Abstract
This study was aimed at the determination of bovine leather moisture sorption characteristics using a static gravimetric method at 30, 40, 50, 60 and 70 °C. The curves exhibit type II behaviour according to the BET classification. The sorption isotherms fitting by seven equations shows that GAB model is able to reproduce the equilibrium moisture content evolution with water activity for moisture range varying from 0.02 to 0.83 kg/kg d.b (0.9898 < R2 < 0.999). The sorption isotherms exhibit hysteresis effect. Additionally, sorption isotherms data were used to determine the thermodynamic properties such as isosteric heat of sorption, sorption entropy, spreading pressure, net integral enthalpy and entropy. Net isosteric heat of sorption and differential entropy were evaluated through direct use of moisture isotherms by applying the Clausius–Clapeyron equation and used to investigate the enthalpy-entropy compensation theory. Both sorption enthalpy and entropy for desorption increase to a maximum with increasing moisture content, and then decrease sharply with rising moisture content. Adsorption enthalpy decreases with increasing moisture content. Whereas, adsorption entropy increases smoothly with increasing moisture content to a maximum of 6.29 J/K.mol. Spreading pressure increases with rising water activity. The net integral enthalpy seemed to decrease and then increase to become asymptotic. The net integral entropy decreased with moisture content increase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Textile drying is among the most energy consuming processes and most important step used in wet processing. Drying optimization is very promising for decreasing energy consumption, improving products quality and improving drying process parameters at the same time [1]. Indeed, heat and mass transfer in the product during drying should be analysed. Coupling simultaneously heat, mass and momentum transfer can clearly explain the complexity of drying phenomena. Dealing with a porous media, many physical changes within the product during this process boosts the problem’s complexity. A good knowledge of the material thermo-physical properties can improve their drying process, drying schedules and can help to efficiently use to achieve optimal qualities.
In fact, moisture transfer in hygroscopic porous media can be the consequence of adsorption/desorption at the surface, moisture diffusion inside the sample and convective exchange between material and the environment [2]. The typical pace of the isotherm curve reflects the way in which the water binds the material [3].
Leather is made by processing the animals ‘skin slaughtered for their meat and skin. Indeed, leather is used in various industries as an important raw material including clothing, shoes fashion accessories, furniture covering and leather goods industries. Drying has an essential role in leather manufacturing process as a means of improving preservation and storage [4]. Many internal and external parameters could influence directly the leather drying behaviour. External parameters include temperature, velocity and air relative humidity. Although, internal parameters include density, porosity, permeability, adsorption–desorption characteristics and thermo physical properties of the material being dried.
Water activity (aw) is derived from fundamental principles of thermodynamics and physical chemistry. It depends on the temperature, composition and physical state of the compounds. [5] It is defined as the ratio of water partial vapour pressure in the material and the standard state partial vapour pressure of pure water at the same temperature. Water activity is one of the most important intrinsic properties used for predicting the microorganisms’ presence inside the product. In fact, the term water activity refers to the free water which is unbound to material molecules and consequently can support the bacteria, yeasts and moulds growth. Reducing the not bound water quantity could also minimize other undesirable chemical changes that occur during preservation. It is related to water content in a non-linear relationship known as a moisture sorption isotherm curve. Isotherms can predict product stability over time in different storage conditions.
Several models have been developed in the literature to predict the relationship between equilibrium moisture content, water activity and temperature. The models ‘equations constitute an essential component of the overall drying theory and provide information directly useful in accurate and optimum design of drying equipment and for the drying process macroscopic modelling. They are needed in the analytical determination of the thermodynamic properties related to moisture sorption [6]. These models can be theoretical [7,8,9], semi-theoretical [10,11,13, 57] or empirical [11–14, 35]. The fitting equation model choice depends on the studied humidity range, the nature of the solvent and the nature of the product. goodness
The thermodynamic properties comprise differential parameters such as net isosteric heat of sorption and differential entropy and integral parameters such as integral enthalpy and integral entropy could readily be computed from the sorption isotherms at given temperature. They are primordial to interpret experimental results and thus understand the water properties and calculate the heat and mass transfer energy requirements during the process which help to comprehend and control the materials ‘drying.
The net isosteric heat of sorption or differential enthalpy gives the binding strength of intermolecular attraction forces between the sorptive sites and sorbate in the solid at different moisture contents [15]. The knowledge of the isosteric heat magnitude, and also the differential entropy change, is interesting because it offers an additional information level to characterize the two molecules association [16]. It is proportional to the available sorption sites number at specific molecules ‘energy level [17].
In general, the sorption isosteric heat can be determined using two methods. The first method estimates the sorption isosteric heat by means of calorimetric techniques and Riedel equation [18, 19]. The second method is based on the Clausius–Clapeyron equation application to sorption isotherms.
The integral parameters permit qualitative analysis of desorption and adsorption isotherms. The net integral enthalpy represents the moisture–solid bindings strength [20], whereas the integral entropy, at any temperature, provides the energy measure that is not available to perform work. Integral entropy describes the disorder degree or motion randomness and other statistical approach related to water sorption by products [21]. Hence, the integral quantities are useful in the physical phenomena interpretation associated with sorbed molecules.
To our knowledge, data concerning leather moisture sorption feature is very scarce in literature. Agbaji [22] determined the sorption isotherms of raw hides and skins, leather and presented the temperature effect on sorption isotherm for untanned and tanned (chrome and vegetable) goat skins and cow hides [23]. Ben Makhlouf [24] also presented sorption isotherms for wet blue and semi-finished bovine leather.
Therefore, the present work aims to the key relationships between moisture content, water activity and temperature using gravimetrical method to provide a better knowledge of the bovine leather thermodynamic properties.
2 Materials and methods
2.1 Raw material
Bovine leather was collected from: «Tannerie Megisserie du Magreb»: a local industry in Grombalia, Tunisia. The variety used in this study was “JAZEERA”. The chrome tanned hides were collected just before drying process. They were kept in plastic bags and frozen at 4 °C until its use.
The bovine leather initial moisture content value was 1.6 kg/kg d.b. Before starting each experiment, the samples were cut in small pieces. The size of the sample was small enough to be sure to reach uniform equilibrium moisture content [25].
2.2 Experimental set up
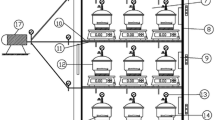
The sorption isotherms were investigated using gravimetrical static method. They were determined at five temperatures (30; 40; 50; 60 and 70 °C). In this method, diffusion is accepted the only way of mass transfer inside the sample. Dilute sulphuric acid solutions were used in order to maintain constant a specific relative humidity [26, 27] inside hermetic and thermostatic desiccators as shown in Fig. 1. Twelve solutions were prepared by adding sulphuric acid to distilled water in order to obtain solutions with different acid fractions. The relative humidity range as a function of temperature and acid concentration inside the sealed measurement chamber was from 5 to 100% (Table 1 [28]). Jars were placed in oven to provide constant temperature environment. So, the specimen was equilibrated with the environment inside the hermetic jars under constant relative humidity and temperature until reaching a constant weight. The weight was determined using a balance “KERN 770” of precision 10−3.
Before conducting the desorption isotherm, the samples were previously placed in distilled water during 24 h at ambiance temperature. The experimental protocol consists of keeping the wet samples in the lower temperature (30 °C) at the beginning. The samples weights were measured daily until the variation became negligible. Once the equilibrium is established, we change to the higher temperature until reaching 70 °C. On the other hand, for adsorption isotherm, the samples were dehydrated before starting experiment. The equilibrium was attained when the difference between two consecutive weightings is constant. Then, the samples were putted into 105 °C until obtaining constant mass to determine the dry mass. Samples were equilibrated for 3 or 4 days depending on experiment conditions.
Usually, to evaluate the experimental uncertainties, two kinds of uncertainties are distinguished: random or A-type uncertainty (uA) and systematic or B-type uncertainty (uB).uA represents the dispersion of the results from one experiment to another while uB derives from the known accuracy of the different elements of the measuring process. The extended uncertainty U is defined as the combination of these two, given by [29]:
2.3 Sorption isotherms data and determination of thermodynamic properties
2.3.1 Modelling equation
Several theoretical model (BET, Langmuir, etc.), semi theoretical (GAB, Chung and Pfost, Modified Halsey, etc.), and empirical equations (Adam and Shove, Oswin, Peleg, etc.) are available to model the moisture sorption behaviour data.
In this study, seven isotherm models were applied to fit the relationship between equilibrium moisture content and the environment conditions data for Bovine leather, as presented in Table 2. w, wm, and aw are respectively equilibrium moisture content, monolayer moisture content and water activity. The other parameters are the model constants. Two equations of the models tested consider the effect of temperature on the moisture sorption behaviour.
These models, based on mathematical functions, were fitted to the experimental data using MATLAB software and the parameters were estimated by means of nonlinear regression analysis. Statistical criteria were used to fit the goodness of different models between the experimental and predicted equilibrium moisture content including correlation coefficient (r2)(Eq. (2)), the root mean square error (RMSE) (Eq. (3)) and sum squared errors (SSE) (Eq. (4)).
Where
Where wcalc,i is the predicted equilibrium moisture, wexp,i is the experimental equilibrium moisture, \( \overline{{\mathrm{w}}_{\exp, \mathrm{i}}} \) is the average value of experimental equilibrium moisture and N is the data values number.
The GAB model, based on physicochemical phenomena, is a multimolecular, localised, homogenous adsorption model. As a refined extension of the BET model, it is the most versatile sorption theory taking into account multilayer adsorption at high value of relative humidity.
In this model, wm presents the monolayer moisture content on the internal surface (kg water/kg solid), C is a constant related to the heat of the first layer sorption and k is a factor related to the heat of the multilayer sorption. The monolayer value of indicates the amount of water strongly adsorbed in specific sites. In fact, it is considered to be the value at which a product is the most stable. In the GAB model, the two parameters C and k are associated to temperature by the Arrhenius equation as presented in Eqs. (5) and (6) [30, 31]
C0 and k0 are entropic accommodation constants; qm, qn and qc are respectively the sorption heat of the monolayer absorbed water and multilayer absorbed water and bulk liquid (heat of water vapour condensation); R is the universal gas constant and T is the temperature.
2.3.2 Net isosteric heat of sorption
The drying operation is accompanied by the heat required to remove water from the material. The net isosteric heat of sorption is defined as the energy amount needed to change the state of a product unit mass from liquid to vapour at constant temperature and water activity [38] . It defined the energy amount when the heat of moisture vaporization exceeds the pure water latent heat. It can be useful to improve the characterization of water molecules binding at the solid particles surface and thus a measure of the material chemical, physical and microbiological stability, and for the time and the energy requirements determination for drying process [39].
Clausius–Clapeyron equation is used to determine the net isosteric heat of sorption by applying Eq. (7) [40] using data obtained from the best fitting desorption model.
Where Qst is the net isosteric heat of sorption (J/mol), qst is the total isosteric heat of sorption (J/mol), R is the gas constant (8.314 J/mol K) and Lv is the latent heat of vaporization (J/mol).
Based on Eq. (8), Qst was obtained from the slope of the plot representing ln(aw) versus (1/T) at a fixed value of equilibrium moisture content. This procedure is repeated for different values of equilibrium moisture content determined by the best fit sorption model to determine its effect on the net isosteric heat of sorption.
Several studies neglected the temperature impact on isosteric heat [41]. In our study, the temperature effect on the isosteric heat was determined at five temperature levels.
2.3.3 Differential entropy
Entropy characterizes spatial arrangement as the order or randomness degree in the water-sorbent relationship which can help interpreting many processes such as dissolution, crystallization and swelling [42].
The molar differential entropy of sorption (ΔS) is associated to the available sorption sites number at a specific energy level [43]. In fact, it is related to the forces of water molecules (attraction or repulsion) to the sample components. The changes in water sorption on free energy are usually accompanied by changes on both the enthalpy and the entropy.
The differential entropy can be calculated from the Gibbs–Helmholtz equation (Eq. (9)) [42]:
ΔG is the Gibbs free energy. It is defined as:
For a thermodynamic system, the net isosteric entropy at each moisture is given by combining (Eqs. (9)) and (10) to the fitting equilibrium data:
From the ln(aw) versus (1/T) plot for various equilibrium moisture content, the differential entropy can be found as the intercept of the linearized experimental curve and the aw axis.
2.3.4 Spreading pressure
The spreading pressure (ϕ) called ‘surface potential’ is the force applied in the surface plane perpendicularly to each edge unit length to keep the surface from spreading. It is the pressure two-dimensional analogue, having units of force per unit length. It is defined as increase in surface tension of bare sorption sites due to the sorbed molecules and considered as the adsorption free surface energy [20].
It was estimated by the following equation as described by Iglesias et al. [6]
Kb is the Boltzmann constant (1.38 х 10−23 J/K); Am is the water molecule area (1.06 х 10−19 m2).
The Bovine leather spreading pressure property ɸ was determined using its desorption equilibrium moisture content data, obtained in the temperature range of 30–70 °C.
2.3.5 Net integral enthalpy and entropy
Conventionally, the water net integral enthalpy of sorption provides an indication of the binding strength and water molecules affinity to the solid structure and has some bearing on both drying and freezing processes energy balance [44].
The net integral enthalpy Qin (J/mol) was determined by a technique similar to that used for the isosteric heat but at constant spreading pressure using Eq. (15) derived from the first law of thermodynamics [45]. At constant spreading pressure, the ln(aw) versus 1/T slope yielded the net integral enthalpy.
Net integral entropy describes the disorder degree, motion randomness and other statistical approach related to water sorption [46]. Thus, this parameter quantifies the adsorbed water molecules mobility and the integral enthalpy is needed in its determination in a sorption process [20].
Net integral entropy ΔSin (J/K.mol) is given by:
Where (aw)* is the geometric mean water activity obtained at constant spreading pressure.
3 Results and discussion
3.1 Adsorption and desorption isotherms
The experimental results for the equilibrium moisture content as a function of water activity for adsorption and desorption obtained at five temperatures (30, 40, 50, 60 and 70 °C) are presented in Fig. 2. The equilibrium moisture content is defined in the dry basis (kg water per kg dry material).
The isotherm typical shape reflects the way in which the water binds the system. Weaker water molecule interactions generate a greater water activity and consequently, the product becomes more unstable. The studied species sorption isotherm patterns show the typical sigmoid curve of type II behaviour [47] according to the BET classification [48]. In the first segment (low aw) of the S-shaped sorption isotherm curves, bovine leather adsorbed relatively lower values of moisture. While, larger moisture amount was adsorbed at higher aw values.
Analogous behaviour was obtained with the experimental sorption isotherms curves for both wet-blue bovine leather, and semi-finished cowhide [24]. This type is typical of many other products like cement-based materials and foods [40, 49,49,50,52].
The two curves show that the equilibrium moisture content increases with the water activity at a constant temperature. Whereas, at given air relative humidity, equilibrium water content decreases with increasing temperature, thus bovine leather is less hygroscopic at higher temperature [50].
This trend is due to the water molecules excitation states by increasing temperature. In fact, heating up affects directly the water molecules mobility inside the product and the dynamic equilibrium between adsorbed phases and vapour. Subsequently, molecules will be thermally excited and this thermal energy will reduce the total number of active sites for water binding by allowing them to break away from their sorption sites and decrease attractive forces between them [53]. Water will change of state from liquid to vapour leading to the reduction in moisture content. Thus, rising the temperature declines the water desorption degree.
We can clearly notice that the temperature effect on the sorption isotherms is lower in the adsorption than desorption isotherms. On desorption, moisture content varies from 0.8378 to 0.0636 kg/kg d.b, while for adsorption, it varies from 0.5576 to 0.0219 kg/kg d.b.
The values obtained for the extended uncertainty U (Eq. (1)) are the same (0.006) for (0.05 ≤ aw < 1).
3.2 Moisture sorption hysteresis
Hysteresis is not a well understood phenomena, although there is general agreement that some thermodynamically irreversible processes must appear during desorption and adsorption. Igleasis and Chirife [12] stated that no theory could explain the origin of the complex phenomena of the hysteresis.
Several theories were suggested to analyse this problem, and the most favoured proposes that polar sites in the material molecular structure are almost satisfied by adsorbed water. Due to the non-reversible physical changes caused by drying and shrinkage (changes in structure in desorption and penetration of the adsorbate in adsorption), the molecules and their water holding sites are closely enough together to satisfy each other. This reduces the material water holding capacity upon subsequent adsorption [54].
On the other hand, capillary condensation can cause the hysteresis: molecules on the surface get adsorbed layer by layer due to the force field exerted on them filling higher energy sites near pore wall then lower energy sites far away from pore wall. Thus, desorption phenomenon is slower than adsorption and the hysteresis loop is formed. So, the same molecules desorb at lower pressure than adsorb. This phenomenon can be also caused by tensile strength effect. It can be explained by the pore ink bottle shape and having throat diameter smaller than the size of the pore body.
The Fig. 3 below displays hysteresis effect between adsorption and desorption over the studied range of water activity at 30, 50 and 70 °C. The same behaviour was observed for the other temperatures. The data also indicated that equilibrium moisture content for adsorption was lower than desorption for the same water activity level. The hysteresis loop magnitude is higher for the lowest temperature (30 °C). It can be due to more strongly associated molecules at the highest temperature (70 °C).
The adsorption/desorption hysteresis is commonly observed for different other porous materials [40, 50].
3.3 Mathematical modelling
Seven isotherm models were tested to fit the sorption isotherms data for bovine leather. The adjustment results at different temperatures and statistical calculations for the sorption models are shown in Table 3. The model fit is good enough for higher value of r2 and lower value of RMSE.
The results examination indicates that Owsin, Modified Henderson, Peleg and GAB models are able to reproduce experimental data within an acceptable precision. GAB model is the most suitable model that describes the experiment, throughout the studied water activity range and over the temperature range, as long as it permits physical interpretation of the sorption phenomenon [51]. Also, this model has many advantages acting as a viable theoretical background, as it is a refinement of BET and Langmuir theories of physical adsorption.
This model was found to be suitable with different materials like foodstuff, [51, 55] cement based materials [49], and clays [56]. Agbaji [23] showed that Henderson equation [12] provides a suitable experimental sorption isotherms fitting for untanned and tanned (chrome and vegetable) goat skins and cow hides at three temperatures (20, 30 and 40 °C).
Ben makhlouf [24] found that Dent model is considered to be the best model for describing the relationship between the equilibrium moisture content, water activity and temperature for bovine wet-blue and semi-finished leather.
The GAB model gives r2 values close to unit ranging from 98.98% to 99.9%. A comparison between calculated curves from GAB model and experimental sorption data are presented in Fig. 4.
For desorption, the k values are lower than that for adsorption contrary to wm. The k parameter is related to the chemical potential difference in the pure liquid adsorbed and in upper layers. Hence, it is near but less than unit, as presented in Table 3, which is the GAB model definitive characteristic [16].
The monolayer moisture content is of practical use as it is the optimal moisture content for storage and preservation and of its relationship with several aspects of the physical and chemical dry product deterioration [37]. In fact, It is considered as the adsorbent sorption capacity and the available polar sites indicator for binding water vapour [57]. The monolayer values for desorption obtained in this study are in the range of data noted in other works in leather domain in the same range of temperatures [24].
It is easy to notice that wm is lower at higher temperatures. Such decreasing trends reveal that water molecules in the vapour state surrounding the sample with their higher kinetic energy at risen temperature result in declined monolayer moisture with temperature increase [58].
3.4 Isosteric heat of sorption and sorption entropy
The water net isosteric heat of adsorption and desorption at constant equilibrium moisture content were determined by applying Eq. (7) to the equilibrium data using the GAB model that gave previously the best equilibrium moisture content data fitting in the temperature range varying from 30 to 70 °C. The isosteric heats of sorption have a strong dependence on moisture content, with a boost of the energy required for desorption (more than the latent heat) at low equilibrium moisture contents.
The values of calculated enthalpy and entropy changes of desorption and adsorption are presented in Figs. 5 and 6.
As seen in Fig. 5, for desorption, the heat of sorption increases to a maximum with increasing moisture content, and then decreases sharply in magnitude with further increase in moisture content. The trend seemed to become asymptotic and tends to zero. On the other hand, the maximum isosteric of desorption heat was found 54.05 kJ/mol in the equilibrium moisture content of 0.09 kg/kg d.b.
This fact may be explained based on the assumption that the maximum value illustrates the strongest binding sites covering through the solid monolayer surface by desorbed water (wm = 0.09 kg/kg d.b) and greatest water-solid interaction. At low moisture levels the sorption is mainly at the monomolecular layer where the sorption sites are usually active.
The heat of desorption decrease below this value was qualitatively explained by considering that the desorption takes place at the beginning in the most active available sites (monolayer region) involving high interaction energies. When all these sites become occupied, multilayers were formed and desorption takes place on the less active site involving reduced interaction energies [19]. In fact, the energy required for water evaporation is very low due to the free water easy to remove.
An almost constant energy input of 5070 J/mol for desorption is needed to desorb moisture from bovine leather above moisture content of 0.33 kg/kg d.b.
The increase in isosteric heat when the material presents low moisture contents can be explained considering that binding is essentially governed by interaction between the water molecules and the adsorbent surface. A plausible reason for the curve pace could be the differences in hydrogen bonding as moisture is desorbed due to differences in attractive forces between water molecules and sorption sites and between water molecules themselves. This observation indicates a greater pattern for water desorption probably due to the cooperative and non-cooperative macromolecules ability resulting in higher binding energy at lower moisture levels. Many authors have proposed that the enthalpy of sorption reduction could be explained by the relaxation phenomenon and phase transition associated with weak water mobility. Therefore, the material evolution toward the equilibrium at the time of sorption curves establishment is disturbed [59].
The same phenomenon was observed by other products, such as food, as the case of apple [51], mulberry [39] and cookies and corn snacks [60]. The same trend was also observed with clays [58]. Agbaji reported that net isosteric heat of desorption decreases with increasing moisture content for nigerian hides, skins and leathers [23]. For adsorption, net isosteric heat of adsorption decreased rapidly and remained constant above moisture content of 0.09 kg/kg d.b.
It can be seen from Fig. 5 that adsorption and desorption data show that, at a specific moisture content, the isosteric heat of desorption is higher than the corresponding adsorption value. The increased energy requirements of the desorption process are characteristic of more polar sites on the product surface and then reduced water molecules mobility. Medeni Maskan and Fahrettin Gogus [39] state that this gap is probably caused by the extensive heat treatment during the sample drying before adsorption process, which could have damaged the sorption sites and makes the water removal easier.
For equilibrium moisture content higher than 0.09 kg/kg db, the difference in heat values decreases between the two processes with increasing moisture content. At a moisture content value of 0.15 kg/kg d.b, the difference is 17.11; whereas it declines to 3.04 at a moisture content of 0.33 kg/kg d.b. The same observations were reported by Hossain et al. [61] for pineapple and McMinn and Magee for potato [15].
Figure 6 reveals the differential entropy production for bovine leather as a function of moisture content. The results show that entropy data display strong dependence on moisture content. As sorption enthalpy, the sorption entropy of desorption exhibits higher magnitude than that for adsorption.
The difference between the two processes decreases with increasing moisture content to become negligible and remains nearly constant until a moisture content of 0.39 kg/kg d.b. This behaviour indicates that water molecules are more mobile during desorption than adsorption.
The evolution of desorption entropy versus w, is similar to that of isosteric sorption enthalpy, and shows a maximum at the same equilibrium moisture content value. At the beginning, the desorption entropy was low at the lowest moisture content. It increased rapidly to a maximum of 144.94 J/K.mol and then decreased gradually with the increase in moisture content to tend asymptotically towards a final value.
Adsorption entropy increased smoothly with increasing moisture content to a maximum of 6.29 J/K.mol at a moisture content value of 0.39 kg/ kg d.b. At low moisture content, the water molecules are adsorbed on the most accessible sorption sites, reducing its motion and losing its sorption entropy. As the moisture content increases, the available sites become saturated and are less accessible. Water molecules form multilayer on the top of the tightly bound first layer whose spatial arrangements allow more molecular motion of adsorbed molecules, increasing the sorption entropy [51, 62].
3.5 Enthalpy-entropy compensation theory
There are stronger intermolecular interactions or bonding related to the enthalpy, which point to a greater reduction in the configuration freedom and hence to a greater system order related to the entropy. This relationship permits the enthalpy- entropy compensation theory application [63, 64].
The relationship between the net isosteric heat of sorption (Qst) and the differential entropy of sorption (∆S) is obtained by applying Eq. (11) for fixed values of moisture content.
The compensation theory allows the Qst and ∆S dependence and proposes a linear relationship between them:
Where Tβ is the isokinetic temperature and α is a constant.
Tβ represents the temperature to which all the series reactions progress to the same rate, and the free energy α at the temperature Tβ provides a criterion to evaluate if the water sorption process is spontaneous (−α) or not (+α).
It was assumed that Qst and ∆S did not vary with temperature, at specific moisture content [65].
Figure 7 shows the linear relation between Qst and ∆S for desorption at all temperatures, which indicates that compensation exists and confirms the enthalpy–entropy compensation hypothesis.
The parameters Tβ and α were calculated from desorption data by linear regression and the result is given by Eq. (18). Hence, the characteristic parameters of the enthalpy–entropy relationship for bovine leather are:
In our case, α was found negative (α = −364.8 J/mol) which is spontaneous reaction characteristic. Similar results for guar have been obtained by Vishwakarma et al. [66] and for cassava reported by Kamenan Koua et al. [67].
3.6 Spreading pressure
Spreading pressure at different temperatures is presented in Fig. 8. It was observed that the spreading pressure increases with increasing water activity. Similar response has been reported on hardened cement paste and mortar [49], cereal grains and starchy materials [68] and sweet potato [69].
Also, spreading pressure increases with rising temperature, at a given water activity. The temperature effect on spreading pressure does not seems to be significant at 40, 50, 60 and 70 °C, especially in the water activity range of [0; 0.5]. Whereas, on desorption, significant temperature effect was detected with decreasing temperature from 40 °C to 30 °C. The temperature effect becomes more pronounced on adsorption than desorption in the high water activity range.
Desorption spreading pressure isotherms were nearly higher than the corresponding adsorption isotherms. This could be attributed to changes in leather matrix structure induced by drying before adsorption process. Toğrul and Arslan [50] states data showing that the walnut kernels spreading pressure in desorption was higher than that in adsorption at the same water activity and temperature.
The water surface energies during adsorption and desorption were higher than that of pure water (0.072 J/m2) [70] at water activities above 0.3. Thus, leather has higher surface excess energy than that of pure water during adsorption and desorption.
3.7 Net integral enthalpy and entropy
Figure 9 shows the net integral enthalpy plots for desorption and adsorption as moisture content function. These curves represent a measure of sorption sites accessibility and bound water location. Net integral enthalpy of bovine leather decreased with increase in moisture content from a value of −11.3 and −8.12 kJ/mol respectively for desorption and adsorption at moisture content of 0.02 kg/k d.b. The net integral enthalpy value seemed to increase asymptotically as the moisture content increased beyond 0.12 and 0.05 kg/kg d.b for desorption and adsorption respectively.
This trend may be due to the fact that, as the leather moisture increased, less favourable sites were covered with water and multiple sorbed water layers were formed on those sites. Water molecules become more held to the particles by surface tension than by chemical adsorption. Hence, weaker surface tensional forces are easily broken, and this results in the integral enthalpy lowering [71].
The net integral enthalpies values for both desorption and adsorption are negative. This signifies that water molecules lose energy upon being transferred from bulk water into the adsorbed state.
The results show that the net integral enthalpy of adsorption is higher than that of desorption. For this case, chain rupture or irreversible swelling may be the cause for a heat of desorption being smaller than a heat of adsorption [72].
The net integral entropy variations for desorption and adsorptions with moisture content for bovine leather are shown in Fig. 10. Net integral entropy decreased continually with moisture content. The entropy decrease is due to the adsorbed water localization (loss of rotational freedom) caused by the strongest binding sites with water molecules and solid, and structural alteration of the adsorbing material towards increased crystallinity [73].
The net integral entropy of adsorption is lower than the net integral entropy of desorption but remain positive values. This is an indication of thermodynamic compensation between enthalpy and entropy.
4 Conclusion
This study determined the bovine leather thermodynamic properties associated with moisture sorption isotherms for temperatures 30, 40, 50, 60 and 70 °C. These thermodynamics parameters can be used to promote the leather use and to improve their drying process with a comprehensive drying model. The isotherms behaviours were manifested a sigmoidal shaped curve form reflecting a Type II isotherm which is common for many hygroscopic materials. The hysteresis phenomenon was distinctly observed. Temperature has an effect on desorption and adsorption isotherms. A good agreement between experimental and predicted data of desorption/adsorption isotherm was found using GAB model by using a nonlinear procedure.
The sorption isotherms data were used to determine some bovine leather thermodynamic properties. The isosteric enthalpy evolution for desorption versus moisture content shows that the energetic water/adsorbent interactions reach a maximum value in the moisture content of 0.09 kg/kg d.b and then decrease to tend asymptotically towards a final value. The same trend was observed with desorption entropy, it increases until the same moisture content value and then decreasing sharply. Adsorption enthalpy decreases with increasing moisture content. While, adsorption entropy increases smoothly with increasing moisture content. The compensation theory allows a linear relationship between isosteric enthalpy and entropy of desorption.
The spreading pressure of bovine leather increased with increase in temperature and increased with increase in water activity more significantly on adsorption than desorption in the high range of water activity.
The net integral enthalpy variation decreased and then increased with moisture content. Net integral entropy declined with increase in moisture to a certain moisture level value.
Abbreviations
- aw :
-
Water activity
- Am :
-
Area of water molecule (m2)
- C :
-
Constant related to the heat of sorption of the first layer
- C0 and k0 :
-
Entropic accommodation constants
- k :
-
Factor related to the heat of sorption of the multilayer
- Kb :
-
Boltzmann constant (J/K)
- Lv :
-
Latent heat of vaporization (J/mol)
- N :
-
Number of data values
- qm :
-
Sorption heat of the monolayer absorbed water (J/mol)
- qn :
-
Sorption heat of multilayer absorbed water (J/mol)
- qc :
-
Sorption heat of the bulk liquid (J/mol)
- Qst :
-
Net isosteric heat of sorption (J/mol)
- qst :
-
Total isosteric heat of sorption (J/mol)
- Qin :
-
Net integral enthalpy(J/mol)
- r2 :
-
Correlation coefficient
- R :
-
Universal gas constant (J/mol.K)
- T :
-
Temperature (K)
- Tβ :
-
Isokinetic temperature (K)
- w :
-
Equilibrium moisture content (kg/kg d.b)
- wm :
-
Monolayer moisture content (kg water/kg solid)
- ΔS :
-
Molar differential entropy of sorption (J/K.mol)
- ΔSin :
-
Net integral entropy(J/K.mol)
- ΔG :
-
Gibbs free energy (J/mol)
- α :
-
constant free energy (J/mol)
- θ :
-
Moisture ratio
- ɸ :
-
Spreading pressure (J.m−-2)
- \( \overline{{\mathrm{w}}_{\exp, \mathrm{i}}} \) :
-
Average value of experimental equilibrium moisture
- ()*:
-
The geometric mean
- calc,i:
-
predicted
- exp.,i:
-
experimental
References
Aouaini F, Knani S, Yahia MB, Bahloul N, Lamine AB, Kechaou N (2016) New insights on energetic analysis of water adsorption isotherms of the Pelargonium graveolens: modeling, interpretations and pore sizes distribution based on statistical physics approach. RSC Adv 6:58796–58804
Ben Abdelhamid M, Mihoubi D, Sghaier J, Bellagi A (2016) Water sorption isotherms and thermodynamic characteristics of hardened cement paste and mortar. Trans Porous Media 1–19. https://doi.org/10.1007/s11242-016-0694-y
Andrade PRD, Lemus MR, Pérez CCE (2011) Models of sorption isotherms for food: uses and limitations. Vitae 18:325–334
Fakhfakh R, Pierre F, Kechaou N, Perre P (2016) Simultaneous measurement of drying kinetics and shrinkage of Tunisian bovine leather during convective dryingThe 20th international drying symposium, Gifu, Japan, pp p2–50
Fabra MJ, Talens P, Moraga G, Martínez-Navarrete N (2009) Sorption isotherm and state diagram of grapefruit as a tool to improve product processing and stability. J Food Eng 93:52–58. https://doi.org/10.1016/j.jfoodeng.2008.12.029
Iglesias HA, Chirife J, Viollaz P (1976) Thermodynamics of water vapour sorption by sugar beet root. Int J Food Sci Technol 11:91–101. https://doi.org/10.1111/j.1365-2621.1976.tb00705.x
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Brunauer S, Skalny J, Bodor EE (1969) Adsorption on nonporous solids. J Colloid Interface Sci 30:546–552. https://doi.org/10.1016/0021-9797(69)90423-8
Do Sup C, Harry BP (1967) Adsorption and desorption of water vapor by cereal grains and their products part ii: development of the general isotherm equation10. 10.13031/2013.39727
Iglesias HA, Chirife, J. (1978) An empirical equation for fitting water sorption isotherms of fruits and related products. Canadian Institute of Food Science and Technology Journal 11:12-15.
Iglesias HA, Chirife J (1976) A model for describing the water sorption behavior of foods. J Food Sci 41:984–992
Henderson S (1952) A basic concept of equilibrium moisture. Agric Eng 33:29–32
Oswin C (1946) The kinetics of package life. III. The isotherm. J Chem Technol Biotechnol 65:419–421
McMinn WAM, Magee TRA (2003) Thermodynamic properties of moisture sorption of potato. J Food Eng 60:157–165. https://doi.org/10.1016/S0260-8774(03)00036-0
Simal S, Femenia A, Castell-Palou Á, Rosselló C (2007) Water desorption thermodynamic properties of pineapple. J Food Eng 80:1293–1301
Al-Muhtaseb A, McMinn W, Magee T (2004) Water sorption isotherms of starch powders: part 1: mathematical description of experimental data. J Food Eng 61:297–307
Riedel L (1977) Calorimetric measurements of heats of hydration of foods. Chemie Microbiologie und Technologie der Lebensmittel 5:101
Sánchez ES, Juan NS, Simal S, Rosselló C (1997) Calorimetric techniques applied to the determination of isosteric heat of desorption for potato. J Sci Food Agric 74:57–63
Fasina O, Ajibola O, Tyler R (1999) Thermodynamics of moisture sorption in winged bean seed and gari. J Food Process Eng 22:405–418
Fasina O, Sokhansanj S, Tyler R (1997) Thermodynamics of moisture sorption in alfalfa pellets. Dry Technol 15:1553–1570
Agbaji A, Agbaji E (1989) Moisture sorption isotherms of some Nigerian hides, skins and leather. J Stored Prod Res 25:57–58
Agbaji A, Agbaji E (1990) Influence of temperature on moisture sorption isotherms of nigerian hides, skins and leathers. J Stored Prod Res 26:81–87
Benmakhlouf N, Azzouz S, Khedhira H, Elcafsi A (2016) Moisture sorption isotherms of leather. J Soc Leather Technol Chem 100:77–83
Lartigue C (1987) Mécanismes élémentaires mises en jeux lors du séchage du bois de Pin maritime PhD thesis. University of Bordeaux I, France
Demchick PH (1984) Taking control of chamber humidity. Sci Teach 51:29–31
Blandamer MJ, Engberts JB, Gleeson PT, Reis JCR (2005) Activity of water in aqueous systems; a frequently neglected property. Chem Soc Rev 34:440–458
Rüegg M (1980) Calculation of the activity of water in sulphuric acid solutions at various temperature. Lebensmittel-Wissenschaft und-Technologie 13:22–24
Hens HSL (2012) Building physics - heat, air and moisture: fundamentals and engineering methods with examples and exercises Wiley
Maroulis Z, Tsami E, Marinos-Kouris D, Saravacos G (1988) Application of the GAB model to the moisture sorption isotherms for dried fruits. J Food Eng 7:63–78
Moreira R, Chenlo F, Torres M, Vallejo N (2008) Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. J Food Eng 88:514–521
Berg CVD, Bruin S (1981) Water activity and its estimation in food systems: theoretical aspects. In: Rockland LB, Stewart GF (Eds) Water activity: Influences on food quality, Academic Press, New York, pp. 2-61
Thompson T, Peart R, Foster G (1968) Mathematical simulation of corn drying: a new model. Trans ASAE 11:582–586
Iglesias H, Chirife J (1976) Prediction of the effect of temperature on water sorption isotherms of food material. Int J Food Sci Technol 11:109–116
Oswin CR (1946) The kinetics of package life III. The isotherm. J Chem Ind 65:419–421
Peleg M (1993) Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J Food Process Eng 16:21–37
Chirife J, Iglesias HA (1978) Equations for fitting water sorption isotherms of foods: part 1 - a review. J Food Technol 13:159–174
Aviara N, Ajibola O (2002) Thermodynamics of moisture sorption in melon seed and cassava. J Food Eng 55:107–113
Maskan M, Göğüş F (1998) Sorption isotherms and drying characteristics of mulberry (Morus Alba). J Food Eng 37:437–449
Nourhène B, Neila B, Mohammed K, Nabil K (2008) Sorptions isotherms and isosteric heats of sorption of olive leaves (Chemlali variety): experimental and mathematical investigations. Food Bioprod Process 86:167–175
Tsami E (1991) Net isosteric heat of sorption in dried fruits. J Food Eng 14:327–335
McMinn W, Al-Muhtaseb A, Magee T (2005) Enthalpy–entropy compensation in sorption phenomena of starch materials. Food Res Int 38:505–510
Madamba P, Driscoll R, Buckle K (1996) Enthalpy-entropy compensation models for sorption and browning of garlic. J Food Eng 28:109–119
Gal S (2012) Recent advances in techniques for the determination of sorption isotherms water relations of foods: proceedings of an international symposium held in Glasgow, September 1974 Elsevier, pp 139
Rizvi SS (1986) Thermodynamic properties of foods in dehydration. Engineering properties of. foods 2:223–309
Mazza G, LeMaguer M (1978) Water sorption properties of yellow globe onion (Allium cepa L.) Can Inst Food Sci Technol J 11:189–193
Marinos-Kouris D, Maroulis ZB (1995) Transport properties in the drying of solids. In: Mujumdar AS (ed) In Handbook of Industrial Drying. 2nd (ed) Marcel Dekker, New York, 1993
Brunauer S, Deming LS, Deming WE, Troller E (1940) On the theory of Van der Waals adsorption of gases. J Am Chem Soc 62:1723–1732
Abdelhamid MB, Mihoubi D, Sghaier J, Bellagi A (2016) Water sorption isotherms and thermodynamic characteristics of hardened cement paste and mortar. Transp Porous Media 113:283–301
Toğrul H, Arslan N (2007) Moisture sorption isotherms and thermodynamic properties of walnut kernels. J Stored Prod Res 43:252–264
Moraes MA, Rosa GS, Pinto LA (2008) Moisture sorption isotherms and thermodynamic properties of apple Fuji and garlic. Int J Food Sci Technol 43:1824–1831
Bahloul N, Boudhrioua N, Kechaou N (2008) Moisture desorption–adsorption isotherms and isosteric heats of sorption of Tunisian olive leaves (Olea europaea L.) Ind Crop Prod 28:162–176
Palipane K, Driscoll R (1993) Moisture sorption characteristics of in-shell macadamia nuts. J Food Eng 18:63–76
Tscheuschner HD, Mohsenin NN (1986) Physical properties of plant and animal materials Gordon and Breach Science Publishers, New York
Bejar AK, Mihoubi NB, Kechaou N (2012) Moisture sorption isotherms–experimental and mathematical investigations of orange (Citrus sinensis) peel and leaves. Food Chem 132:1728–1735
Hammouda I, Mihoubi D (2014) Thermodynamic and mechanical characterisation of kaolin clay. Polish J Chem Technol 16:28–35. https://doi.org/10.2478/pjct-2014-0005
Chung DS, Pfost HB (1967) Adsorption and desorption of water vapor by cereal grains and their products. Part I: heat and free energy changes of adsoption and desorption. Trans ASAE 10:549–551
Mihoubi D, Bellagi A (2006) Thermodynamic analysis of sorption isotherms of bentonite. J Chem Thermodyn 38:1105–1110
Franks F (1991) Hydration phenomena: an update and implications for the food processing industry. In: Levine H, Slade L (Eds.) Water relationships in foods: Advances in the 1980s and Trends for the 1990s Springer US, Boston, MA pp 1–19
Palou E, Lopez-Malo A, Argaiz A (1997) Effect of temperature on the moisture sorption isotherms of some cookies and corn snacks. J Food Eng 31:85–93
Hossain M, Bala B, Hossain M, Mondol M (2001) Sorption isotherms and heat of sorption of pineapple. J Food Eng 48:103–107
Arslan N, Toğrul H (2005) Modelling of water sorption isotherms of macaroni stored in a chamber under controlled humidity and thermodynamic approach. J Food Eng 69:133–145
Krug R, Hunter W, Grieger R (1976) Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of van't Hoff and Arrhenius data. J Phys Chem 80:2335–2341
Krug R, Hunter W, Grieger R (1976) Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effect. J Phys Chem 80:2341–2351
Aguerre R, Suarez C, Viollaz P (1986) Enthalpy-entropy compensation in sorption phenomena: application to the prediction of the effect of temperature on food isotherms. J Food Sci 51:1547–1549
Vishwakarma R, Shivhare U, Nanda S (2011) Moisture adsorption isotherms of guar (Cyamposis tetragonoloba) grain and guar gum splits. LWT-Food Sci Technol 44:969–975
Koua BK, Koffi PME, Gbaha P, Toure S (2014) Thermodynamic analysis of sorption isotherms of cassava (Manihot esculenta). J Food Sci Technol 51:1711–1723
Tolaba M, Suarez C, Viollaz P (1995) Spreading pressure-water activity and moisture relationships in starchy materials. Dry Technol 13:2097–2111
Fasina O (2006) Thermodynamic properties of sweetpotato. J Food Eng 75:149–155
Aviara NA, Ojediran JO, Sa'id UM, Raji AO (2016) Effect of moisture sorption hysteresis on thermodynamic properties of two millet varieties. Agric Eng Int CIGR J 18:363–383
Aviara N, Ajibola O, Oni S (2004) Sorption equilibrium and thermodynamic characteristics of soya bean. Biosyst Eng 87:179–190
Rizvi S, Benado A (1983) Thermodynamic analysis of drying foods. Dry Technol 2:471–502
Kapsalis JG (1987) Influences of hysteresis and temperature on moisture sorption isotherms. Water activity: theory and applications to food: 173–213
Acknowledgements
This work has been made possible through the financial support and research partner of TMM industry. The authors would like to thank all the ‘Tannerie Megisserie du Maghreb’ industry team and especially Mr. Hechmi khedira, a responsible management environment in TMM industry, for the leather sampling collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fakhfakh, R., Mihoubi, D. & Kechaou, N. Moisture sorption isotherms and thermodynamic properties of bovine leather. Heat Mass Transfer 54, 1163–1176 (2018). https://doi.org/10.1007/s00231-017-2223-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-017-2223-0