Abstract
Sorption isotherms of cassava were determined experimentally using a static gravimetric method at 30, 45 and 60 °C and within the range of 0.10–0.90 water activity. At a constant water activity, equilibrium moisture content decreased with increasing temperature. The equilibrium moisture content increased with increasing water activity at a given temperature. The experimental results were modelled using seven sorption models using non-linear regression technique. Results demonstrated that the GAB model adequately predicted equilibrium moisture content of cassava for the range of temperatures and water activities studied. The thermodynamic functions such as net isosteric heat of sorption, differential entropy of sorption, net integral enthalpy and entropy were evaluated to provide an understanding of the properties of water and energy requirements associated with the sorption behaviour. Net isosteric heat and differential entropy decreased with increasing equilibrium moisture content. The net integral enthalpy decreased while net integral entropy increased with increasing equilibrium moisture content. Net integral entropy was negative in value. All thermodynamic functions were adequately characterised by a power law model. The point of maximum stability was found between 0.053 and 0.154 kg water/kg db for cassava.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cassava (Manihot esculenta) is a perennial shrub of the Euphorbiaceae family, native of Guyana. It is now widely cultivated and harvested as annual plant in the tropical and subtropical regions. Cassava is becoming an increasingly important crop in animal feed and human diet in West Africa (Olufayo and Ogunkunle 1996). Cassava tubers are processed in various ways for human consumption.
Cassava has a moisture content of 63 % wet basis at harvest. Therefore, they cannot be preserved for more than a few days under ambient conditions. Compared to fresh products which can only be kept for a few days under ambient conditions, dry products can be stored for months or even years without appreciable loss of nutrients (Shivhare et al. 2000; Mwithiga and Olwal 2005).
However, the knowledge of sorption isotherms is essential for various foodstuff processes such as drying, storage and packaging. Sorption isotherms describe the relationship between the equilibrium moisture content and the water activity at constant temperatures. They give information about the water sorption mechanism and interactions between food components and water. They are also extremely important in modelling of the drying process, in design and optimisation of drying equipment, in predicting shelf-life stability, in determining critical moisture, in selecting appropriate packaging material and water activity for acceptability of products that deteriorate mainly by moisture gain (Van den Berg and Bruin 1981; Gal 1987; Palou et al. 1997; Arora et al. 2003; Kaymak-Ertekin and Gedik 2004).
Relevant work has been carried out on sorption isotherms characteristics of foodstuffs
(Mir and Nath 1995; Ayranci 1995; Myhara et al. 1998; Tsami et al. 1999; Ahouannou et al. 2000; Talla et al. 2001; Lahsani et al. 2003; Talla et al. 2005; Kaur et al. 2006; Yazdani et al. 2006; Vishwakarma et al. 2007; Jain et al. 2010; Jagadish et al. 2010; Chenlo et al. 2011).
Many empirical and semi-empirical mathematical models for description of the sorption behaviour of foodstuffs are available in literature. Criteria used for selecting the most appropriate sorption model are the degree of fit to the experimental data and the simplicity of the model (Kaymak-Ertekin and Gedik 2004; Basu et al. 2006; Chen and Weng 2010; Shafiq Alam and Singh 2011).
Knowing the sorption isotherms at different temperatures makes it possible to calculate the thermodynamic functions such as net isosteric heat, differential entropy, net integral enthalpy and entropy. The thermodynamic functions of water sorption in dried foodstuffs have drawn interest because it provides a more through interpretation of the sorption isotherm phenomenon and helps to understand better the sorption mechanism (Beristain et al. 2002; Pérez-Alonzo et al. 2006). The net isosteric heat of sorption can be used to estimate the energy requirements of drying and provides important information on the state of water in foodstuffs. The differential entropy of sorption is proportional to the number of available sorption sites at a specific energy level (Togrul and Arslan 2006; Rosa et al. 2010). Net integral enthalpy and entropy are used to explain the modes of moisture sorption by foodstuffs.
The objectives of this study are to determine the experimental sorption isotherms of cassava at 30, 45 and 60 °C, to evaluate the best sorption isotherm model to fit experimental data and to determine the thermodynamic functions such as net isosteric heat, differential entropy, spreading pressure, net integral enthalpy and entropy for the sorption isotherms of cassava.
Materials and methods
Experimental procedure
The static gravimetric method of the saturated salts solutions was used for adsorption and desorption isotherms determination. Ten saturated salt solutions selected to give different water activities in the range of 0.110–0.900 were used. These include: LiCl, KCH3CO2, MgCl2, K2CO3, Mg(NO3)2, NaBr, SrCl2, NaCl, KCl and BaCl2. The water activity values of the saturated salt solutions at different experimental temperatures were taken from data reported by Kiranoudis et al. (1993) and given in Table 1.
The static gravimetric is a method where diffusion is the only way of mass transfer between the product and the surrounding air. The air relative humidity is fixed by contact with saturated salts solution whose water vapour pressure at a given temperature is perfectly known. This method is commonly used for product sorption isotherms determination (Kouhila et al. 2001; Lahsani et al. 2003; Talla et al. 2005; Yazdani et al. 2006; Brett et al. 2009; Singh et al. 2011) and is recommended by the COST 90 project (Wolf et al. 1985).
The samples were kept in triplicate in desiccators containing saturated salt solutions and placed in a temperature controlled cabinet, with an accuracy of ±1 °C at the selected temperatures 30 °C, 45 °C and 60 °C. For adsorption, the samples were pre-dried in a vacuum oven. The samples were weighed on an electronic balance with an accuracy of 0.01 g (Metter PL 1200 digital) at regular intervals until the equilibrium reached.
The cassava used in this study was purchased from the local retail market. The initial moisture content was determined by the oven-drying method for 24 h at 105 °C. Initial moisture content of cassava was 1.70 kg water/kg dry basis.
Data analysis and thermodynamic properties
Sorption isotherm
The relationship between the equilibrium moisture content and the water activity of the product was predicted by using the equations representing the models commonly used in foodstuff. These models which incorporate the temperature effect have been adopted as standard equations by the ASAE for the description of sorption isotherms (ASAE 1997). In this study, the eight isotherm models used to fit the experimental data are presented in Table 2.
The constants were estimated by fitting the mathematical model to the experimental data, using a non-linear regression analysis with Microsoft Excel 2007 software. The quality of the fitting of different models was evaluated by calculating the correlation coefficient (r2), the mean relative percentage deviation modulus E in % and the root mean square error (RMSE) between the experimental and predicted equilibrium moisture content (Boquet et al. 1978; Basu et al. 2006):
where N is the number of observations; Xeq,exp and Xeq,pre are the experimental and predicted values of the equilibrium moisture content, respectively.
The mean relative percentage deviation modulus is widely adopted throughout the literature, with a modulus value below 10 % indicative of a good fit for practical purposes (Lomauro et al. 1985; Kaymak-Ertekin and Gedik 2004). In the same way, the smaller the RMSE value, the better the fit of the model.
According to Kaleemullah and Kallappan (2004), Aviara et al. (2004) and Basu et al. (2006), statistical parameters like r2, E and RMSE may not be sufficient evidence for the goodness of fit of a moisture sorption model based on experimental data, but the nature of the residual plots should be considered in addition. A model was considered acceptable if the residuals (Xeq,exp – Xeq,pre) are uniformly scattered around the horizontal value of zero, showing no systematic tendency towards a clear pattern.
In the GAB model, Xm is the moisture content corresponding to the formation of a monomolecular layer on the internal surface; G is a constant related to the heat of sorption of the first layer on primary sites and K is a factor correcting properties of the multi-layer molecules with respect to the bulk liquid. The influence of temperature on GAB constants G and K can be expressed with Arrhenius type equations (Bellagha et al. 2005):
where G0 and K0 are the pre-exponential factors, Hm is the sorption heat of the monolayer, Hn is the sorption heat of the multi-layer, HL is the heat of condensation of water vapour (HL = 43.53 KJ.mol−1 at the average temperature 35 °C within the investigate range 25–45 °C), R is the universal gas constant (R = 8.314 J.mol−1 K−1) and T is the absolute temperature.
Bizot (1983) transformed the GAB equation into a quadratic equation:
Where A, B and C are the coefficients dependent of temperature.
By combining Eq. (5) and GAB equation, other expressions to estimate Xm, G and K may be considered:
The BET model can be rearranged as:
with
Treatments of sorption data according to the BET (values for aw up to 0.5) and GAB (values for aw up to 0.95) models allow the evaluation of monolayer moisture content values, Xm, of foodstuffs. The prediction of Xm values is important since deterioration of foodstuffs is very small below Xm. This is because water is strongly bound to the foodstuff below Xm and is not involved in any deteriorative reaction either as solvent or as one of the substrates (Ayranci and Duman 2005). Once the monolayer moisture content was known, the water surface area of product can be determined by the following Eq. (11) (Rosa et al. 2010). The specific surface area plays an important role in determining the water binding properties of particulate products.
where Sa is the product surface area (m2.g−1), Xm is the monolayer moisture content (kg.kg−1 dry basis), \( {{\text{M}}_{{{\text{H}}_2}{\text{O}}}} \)is the molecular weight of water (18 g.mol−1), NA is Avogadro’s number (6 × 1023 molecules.mol−1) and \( {{\text{A}}_{{{\text{H}}_2}{\text{O}}}} \)is the area of one water molecule (1.06 × 10−19 m2).
Isosteric heat of sorption
Isosteric heat of sorption or differential enthalpy is the amount of energy required to change unit mass of a product from liquid to vapour at a particular temperature and water activity (Aviara et al. 2002). The net Isosteric heat of sorption is defined as the isosteric heat of sorption minus the latent heat of the pure water vaporisation. It can be computed from experimental sorption isotherm data using the Clausius-Clapeyron equation in the form (Kaya and Kahyaoglu 2007; Singh et al. 2011; Chenlo et al. 2011):
where qst is the net isosteric heat of sorption (kJ.mol−1); Qst is the isosteric heat of sorption (kJ.mol−1); Lv is the latent heat of the pure water vaporisation (kJ.mol−1).
The aforementioned procedure assumes that qst is independent of temperature; although this is not always true, it has often been accepted (Iglesias et al. 1989). The application of this method requires data at least at two or more experimental temperatures. On the other hand the integrating of Eq. (13) between the two equilibrium states (T1, aw1) and (T2, aw2) for any arbitrary constant moisture content, X, leads to Eq. (14):
Where awi is the measurement of water activity at the absolute temperature Ti for constant moisture content.
Differential entropy
Entropy change plays an important role in the energy analysis of food processing systems. The differential entropy or sorption entropy is proportional to the number of available sorption sites at a specific energy level (Togrul and Arslan 2006). The differential entropy (ΔS) was obtained by fitting Eq. (15) to equilibrium moisture content data from the best-fitting equation (Madamba et al. 1996).
The net isosteric heat of sorption and differential entropy can be calculated from Eq. (15) by plotting ln(aw) versus 1/T, for given a moisture content and then determining the slope (−qst/R) and the linear coefficient (ΔS/R).
Enthalpy-entropy compensation theory
The compensation theory was further used to evaluate the effect of temperature on the sorption behaviour. It proposes a linear relationship between qst and ΔS (McMinn and Magee 2003):
The isokinetic temperature, Tβ, has an important physical meaning as it represents the temperature at which all reactions in the series proceed at the same rate. The free energy, ΔGβ, at temperature Tβ provides a criterion to estimate if the water sorption process is spontaneous (−ΔGβ) or no (+ΔGβ).
Since there is a high degree of linear correlation between enthalpy and entropy, the compensation theory was assumed to be valid for sorption (Madamba et al. 1996; Vishwakarma et al. 2011). To corroborate the compensation theory, a statistical analysis test (Eq. (17)) was proposed by Krug et al. (1976a, b). This involves a comparison of the isokinetic temperature (Tβ) with the harmonic mean temperature (Thm). The compensation theory only applies if Tβ ≠ Thm.
Spreading pressure
The spreading pressure is the two-dimensional analogue of pressure, having units of force per unit length, akin to surface tension. It can be viewed as the force in the plane of the surface that must be exerted perpendicular to each unit length of edge to keep the surface from spreading (Smith et al. 2001). Skaar and Babiak (1982) used the spreading pressure concept as the driving force in developing transport models during diffusion in porous solid. The spreading pressure (surface potential) which is the surface excess free energy is calculated using an analytical procedure described by Iglesias et al. (1976):
with the moisture ratio θ is:
where KB is the Boltzmann’s constant (KB = 1.38 × 10−23 JK−1) and π is the spreading pressure (J.m−2).
The value of aw = 0 will make the integral of Eq. (18) to be indeterminate. Therefore, the GAB model (Eq. (20)) was used since the calculation of the integral in Eq. (18) changes from a numerical procedure to assume an empirical relationship between water activity and equilibrium moisture content.
Substituting Eq. (20) into Eq. (18), rearranging and then integrating gives the spreading pressure at different temperatures on the basis of surface area per sorption site or area per one molecule of water on each sorption site as:
Net integral enthalpy and entropy
The net integral enthalpy represents the total energy available to do work. It provides an indication of the binding strength of water molecules to the solid, and has some bearings on the energy balance of drying and freezing operations (Gal 1975). The net integral enthalpy (qin), a measure of the food-water affinity, was determined in a similar manner as the isosteric heat but at a constant spreading pressure (Togrul and Arslan 2007).
A plot of ln(aw) versus 1/T at constant spreading pressure gives the net integral enthalpy from the slope.
The net integral entropy describes the degree of disorder and randomness of motion of water molecules. It quantifies the mobility of the adsorbed water molecules, and indicates the degree to which the water-substrate interaction exceeds that of the water molecules (Togrul and Arslan 2006). The net integral entropy (ΔSin) is given by (Togrul and Arslan 2006):
where \( {\text{a}}_{\text{w}}^* \) is the geometric mean water activity obtained at constant spreading pressure at different temperatures.
Results and discussions
Sorption isotherm
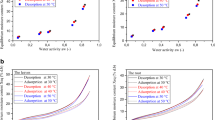
The sorption isotherm representing the variation of the equilibrium moisture content with the water activity is plotted in Fig. 1 for cassava at various temperatures. The isotherm is sigmoidal as usually presented for foodstuff by Iglesias and Chirife (1982). This is typical of type II isotherms and has been reported for starchy products as potato (McMinn and Magee 2003), yellow dent corn (Samapundo et al. 2007), oat flour and rice flour (Brett et al. 2009), tea (Chen and Weng 2010), chestnut flour and starch (Chenlo et al. 2011), guar grains and splits (Vishwakarma et al. 2011). The equilibrium moisture content decreases with the temperature at constant water activity. Equilibrium moisture content increases also with increase in water activity for all temperatures. This behaviour may be attributed to the excitation states of molecules. At increased temperatures, molecules are in an increased state of excitation, thus increasing their distance decreases the attractive forces between them (Kaya and Aydin 2009). This leads to a decrease in the degree of water sorption at a given water activity with increasing temperature (Jamali et al. 2006). Whereas Mazza and Lemaguer (1980) suggested that increase in temperature induces physical and chemical changes in the product that can reduce the number of active sites for water binding. The temperature dependence of the equilibrium moisture content has an important practical bearing on chemical and microbiological reactions associated with spoilage (Al-Muhtaseb et al. 2004). At the same moisture content, higher temperatures entail a higher water activity and consequently faster rates of deterioration (Van den Berg and Bruin 1981).
The comparison of the adsorption and desorption data are illustrated in Fig. 1. The desorption curves clearly lie above the adsorption curves, forming the hysteresis loop. The hysteresis effect extends over the entire water activity range. Hysteresis is not fully understood (Al-Muhtaseb et al. 2004), although there is general agreement that some thermodynamically irreversible processes must occur during desorption or adsorption, or both. One theory used to explain hysteresis suggests that in the wet condition the polar sites in the molecular structure of the material are almost entirely satisfied by adsorbed water. The shrinkage that occurs upon drying draws them closer enabling them to satisfy each other. This reduces the water binding capacity during adsorption (Mohsenin 1986). Similar hysteresis phenomena have been reported for some foods (Johnson and Brennan 2000; Al-Muhtaseb et al. 2004; Togrul and Arslan 2007; Samapundo et al. 2007; Shafiq Alam and Singh 2011).
Table 3 shows the coefficients of the models fitted to the experimental adsorption and desorption data by non-linear regression, the mean relative percentage deviation modulus (E), the root mean square error (RMSE) and the correlation coefficient (r2). All models presented correlation coefficients very close to unity indicating good fit to experimental data. The high value of r2 at different temperatures showed that the good fit of the models is independent of temperature.
According to the results shown in Table 3, for temperatures from 30 to 60 °C and water activities from 0.10 to 0.90, it can be seen that the GAB model obtained the least mean relative percentage deviation modulus (E), least root mean square error (RMSE) and highest correlation coefficient (r2) followed by BET and Smith model that showed very similar fitting parameters.
Plotting residuals of the models against water activity revealed that GAB model, BET model, Smith model, Kuhn model and Oswin model presented a random residual distribution and the other models, Halsey and Iglesias-Chirife model, a patterned residual distribution. According to these results, GAB model was considered the best model for predicting the equilibrium moisture content of cassava. The GAB model has a theoretical basis, while the other models are empirical or semi-empirical (Adebowale et al. 2007). According to Lomauro et al. (1985), the GAB model provides the best fit for more than 50 % of the fruits, meats and vegetables analysed. This model has been widely applied to describe equilibrium moisture content in foodstuffs, and was recommended by the European Project COST90 (Garcia-Pérez et al. 2008).
Monolayer moisture content (Xm) of cassava obtained using GAB and BET models are also given in Table 3. In the adsorption mode, Xm ranged from 6.16 to 3.66 g water/100 g dry basis and 5.55 to 2.64 g water/ 100 g dry basis for the GAB and BET models, respectively. In the desorption mode, Xm ranged from 6.96 to 4.21 g water/100 g dry basis and 6.24 to 2.93 g water/ 100 g dry basis for the GAB and BET models, respectively. The decrease in monolayer moisture contents with increase in temperature, as observed in this study, may be due to a reduction in the total number of active sites for water binding as a result of physical and/or chemical changes in the product induced by temperature (Iglesias and Chirife 1976). Also, according to Palipane and Driscoll (1992), it could be that with increase in temperature, the water molecules got activated due to an increase in their energy level, causing them to become less stable and to break away from the water binding sites of the food, thus decreasing the monolayer moisture content. These observations imply that the anticipated temperature of storage of the cassava would determine the optimum moisture content for maximum self stability.
Table 4 gives the results of the regression analysis of GAB model parameters. The positive value for ΔHG in adsorption and desorption mode indicates that the sorption heat of water monolayer molecules (Hm) is higher than the sorption heat of water multilayer molecules (Hn). The positive value for ΔHk in adsorption and desorption mode indicates that the condensation heat of water vapour (HL is higher than the sorption heat of water multilayer molecules (Hn). The ΔHG and ΔHK values for desorption are higher than the values for adsorption in the case of cassava. The variation in enthalpy values between adsorption and desorption suggests a degree of irreversibility with respect to the water binding properties of the foodstuffs (McMinn and Magee 1999).
Table 5 presents the water surface area of cassava between 30 and 60 °C. It was estimated using Eq. (12) and the monolayer moisture contents of cassava obtained by GAB model. These results (Table 5) indicate that the availability of surface areas for hydrophilic binding decrease with increasing temperature. The calculated surface area values of cassava were within the range commonly obtained for foodstuffs (100–250 m2.g−1) (Cassini et al. 2006). Aguerre et al. (1989) and Calzetta et al. (2000) indicated that the large surface area of many biopolymers is due to the existence of an intrinsic microporous structure in the foodstuffs.
Thermodynamic properties
Net isosteric heat of sorption
The net isosteric heats of adsorption and desorption of water at constant equilibrium moisture content were determined by applying Eqs. (13) and (14) to data calculated by using the GAB model that gave the best fitting of equilibrium moisture content data in the range of temperature from 30 to 60 °C. As can be observed in Table 6, the net isosteric heats for cassava at different equilibrium moisture contents from isotherms through the differential method (Eq. (13)) and by the integral method (Eq. (14)) are very close.
Figure 2 shows the net isosteric heat of adsorption and desorption of cassava as a function of equilibrium moisture content. It can be clearly seen that the net isosteric heat of adsorption and desorption decreased with increasing equilibrium moisture content. This decrease can be qualitatively explained by considering that sorption initially occurs at the most active sites, giving rise to the greatest interaction energy. As the equilibrium moisture content increases, the sites available for water sorption decrease, resulting in lower values of net isosteric heat (Tsami et al. 1990). Similar results have been reported for the foodstuffs in literature (Delgado and Sun 2002; Kaymak-Ertekin and Gedik 2004; Kaya and Kahyaoglu 2007; Rosa et al. 2010).
Comparison of the adsorption and desorption data shows that, at constant equilibrium moisture content, the net isosteric heat of desorption is higher than the corresponding adsorption value. This indicates that the desorption process requires a greater amount of energy than adsorption as stated by Rizvi (1986) for rice, Wang and Brennan (1991) for potatoes, McMinn and Magee (2003) for potato, Kaymak-Ertekin and Gedik (2004) for grapes, apricots, apples and potatoes, Samapundo et al. (2007) for yellow dent corn. The net isosteric heat versus equilibrium moisture content results are adequately represented by a power law relation of the form:
-
Adsorption: \( {{\text{q}}_{\text{st}}} = 2497.38\,{\text{X}}_{\text{eq}}^{ - 0.841} \) with r2 = 0.984
-
Desorption: \( {{\text{q}}_{\text{st}}} = 2807.36\,{\text{X}}_{\text{eq}}^{ - 0.836} \) with r2 = 0.988
Differential entropy
The differential entropy of cassava versus equilibrium moisture content is shows in Fig. 2. The differential entropy decreases with increasing equilibrium moisture content. At low water activity range, a decrease in differential entropy values is possibly caused by the strongest binding sites with water molecules and the solid as suggested by McMinn and Magee (2003). Once again, the differential entropy of desorption is higher than the corresponding adsorption value, at constant equilibrium moisture content. The differential entropy data displays a strong dependence on equilibrium moisture content as stated by Kaya and Kahyaoglu (2007). The differential entropy versus equilibrium moisture content results are adequately represented by a power law relation of the form:
-
Adsorption: \( \Delta {\text{S}} = 8.35\,{\text{X}}_{\text{eq}}^{ - 0.782} \) with r2 = 0.980
-
Desorption: \( \Delta {\text{S}} = 9.35\,{\text{X}}_{\text{eq}}^{ - 0.781} \) with r2 = 0.985
Enthalpy-entropy compensation theory
Figure 3, representing qst as a function of ΔS, shows a linear relation with a correlation coefficient r2 = 0.999. Table 7 gives the parameters Tβ and ΔGβ (Eq. (16)) calculated from the data by linear regression. The adsorption data gives higher values than those for desorption.
Confirmation of the application of the compensation theory is provided by calculation of an harmonic mean temperature (Thm) value of 317.66 K. For both adsorption and desorption Tβ ≠ Thm. The compensation theory can also be applied and the water sorption in cassava is spontaneous (ΔGβ < 0). Similar results for guar have been obtained by Vishwakarma et al. (2011).
Spreading pressure
The spreading pressure values (π) were evaluated using the analytical expression (Eq. (21)). The constants (G, K) in Eq. (21) were determined from the GAB model. The spreading pressure in adsorption and desorption process of cassava are represented in Fig. 4. The results shown that the spreading pressure increased with increasing water activity and, at a given water activity, decreased with increasing temperature. The trends of spreading pressures with respect to temperature and water activity for adsorption and desorption were similar to those determined by Fasina et al. (1999) for winged bean and gari, Aviara and Ajibola (2002) for melon seed and cassava, McMinn and Magee (2003) for potato.
Net integral enthalpy and entropy
The net integral enthalpy variations with respect to equilibrium moisture content are represented in Fig. 2. The net integral enthalpy of adsorption and desorption decreased with increasing equilibrium moisture content. The net integral enthalpy values for desorption were greater than those for adsorption at any equilibrium moisture content. The reason for such behaviour may be chain rupture or irreversible swelling (Togrul and Arslan 2006). The results are similar to those reported by Aviara and Ajibola (2002) for cassava, Togrul and Arslan (2006) for rice. While the net integral enthalpy of adsorption differed from the net enthalpy of desorption in magnitude, the net enthalpies of adsorption and desorption were qualitatively similar with regard to their functionality with equilibrium moisture content. The experimental net enthalpy data conform to a power law relation as represented by the equation:
-
Adsorption: \( {{\text{q}}_{\text{in}}} = 3320.93\,{\text{X}}_{\text{eq}}^{ - 0.584} \) with r2 = 0.993
-
Desorption: \( {{\text{q}}_{\text{in}}} = 3695.98\,{\text{X}}_{\text{eq}}^{ - 0.595} \) with r2 = 0.993
Figure 2 shows the variation in the net integral entropy with equilibrium moisture content at 30 °C, for cassava. The net integral entropy of adsorption and desorption increased with increasing equilibrium moisture content. The net integral entropy values of adsorption are found to be greater than those for desorption at a constant equilibrium moisture content. As cassava adsorbed moisture, the entropy diminished to a minimum point that is considered as that of maximum stability, because it is where the water molecules achieve a more ordered arrangement within the solid. The minimum integral entropy value found at 30 °C was 0.154 kg water/kg db for cassava. The minimum entropy can be interpreted as the monolayer moisture content. This minimum value was expected to arise where strong bonding occurs between adsorbent and adsorbate which corresponds to less water being available for spoilage reactions. It can also be seen from Fig. 2 that the equilibrium moisture content corresponding to the minimum integral entropy value for cassava to achieve maximum stability was greater than corresponding to the GAB monolayer. The conditions for maximum storage stability of the cassava were shown in Table 8. As can be appreciated, as temperature increases the water activity decreases.
For all of the equilibrium moisture content range the net integral entropy values were negative. Negative net integral entropy values were attributed to the existence of chemical adsorption and/or structural modifications of the absorbent (Iglesias et al. 1976). The net integral entropy data conform to a power law relation as represented by the equation:
-
Adsorption: \( \Delta {{\text{S}}_{\text{in}}} = - 14.85\,{\text{X}}_{\text{eq}}^{ - 0.412} \) with r2 = 0.978
-
Desorption: \( \Delta {{\text{S}}_{\text{in}}} = - 16.26\,{\text{X}}_{\text{eq}}^{ - 0.439} \) with r2 = 0.981
The thermodynamic analysis of moisture sorption in dried foodstuffs has drawn interest because it provides a more thorough interpretation of the sorption isotherm phenomenon and helps to understood better the sorption mechanism (Beristain et al. 2002). Thermodynamic parameters such as net isosteric heat, differential entropy, net integral enthalpy and entropy could be used to determine interactions of water and food substances and to provide information useful for assessing food processing operations such as drying, mixing and storage. These parameters also improve the design of the food drying process, for instance establishing the drying conditions, to fix the end-point of the process or the determination of the energy requirements.
Conclusion
In this study, the sorption isotherms of cassava were determined at the temperatures of 30, 45 and 60 °C over the water activity range of 0.10 to 0.90, using the static gravimetric method. The sorption isotherms of cassava presented a sigmoidal shape. Equilibrium moisture contents were found to decrease with increasing temperature at constant water activity. They were also found to increase with increasing water activity at a given temperature. Hysteresis is evident over the entire range of water activity. All models presented a good fit to the experimental data. However, the GAB model described well the sorption isotherms of cassava.
On the basis of the thermodynamic analysis the following conclusions can be also drawn:
-
The net isosteric heats of adsorption and desorption decreased with increasing equilibrium moisture content. The net isosteric heat is higher for desorption than adsorption at a constant equilibrium moisture content indicating that the desorption process involves more energy than adsorption.
-
The differential entropy of cassava decreased with increasing equilibrium moisture content.
-
The net isosteric heats versus differential entropy data satisfy the enthalpy-entropy compensation theory.
-
Net integral enthalpy of adsorption and desorption decreased with increasing equilibrium moisture content. In a reverse manner, net integral entropy increased with increasing equilibrium moisture content. The minimum integral entropy was proposed as indicative of the most suitable conditions for storage, with the corresponding water activity being available from the isotherm; this is in the range of 0.580–0.691for the temperature range between 30 and 60 °C.
All thermodynamic functions such as net isosteric heat, differential entropy, net integral enthalpy and entropy were adequately characterised by a power law model.
References
Adebowale AR, Sanni I, Awonorin S, Daniel I, Kuye A (2007) Effect of cassava varieties on the sorption isotherm of tapioca grits. Inter J Food Sci Technol 42:448–452
Aguerre RJ, Suarez C, Viollaz PE (1989) Swelling and pore structure in starch materials. J Food Eng 9:71–80
Ahouannou C, Jannot Y, Lips B, Lallemand A (2000) Caractérisation et modélisation du séchage de trois produits tropicaux: manioc, gingembre et gombo. Sci Aliments 20:413–432
Al-Muhtaseb AH, McMinn WAM, Magee TRA (2004) Water sorption isotherms of starch powders. Part I: Mathematical description of experimental data. J Food Eng 61:297–307
Arora S, Shivhare US, Ahmed J, Raghavan GSV (2003) Drying kinetics of agaricus bisporus and pleurotus florida mushrooms. Trans ASAE 46(3):721–724
ASAE D245.5 (1997) Moisture relationship of plant-Based Agricultural products. ASAE Standards 44th Eds., Michigan, USA. St Joseph
Aviara NA, Ajibola OO (2002) Thermodynamics of moisture sorption of melon seed and cassava. J Food Eng 55:107–113
Aviara NA, Ajibola OO, Dairo UO (2002) Thermodynamics of moisture sorption in sesame seed. Biosystems Eng 83:423–431
Aviara NA, Ajibola OO, Oni SA (2004) Sorption equilibrium and thermodynamic characteristics of soya bean. Biosystems Eng 87(2):179–190
Ayranci E (1995) Equilibrium moisture characteristics of dried eggplant and okra. Food/ Nahrung 39(3):228–233
Ayranci E, Duman O (2005) Moisture sorption isotherms of cowpea (Vigna unguiculata L. Walp) and its protein isolate at 10, 20 and 30 °C. J Food Eng 70:83–91
Basu S, Shivhare US, Mujumdar AS (2006) Models for sorption isotherms for foods: a review. Dry Technol 24(8):917–930
Bellagha S, Sahli A, Glenza A, Kechou N (2005) Isohalic sorption isotherm of sardine (Sardinella aurita): experimental determination and modeling. J Food Eng 68:105–111
Beristain CI, Azuara E, Vernon-Carter EJ (2002) Effect of water activity on the stability to oxidation of spray-dried encapsulated orange peel oil using mesquite gum (Prosopis juliflora) as wall material. J Food Sci 67:206–211
Bizot H (1983) Using the GAB model to construct sorption isotherms. In: Jowitt et al (eds) Physical properties of foods. Applied Science, London, pp 43–54
Boquet R, Chirife J, Iglesias HA (1978) Equations for fitting water sorption isotherms of foods. II- Evaluation of various two-parameter models. J Food Technol 13:319–327
Brett B, Figueroa M, Sandoval AJ, Barreiro JA (2009) Moisture sorption characteristics of starchy products: oat flour and rice flour. Food Biophys 4:151–157
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layer. J Am Chem Soc 60(2):309–319
Calzetta Resio AN, Tolaba MP, Suarez C (2000) Some physical and thermal characteristics of amaranth starch. Food Sci Technol Int 6:371–378
Cassini AS, Marczak LDF, Norena CPZ (2006) Water adsorption isotherms of texturized soy protein. J Food Eng 77:194–199
Chen C, Weng Y-K (2010) Moisture sorption isotherms of Oolong tea. Food Bioprocess Technol 3(2):226–233
Chenlo F, Moreira R, Prieto DM, Torres MD (2011) Desorption isotherms and net isosteric heat of chestnut flour and starch. Food Bioprocess Technol 4(8):1497–1504
Delgado AE, Sun D-W (2002) Desorption isotherms for cooked and cured beef and pork. J Food Eng 51:163–170
Fasina O, Ajibola OO, Tyler R (1999) Thermodynamic of moisture sorption in winged bean seed and gari. J Food Process Eng 22:405–418
Gal S (1975) Recent advances in techniques for the determination of sorption isotherms. In: Duckworth RB (ed) Water relations of foods. Academic, London, pp 139–155
Gal S (1987) The need for, and practical applications of sorption data. In: Jowitt R, Escher F, Hallström B, Mefert H, Spiess W, Vos G (eds) Physical properties of foods-2. Elsevier Applied Science, London, pp 13–25
Garcia-Pérez JV, Carcel JA, Clemente G, Mulet A (2008) Water sorption isotherms for lemon peel at different temperatures and isosteric heats. LWT- Food Sci Technol 41(1):18–25
Hasley G (1948) Physical adsorption in non-uniform surfaces. J Chem Phys 16:931–945
Iglesias HA, Chirife J (1976) Local isotherm concept and modes of moisture binding in food products. J Agric Food Chem 24(1):77–79
Iglesias HA, Chirife J (1981) An equation for fitting uncommon water sorption isotherms in foods. LWT 14:111–117
Iglesias HA, Chirife J (1982) Handbook of food isotherms water sorption. Parameters for food and food components. Academic, New York
Iglesias HA, Chirife J, Viollaz P (1976) Thermodynamics of water vapour sorption by sugar beet root. J Food Technol 11:91–101
Iglesias HA, Chirife J, Ferro Fontan C (1989) On the temperature dependence of isosteric heats of water sorption in dehydrated foods. J Food Sci 54(6):1620–1631
Jagadish RS, Rastogi NK, Raj B (2010) Moisture sorption characteristics of chitosan /polyethylene oxide blended films. J Polym Environ 18(3):266–276
Jain SK, Veima RC, Sharma GP, Jain HK (2010) Studies on moisture sorption isotherms for osmotically dehydrated papaya cubes and verification of selected models. J Food SciTechnol 47(3):343–346
Jamali A, Kouhila M, Mohamed IA, Idliman A, Lamharrar A (2006) Moisture adsorption-desorption isotherms of citrus reticulate leaves at three temperatures. J Food Eng 77:71–78
Johnson P-NT, Brennan JG (2000) Moisture sorption isotherm characteristics of plantain (Musa, AAB). J Food Eng 44:79–84
Kaleemullah S, Kallappan R (2004) Moisture sorption isotherms of red chillies. Biosystems Eng 88(1):95–104
Kaur D, Wani AA, Sogi DS, Shivhare US (2006) Sorption isotherms and drying characteristics of tomato peel isolated from tomato pomace. Dry Technol 24(11):1515–1520
Kaya A, Aydin O (2009) An experimental study on drying kinetics of some herbal leaves. Energy Convers Manag 50:118–124
Kaya S, Kahyaoglu T (2007) Moisture sorption and thermodynamic properties of safflower petals and tarragon. J Food Eng 78:413–421
Kaymak-Ertekin F, Gedik A (2004) Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes. LWT- Food Sci Technol 37:429–438
Kiranoudis CT, Maroulis ZB, Tsami E, Marinos-Kouris D (1993) Equilibrium moisture content and heat of desorption of some vegetables. J Food Eng 20:55–74
Kouhila M, Belghit A, Daguenet M, Boutaleb BC (2001) Experimental determination of the sorption isotherms of mint (Mentha viridis), sage (Salvia officinalis) and verbena (Lippia citriodara). J Food Eng 47:281–287
Krug RR, Hunter WG, Grieger RA (1976a) Enthalpy-entropy compensation. 1- some fundamental statistical problems associated with the analysis of Van’t Hoff and Arrhenius data. J Physl Chem 80:2335–2341
Krug RR, Hunter WG, Grieger RA (1976b) Enthalpy-entropy compensation. 2- Separation of the chemical from the statistical effect. J Phys Chem 80:2341–2351
Kuhn I (1967) Generalized potential theory of adsorption: I. The derivation of a general equation for adsorption. J Colloid Interface Sci 23:563
Lahsani S, Kouhila M, Mahrouz M, Fliyou M (2003) Moisture adsorption-desorption isotherms of prickly pear cladode (Opuntia ficus India) at different temperatures. Energy Convers Manag 44:923–936
Lomauro CJ, Bakshi AS, Labuza TP (1985) Evaluation of Food moisture sorption isotherm equations. Part I- Fruit, vegetable and meat products. LWT- Food Sci Technol 18:111–117
Madamba PS, Driscoll RH, Buckle KA (1996) Enthalpy-entropy compensation models for sorption and browning of garlic. J Food Eng 28:109–119
Mazza G, LeMaguer M (1980) Dehydration of onion: some theoretical and practical considerations. J Food Technol 15(2):181–194
McMinn WAM, Magee TRA (1999) Studies on the effect of temperature on the moisture sorption characteristics of potatoes. J Food Process Eng 22:113–128
McMinn WAM, Magee TRA (2003) Thermodynamic properties of moisture sorption of potato. J Food Eng 60:157–165
Mir MA, Nath N (1995) Sorption isotherms of fortified mango bars. J Food Eng 25:141–150
Mohsenin N (1986) Physical properties of plant and animal materials. Gordon & Breach, New York
Mwithiga G, Olwal JO (2005) The drying kinetics of kale (Brassica oleracea) in a convective hot air dryer. J Food Eng 71:373–378
Myhara R-M, Taylor MS, Slominski BA, Al-Bulushi I (1998) Moisture sorption isotherms and chemical composition of omani dates. J Food Eng 37:471–479
Olufayo AA, Ogunkunle OJ (1996) National drying of cassava chips in the humid zone of Nigeria. Bioresour Technol 58:89–91
Oswin CR (1946) The kinetics of package life. III- The isotherm. J Chem Ind 65:419–421
Palipane KB, Driscoll RH (1992) Moisture sorption characteristics of in-shell macadamia nuts. J Food Eng 18:63–76
Palou E, Lopez-Malo A, Argaiz A (1997) Effect of temperature on the moisture sorption isotherms of some cookies and corn snacks. J Food Eng 31:85–93
Pérez-Alonzo C, Beristain CI, Lobato-Calleros C, Rodriguez-Huezo ME, Vernon-Carter EJ (2006) Thermodynamic analysis of the sorption isotherms of pure and blended carbohydrate polymers. J Food Eng 77:753–760
Rizvi SSH (1986) Thermodynamic properties of food in dehydration. In: Rao MA, Rizvi SSH (eds) Engineering properties of foods. Marcel Dekker, New York, pp 133–214
Rosa GS, Moraes MA, Pinto LAA (2010) Moisture sorption properties of chitosan. LWT- Food Sci Technol 43:415–420
Samapundo S, Devlieghere F, De Meulenaer B, Atukwase A, Lamboni Y, Debevere JM (2007) Sorption isotherms and isosteric heats of sorption of whole yellow dent corn. J Food Eng 79:168–175
Shafiq Alam Md, Singh A (2011) Sorption isotherm characteristics of aonla flakes. J Food Sci Technol 48(3):335–343
Shivhare US, Gupta A, Bawa AS, Gupta P (2000) Drying characteristics and product quality of okra. Dry Technol 18(1&2):409–419
Singh KP, Mishra HN, Saha S (2011) Sorption isotherms of barnyard millet grain and kernel. Food Bioprocess Technol 4(5):788–796
Skaar C, Babiak M (1982) A model for bound water transport in wood. Wood Sci Technol 16:123–138
Smith SE (1947) The sorption of water vapour by high polymers. J Am Chem Soc 69:646–651
Smith JM, Van Ness HC, Abbot MM (2001) Introduction to chemical engineering thermodynamics. McGraw-Hill, Boston
Talla A, Jannot Y, Kapseu C, Nganhou J (2001) Experimental study and modelling of the kinetics of drying of tropical fruits. Application to banana and mango. Sci Aliments 21:499–518
Talla A, Jannot Y, Nkeng GE, Puiggali J-R (2005) Experimental determination and modelling of sorption isotherms of tropical fruits: banana, mango and pineapple. Dry Technol 23:1477–1498
Togrul H, Arslan N (2006) Moisture sorption behaviour and thermodynamic characteristics of rice stored in a chamber under controlled humidity. Biosystems Eng 95(2):181–195
Togrul H, Arslan N (2007) Moisture sorption isotherms and thermodynamic properties of walnut kermels. J Stored Prod Res 45:252–264
Tsami E, Maroulis ZB, Marinos-Kouris D, Saravacos GD (1990) Heat of sorption of water in dried fruits. Int J Food Sci Technol 25:350–359
Tsami E, Krokida MK, Drouzas AE (1999) Effect of drying on the sorption characteristics of model fruit powders. J Food Eng 38:381–392
Van den Berg C, Bruin S (1981) Water activity and its estimation in food systems: theoretical aspects. In: Rockland LB, Stewart GF (eds) Water activity: influences on food quality. Academic, New York, pp 147–177
Vishwakarma RK, Goyal RK, Bhargav VK (2007) Moisture sorption isotherms of pigeonpea (Cajanas Cajan) grain and its dehulled splits (Dhal). Am J Food Technol 2(4):228–237
Vishwakarma RK, Shivhare US, Nanda SK (2011) Moisture adsorption isotherms of guar (cyamposis tetragonoloba) grain and guar gum splits. LWT- Food Sci Technol 44(4):969–975
Wang N, Brennan JG (1991) Moisture sorption isotherm characteristics of potatoes at four temperatures. J Food Eng 14:269–287
Wolf W, Spiess WEL, Jung G (1985) Properties of water in foods in relation to food quality and stability. In: Stimatos D, Multon JL (eds) Standardization of isotherm measurement. Martinus Nijhoff Publishers, Dordrecht, pp 661–679
Yazdani M, Sazandehchi P, Azizi M, Ghobadi P (2006) Moisture sorption isotherms and isosteric heat for pistachio. Eur Food Res Technol 223(5):577–584
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koua, B.K., Koffi, P.M.E., Gbaha, P. et al. Thermodynamic analysis of sorption isotherms of cassava (Manihot esculenta) . J Food Sci Technol 51, 1711–1723 (2014). https://doi.org/10.1007/s13197-012-0687-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-012-0687-y