membrane permeability (Esmann and Marsh 2006; Katyukhin et al. 1998; Rodrigo et al. 2007a; Wheeler and Whittam 1970).
It is described that the growth of malignant tumors is closely related to modulations in the lipid profile of erythrocyte membranes (Stepovaya et al. 2003b). In Fig. 3, we demonstrated the occurrence of a rearrangement in membrane lipid composition in an attempt to maintain its integrity after lipid peroxidation is evident. However, a higher concentration of cholesterol (Fig. 2b), together with PC (Fig. 3a) and a decrease in PE (Fig. 3b) causes erythrocyte membranes to become more rigid due to lower fluidity, decreasing their microviscosity and consequently loss of their functionality. Among the few reports in relation to head and neck cancer Stepovaya et al. (2003a), demonstrated the same change in lipid composition in terms of PC and PE fractions in erythrocytes of patients with HNSCC. Our data also corroborate with Schnitzer et al. (2007) which reported that lipid peroxidation alters packaging and polarity of membrane lipids, promoting a variety of changes in physical properties, such as microviscosity alteration, lateral lipid diffusion and fluidity and variation of some phospholipids.
Rheological changes are commonly found in cancer, however, there are very few reports addressing these changes in patients with HNSCC. In our work, we observed that the red blood cells of patients with HNSCC had an altered fluidity, and possibly, the erythrocyte architecture was also impaired, modifying their rheological properties, causing loss of elasticity and the ability to maintain the discoid shape, making it difficult its passage through narrow capillaries which can lead to thrombotic events (Amézaga et al. 2018; Dumez et al. 2004). Although most of these changes are caused by mechanisms that are not fully understood, it has been demonstrated that the degree of these changes in certain tumors is related to the stage of cancer, metastatic events and the prognosis of the disease (Amézaga et al. 2018). Thus, the monitoring of blood viscosity determinants could act as a molecular marker in the progression of HNSCC.
Regarding Na, K-ATPase (Fig. 4), we found a significant increase in its activity in the HNSCC group in relation to the control, where two mechanisms can be involved. This increase may be related to changes in the lipid profile found in the microdomain where the enzyme is inserted; the higher concentration of PC (Fig. 3a) and cholesterol (Fig. 2b) in the erythrocyte membrane are the main factors of this, since the enzyme has a binding site for these lipids that are related to their activation. Habeck et al. (2015) revealed that phospholipid and cholesterol binding occurs at specific enzyme sites and that the catalytic activity is dependent on these specific interactions. The second mechanism for the changes found in Na, K-ATPase, may represent a direct kinetic adaptation to the effects of lipid peroxidation on the membrane, since the erythrocytes of patients with HNSCC are more permeable, leading to an ionic imbalance, responsible for modify intracellular conditions promoting hypoosmotic stress, so in an attempt to counteract passive ions leaks, the enzyme's activity is increased. Koc et al. (2014) also found an increased Na, K-ATPase activity in erythrocytes of patients with lung cancer.
It has been reported that tobacco contains a large amount of ROS (Church and Pryor 1985) and it is well established that oxidative stress provokes the lipid peroxidation of the cellular membrane. Peroxidation of membrane proteins and lipids has been assumed to be a major mechanism of oxidative damage leading to membrane dysfunction and consequently alterations in cellular functions (Hernández et al. 2016). A study demonstrated that the Na, K-ATPase is a target of cigarette smoke and a reduced expression of this enzyme was found in a patient with lung cancer (Huynh et al. 2012). In addition, other study showed that alcohol consumption cause a rise of nitric oxide (NO) production leading to increased lipid peroxidation and decreased the activity of Na, K-ATPase (Maturu et al. 2010). Differently, in our study an augment of Na, K-ATPase of erythrocytes was described, suggesting that something more than the use of alcohol and tobacco interferes with the function of Na, K-ATPase. However, the correlation between oxidative stress and membrane properties is not understood fully in alcoholism and tobacco use, then further studies should be conduct to analyse the membrane properties in this group.
As observed in Fig. 5, the erythrocytes of HNSCC patients are more sensitive to hemolysis. We suggested that the increase of osmotic fragility in cancer patients is a consequence due to the damage mentioned above, in which the products of lipid peroxidation attack the erythrocyte membrane leading to changes in concentration and disposition of phospholipids and cholesterol fundamental to maintain fluidity, forming pores that increase cell permeability and subsequent lysis. Other factors that may cause increased osmotic fragility, such as alteration of endogenous antioxidant synthesis and external chemical agents, such as cigarette and alcohol, substances frequently used by patients with HNSCC, which lead to a decrease in erythrocytes average life in these patients that are already debilitated due to neoplasia (Dobrossy 2005).
The modification of the lipid composition of membranes has been suggested as a possible marker of tumor progression, especially with regard to metastatic ability (Skotland et al. 2020). In addition, changes in intracellular sodium concentration have been linked to invasion and migration. Thus, a recent review addressed the measurement of intracellular sodium as a robust tool to assist in assessing the evolution of various cancers (Poku et al. 2020). Although these studies were performed in situ, others research postulate that the tumor can cause modification in blood cells. Recently was shown that patients with HNSCC have two times more the levels of MDA in plasma comparing to healthy individuals (Aksoy and Kurnaz 2019). Other study showed the levels of 8-Hydroxy-2′-deoxyguanosine was increased in tumor but not in non-tumor tissue, and was also increased in the blood and urine comparing with health subjects (Mazlumoglu et al. 2017). Considering the fact that tumors can secrete molecules that will have action in other tissues such as blood cells (Arayataweegool et al. 2020), these findings together, allowed us to suggest that the lipid composition of the membrane and alteration of Na, K-ATPase function of erythrocytes present a potential use as a biomarker of head and neck cancer progression.
Most importantly, our results indicate that the membrane lipid profile and Na, K-ATPase function from HNSCC patients erythrocytes is altered by alcohol, tobacco use and tumor presence. Further studies are need to examine the alcohol and tobacco effect without tumor to better understand the tumor action on this system and reinforce the potential of erythrocytes membrane as a HNSCC biomarker.
Conclusion
Our results suggest that alcohol and tobacco use and tumor secretion are able to alter the lipid profile and Na, K-ATPase function of erythrocytes in patients with HNSCC compared to healthy subjects. The combination of several factors, such as elevated levels of TBARS, changes in the content of phospholipids, cholesterol, PC and PE result in loss of fluidity membrane, promoting an increase in permeability, and consequently, makes erythrocytes more sensitive to hyposmotic stress. Thus, the erythrocyte changes found in this study could act as a new approach for non-invasive diagnosis of this type of cancer. Due to the dismal prognosis of advanced HNSCC, these new findings highlight the strong potential of erythrocytes as a non-invasive tumor progression biomarker.
References
Abou-Seif MA, Rabia A, Nasr M (2000) Antioxidant status, erythrocyte membrane lipid peroxidation and osmotic fragility in malignant lymphoma patients. Clin Chem Lab Med 38:737–742
Aksoy A, Kurnaz S (2019) An investigation of oxidative stress and coenzyme Q10 levels in patients with head and neck squamous cell carcinomas. Eur Arch Otorhinolaryngol 276:1197–1204
Amézaga J, Arranz S, Urruticoechea A, Ugartemendia G, Larraioz A, Louka M, Uriarte M, Ferreri C, Tueros I (2018) altered red blood cell membrane fatty acid profile in cancer patients. Nutrients 10:1853
Arayataweegool A, Srisuttee R, Bin-Alee F, Mahattanasakul P, Tangjaturonrasme N, Kerekhanjanarong V, Mutirangura A, Kitkumthorn N (2020) Induction of ZCCHC6 expression in peripheral blood mononuclear cells by HNSCC secretions. Gene 754:144880
Boffetta P, Hashibe M (2006) Alcohol and cancer. Lancet Oncol 7:149–156
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bulle S, Reddy VD, Padmavathi P, Maturu P, Puvvada PK, Nallanchakravarthula V (2017) Association between alcohol-induced erythrocyte membrane alterations and hemolysis in chronic alcoholics. J Clin Biochem Nutr 60:63–69
Catalá A (2009) Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids 157:1–11
Chen P, Toribara Tt, Warner H (1956) Microdetermination of phosphorus. Anal Chem 28:1756–1758
Church DF, Pryor WA (1985) Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 64:111–126
Cooper RA (1977) Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med 297:371–377
Costa CMd, dos Santos RC, Lima ES (2006) A simple automated procedure for thiol measurement in human serum samples. J Bras Patol Med Lab 42:345–350
de Castro J, Rodríguez MC, Martínez-Zorzano VS, Hernández-Hernández A, Llanillo M, Sánchez-Yagüe J (2008) Erythrocyte and platelet phospholipid fatty acids as markers of advanced non-small cell lung cancer: comparison with serum levels of sialic acid, TPS and Cyfra 21–1. Cancer Invest 26:407–418
Dobrossy L (2005) Epidemiology of head and neck cancer: magnitude of the problem. Cancer Metastasis Rev 24:9–17
Dumez H, Reinhart WH, Guetens G, de Bruijn EA (2004) Human red blood cells: rheological aspects, uptake, and release of cytotoxic drugs. Crit Rev Clin Lab Sci 41:159–188
Dyńska-Kukulska K, Ciesielski W (2012) Methods of extraction and thin-layer chromatography determination of phospholipids in biological samples. Rev Anal Chem 31:43–56
Eritsland J (2000) Safety considerations of polyunsaturated fatty acids. Am J Clin Nutr 71:197s–201s
Esmann M, Marsh D (2006) Lipid-protein interactions with the Na, K-ATPase. Chem Phys Lipids 141:94–104
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T, Gasparovic AC, Cuadrado A, Weber D, Poulsen HE, Grune T, Schmidt HH, Ghezzi P (2015) Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal 23:1144–1170
Gut J, Kawato S, Cherry RJ, Winterhalter KH, Richter C (1985) Lipid peroxidation decreases the rotational mobility of cytochrome P-450 in rat liver microsomes. Biochim Biophys Acta 817:217–228
Habeck M, Haviv H, Katz A, Kapri-Pardes E, Ayciriex S, Shevchenko A, Ogawa H, Toyoshima C, Karlish SJ (2015) Stimulation, inhibition, or stabilization of Na, K-ATPase caused by specific lipid interactions at distinct sites. J Biol Chem 290:4829–4842
Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wünsch-Filho V, Franceschi S, Hayes RB, Herrero R, Koifman S, La Vecchia C, Lazarus P, Levi F, Mates D, Matos E, Menezes A, Muscat J, Eluf-Neto J, Olshan AF, Rudnai P, Schwartz SM, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Talamini R, Wei Q, Winn DM, Zaridze D, Zatonski W, Zhang ZF, Berthiller J, Boffetta P (2007) Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 99:777–789
Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wünsch-Filho V, Franceschi S, Hayes RB, Herrero R, Kelsey K, Koifman S, La Vecchia C, Lazarus P, Levi F, Lence JJ, Mates D, Matos E, Menezes A, McClean MD, Muscat J, Eluf-Neto J, Olshan AF, Purdue M, Rudnai P, Schwartz SM, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Talamini R, Wei Q, Winn DM, Shangina O, Pilarska A, Zhang ZF, Ferro G, Berthiller J, Boffetta P (2009) Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev 18:541–550
Hernández JA, López-Sánchez RC, Rendón-Ramírez A (2016) Lipids and oxidative stress associated with ethanol-induced neurological damage. Oxid Med Cell Longev 2016:1543809
Huang G, Pan ST (2020) ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid Med Cell Longev 2020:5047987
Huynh TP, Mah V, Sampson VB, Chia D, Fishbein MC, Horvath S, Alavi M, Wu DC, Harper J, Sarafian T, Dubinett SM, Langhans SA, Goodglick L, Rajasekaran AK (2012) Na, K-ATPase is a target of cigarette smoke and reduced expression predicts poor patient outcome of smokers with lung cancer. Am J Physiol Lung Cell Mol Physiol 302:L1150–L1158
Katyukhin LN, Kazennov AM, Maslova MN, Yu AM (1998) Rheologic properties of mammalian erythrocytes: relationship to transport ATPases. Comp Biochem Physiol B Biochem Mol Biol 120:493–498
Khoshbin AR, Mohamadabadi F, Vafaeian F, Babania A, Akbarian S, Khandozi R, Sadrebazaz MA, Hatami E, Joshaghani HR (2015) The effect of radiotherapy and chemotherapy on osmotic fragility of red blood cells and plasma levels of malondialdehyde in patients with breast cancer. Rep Pract Oncol Radiother 20:305–308
Knapp HR, Hullin F, Salem N Jr (1994) Asymmetric incorporation of dietary n-3 fatty acids into membrane aminophospholipids of human erythrocytes. J Lipid Res 35:1283–1291
Koc M, Cetinkaya CD, Gurbilek M, Adli M (2014) Erythrocyte Membrane Na+/K+-atpase activity in locally advanced non-small cell lung cancer patients treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys 90:S620–S621
Kolanjiappan K, Manoharan S, Kayalvizhi M (2002) Measurement of erythrocyte lipids, lipid peroxidation, antioxidants and osmotic fragility in cervical cancer patients. Clin Chim Acta 326:143–149
Maia GA, Renó Cde O, Medina JM, Silveira AB, Mignaco JA, Atella GC, Cortes VF, Barbosa LA, Santos Hde L (2014) The effect of gamma radiation on the lipid profile of irradiated red blood cells. Ann Hematol 93:753–760
Manoharan S, Kolanjiappan K, Kayalvizhi M (2004) Enhanced lipid peroxidation and impaired enzymic antioxidant activities in the erythrocytes of patients with cervical carcinoma. Cell Mol Biol Lett 9:699–707
Maturu P, Vaddi DR, Pannuru P, Nallanchakravarthula V (2010) Alterations in erythrocyte membrane fluidity and Na+/K+-ATPase activity in chronic alcoholics. Mol Cell Biochem 339:35–42
Maturu P, Vaddi DR, Pannuru P, Nallanchakravarthula V (2013) Modification of erythrocyte membrane proteins, enzymes and transport mechanisms in chronic alcoholics: an in vivo and in vitro study. Alcohol Alcohol 48:679–686
Mazlumoglu MR, Ozkan O, Alp HH, Ozyildirim E, Bingol F, Yoruk O, Kuduban O (2017) Measuring oxidative DNA damage with 8-hydroxy-2′-deoxyguanosine levels in patients with laryngeal cancer. Ann Otol Rhinol Laryngol 126:103–109
Padmavathi P, Reddy VD, Kavitha G, Paramahamsa M, Varadacharyulu N (2010) Chronic cigarette smoking alters erythrocyte membrane lipid composition and properties in male human volunteers. Nitric Oxide 23:181–186
Parpart AK, Lorenz PB, Parpart ER, Gregg JR, Chase AM (1947) The osmotic resistance (fragility) of human red cells. J Clin Investig 26:636–640
Poku LO, Phil M, Cheng Y, Wang K, Sun X (2020) (23) Na-MRI as a noninvasive biomarker for cancer diagnosis and prognosis. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27147
Pretorius E, du Plooy JN, Soma P, Keyser I, Buys AV (2013) Smoking and fluidity of erythrocyte membranes: a high resolution scanning electron and atomic force microscopy investigation. Nitric Oxide 35:42–46
Puttipanyalears C, Kitkumthorn N, Buranapraditkun S, Keelawat S, Mutirangura A (2016) Breast cancer upregulating genes in stromal cells by LINE-1 hypermethylation and micrometastatic detection. Epigenomics 8:475–486
Rodrigo R, Bächler JP, Araya J, Prat H, Passalacqua W (2007a) Relationship between (Na + K)-ATPase activity, lipid peroxidation and fatty acid profile in erythrocytes of hypertensive and normotensive subjects. Mol Cell Biochem 303:73–81
Rodrigo R, Bächler JP, Araya J, Prat H, Passalacqua W (2007b) Relationship between (Na+ K)-ATPase activity, lipid peroxidation and fatty acid profile in erythrocytes of hypertensive and normotensive subjects. Mol Cell Biochem 303:73–81
Rose HG, Oklander M (1965) Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res 6:428–431
Sánchez-Rodríguez P, Rodríguez MC, Sánchez-Yagüe J (2015) Identification of potential erythrocyte phospholipid fatty acid biomarkers of advanced lung adenocarcinoma, squamous cell lung carcinoma, and small cell lung cancer. Tumour Biol 36:5687–5698
Schnitzer E, Pinchuk I, Lichtenberg D (2007) Peroxidation of liposomal lipids. Eur Biophys J 36:499–515
Skotland T, Kavaliauskiene S, Sandvig K (2020) The role of lipid species in membranes and cancer-related changes. Cancer Metastasis Rev 39:343–360
Sousa L, Garcia IJ, Costa TG, Silva LN, Renó CO, Oliveira ES, Tilelli CQ, Santos LL, Cortes VF, Santos HL (2015) Effects of iron overload on the activity of Na, K-ATPase and lipid profile of the human erythrocyte membrane. PLoS ONE 10:e0132852
Stepovaya E, Novitskii V, Ryazantseva N, Gol’dberg V, Tkachenko S, Kolosova M (2003) Structure and properties of lipid bilayer of erythrocyte membranes in patients with malignant tumors. Bull Exp Biol Med 136:490–493
Stepovaya EA, Novitskii VV, Ryazantseva NV, Goldberg VE, Tkachenko SB, Kolosova MV (2003) Structure and properties of lipid bilayer of erythrocyte membranes in patients with malignant tumors. Bull Exp Biol Med 136:490–493
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
van Ginkel G, Sevanian A (1994) Lipid peroxidation-induced membrane structural alterations. Methods Enzymol 233:273–288
Vokurková M, Nováková O, Kunes J, Zicha J (2005) Relationships between membrane lipids and ion transport in red blood cells of Dahl rats. Life Sci 77:1452–1464
Wheeler KP, Whittam R (1970) The involvement of phosphatidylserine in adenosine triphosphatase activity of the sodium pump. J Physiol 207:303–328
Acknowledgements
We would like to thank all the patients and volunteers for their contribution to this study.
Funding
This work was funded by FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais) APQ-00290-16, PPM-00307-18, APQ-00855-19; CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) Finance Code 01, and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) 401914/2016-0, 305173/2018-9.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was approved by the Ethics in Research involving Human Subjects of the Universidade Federal de São João del-Rei, Campus Centro-Oeste Dona Lindu - CEPES/CCO (Protocol Number 07004713.9.0000.5545). All of the procedures followed in this study were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. All patients were informed about the study and consented to participate by signing an Informed Consent Form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Souza Gonçalves, B., Toledo, M.M., Colodette, N.M. et al. Evaluation of the Erythrocyte Membrane in Head and Neck Cancer Patients. J Membrane Biol 253, 617–629 (2020). https://doi.org/10.1007/s00232-020-00147-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-020-00147-w