Abstract
Purpose
The aim of this meta-analysis of randomized controlled trials was to evaluate the effect of hydroxychloroquine on glucose control.
Methods
Randomized controlled trials examining the impact of hydroxychloroquine on glycemic markers were searched in PubMed, Web of Science, Scopus, and Google Scholar databases. Meta-analysis was performed using a random-effects model and sensitivity analysis through the leave-one-out method.
Results
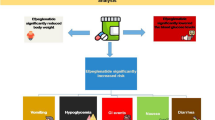
Meta-analysis revealed a significant reduction of fasting glucose (WMD: − 8.05 mg/dl; 95% CI: − 11.17, − 4.93; I2 = 75%; p ˂0.0001), 2-h postprandial glucose (WMD: − 15.52 mg/dl; 95% CI: − 20.61, − 10.42; I2 = 53%; p ˂0.00001), and glycated hemoglobin (HbA1c) values (WMD: − 0.19%, 95% CI: − 0.37, − 0.02; I2 = 94%; p = 0.03) after hydroxychloroquine treatment. Otherwise, meta-analysis showed no significant effect of hydroxychloroquine on insulin levels (WMD: 16.52 μUI/ml; 95% CI: − 16.35, 49.40; I2 = 90%; p = 0.32) and HOMA-β (WMD: − 14.62; 95% CI: − 45.84, 16.59; I2 = 0%; p = 0.36).
Conclusion
The present meta-analysis revealed that treatment with hydroxychloroquine improves glucose control through the reduction of fasting glucose, 2-h postprandial glucose, and HbA1c values. Given that the effect of hydroxychloroquine on beta-cell function is based only on two clinical trials, it is not possible to draw definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes, a chronic noncommunicable disease, is one of the main causes of morbidity and mortality in the USA [1]. Additionally, the global prevalence was estimated at 9.3%, 10.2%, and 10.9% by 2019, 2030, and 2045, respectively [2].
Type 2 diabetes is characterized by insulin resistance in the muscle, adipose, and liver tissues, and β-cell dysfunction. Nonetheless, the pathophysiology of type 2 diabetes is very complex, including the interaction between genetic and environmental factors [3]. Therefore, the optimal treatment to achieve the therapeutic goal is currently a challenge. Accordingly, this complex pathophysiology requires an elaborate scheme of drugs to achieve and maintain the therapeutic goals of its treatment. Although there is a wide variety of therapeutic options for the management of type 2 diabetes, the glycemic control is still difficult to maintain in these patients [4]. Thus, alternative antidiabetic drugs capable of improving glucose metabolism are of great interest.
Hydroxychloroquine is a drug that was initially introduced as antimalarial; however, due to its immunoregulatory and anti-inflammatory properties, it is also indicated for the treatment of systemic lupus erythematosus and rheumatoid arthritis [5]. Besides, it has been reported that hydroxychloroquine may have hypoglycemic effects [6, 7]; even it was approved for the management of type 2 diabetes as third-line drug in uncontrolled patients despite lifestyle management plus metformin and sulfonylurea combination therapy in India [8, 9]. However, the underlying mechanisms involved in the antidiabetogenic properties of hydroxychloroquine are still unclear and poorly investigated. Therefore, this meta-analysis of randomized controlled trials aimed to evaluate the effect of the hydroxychloroquine on glucose control.
Methods
Search strategy
According to the guidelines of the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [10], the present study was carried out. PubMed-Medline, Web of Science, SCOPUS, and Google Scholar databases were searched using the following search terms within titles and abstracts (also in combination with MESH terms): (hydroxychloroquine) AND (glucose OR postprandial glucose OR insulin OR insulin resistance OR insulin sensitivity OR “HOMA” OR HbA1c OR hemoglobin A1c OR glycated hemoglobin OR glycosylated hemoglobin). The wild-card term ‘‘*’’ was used to increase the sensitivity of the search strategy. There was no language restriction in the search process. The literature was searched from inception to August 7, 2020.
Study selection
Eligible studies were selected when they met the following inclusion criteria: (1) being a randomized controlled trial; (2) examining the impact of hydroxychloroquine on either fasting glucose, 2-h postprandial glucose, insulin, or glycated hemoglobin (HbA1c) values; (3) treatment period of at least one month; (4) providing sufficient information on fasting glucose, 2-h postprandial glucose, insulin or HbA1c values at baseline and at the end of follow-up in each group or presenting the net change values. Exclusion criteria were (1) non-interventional trials; (2) lack of a placebo or control group for hydroxychloroquine treatment; (3) observational studies with case–control, cross-sectional or cohort design; and (4) insufficient data on the baseline or follow-up fasting glucose, 2-h postprandial glucose, insulin or HbA1c values. Study selection was performed by two independent authors, and disagreements were resolved with a third author.
Results
Flow and characteristics of included studies
A total of 88 studies were identified in the multiple databases after search strategy. Then, 71 were excluded following the screening of titles and abstracts. Next, 17 full-text articles were carefully reviewed for eligibility and 6 studies were excluded for treatment duration < 1 month (n = 1), not presenting numerical values (n = 2), and incomplete data on glycemic parameters (n = 3). Thus, 11 clinical trials were selected for meta-analysis (Fig. 1).
Table 1 summarizes the characteristics of the included studies and baseline parameters. A total of 2208 subjects were included from 11 randomized controlled trials, comprising 1172 and 1036 participants in the treatment and control arms, respectively. Selected studies were published between 2014 and 2020. The range of intervention periods was from 8 to 24 weeks [6, 7, 11,12,13,14,15,16,17,18,19]. Almost all included clinical trials had a parallel design; only one study was cross-over [19]. Selected studies enrolled patients with type 2 diabetes [11,12,13,14,15,16, 18], primary dyslipidemia [17], prediabetes [7], rheumatoid arthritis [19], and non-diabetic individuals [6].
Risk of bias assessment
Four studies [11, 12, 14, 18] were characterized by lack of information regarding sequence generation. Additionally, several trials exhibited insufficient information for allocation concealment. Also, five studies [11, 12, 14, 15, 18] had a high risk of bias with respect to the blinding of participants, personnel, and outcome assessors. However, all included studies showed a low risk of bias according to incomplete outcome data and selective outcome reporting. The quality of bias assessment for each study is provided in Table 2.
Effect of hydroxychloroquine on glycemic parameters
A total of ten, seven, ten, two, and two clinical trials reported data for fasting glucose, 2-h postprandial glucose, Hb1Ac, insulin, and HOMA-β, respectively. Meta-analysis revealed a significant reduction of fasting glucose (WMD: − 8.05 mg/dl; 95% CI: − 11.17, − 4.93; I2 = 75%; p ˂0.0001; Fig. 2), 2-h postprandial glucose (WMD: − 15.52 mg/dl; 95% CI: − 20.61, − 10.42; I2 = 53%, p ˂0.00001; Fig. 3), and HbA1c values (WMD: − 0.19%; 95% CI: − 0.37, − 0.02; I2 = 94%; p = 0.03; Fig. 4) after hydroxychloroquine treatment. The leave-one-out sensitivity analysis was robust for fasting glucose and 2-h postprandial glucose (Table S1 and S2), while the estimated effect size was sensitive to four studies for HbA1c [6, 7, 18, 20] (Table S3). Otherwise, meta-analysis showed no significant effect of hydroxychloroquine on insulin levels (WMD: 16.52 μUI/ml; 95% CI: − 16.35, 49.40; I2 = 90%; p = 0.32; Fig. 5) and HOMA-β (WMD: − 14.62; 95% CI: − 45.84, 16.59; I2 = 0%; p = 0.36; Fig. 6).
Publication bias
Funnel plots were generated only for fasting glucose (Fig. S1A) and Hb1Ac (Fig. S1B). After visual inspection, asymmetry was not observed for fasting glucose; however, one study [14] was in the non-significance area (right) for Hb1Ac.
Discussion
This meta-analysis supports that hydroxychloroquine lowers fasting glucose, 2-h postprandial glucose, and HbA1c values, but it does not affect insulin levels and HOMA-β.
Previously, experimental studies in induced diabetic models suggested beneficial effects of hydroxychloroquine on glucose control [20]. Then, observational and cohort studies showed a significant decrease in fasting glucose, 2-h postprandial glucose, and HbA1c values in patients with type 2 diabetes [21, 22]. Additionally, in line with our results, a recent systematic review of preclinical and clinical studies described that treatment with hydroxychloroquine improves fasting plasma glucose, 2-h postprandial glucose, and HAb1c concentrations [23]. Although the authors of this review included 15 clinical studies, only 6 were randomized controlled trials and the meta-analysis was not performed.
We found a robust effect of hydroxychloroquine on both fasting glucose and 2-h postprandial glucose, although the effect size was sensitive for HbA1c. It is noteworthy that only the study by Hsia et al. [14] had conflicting results for HbA1c; this could be explained by the small sample size including 15 subjects in the hydroxychloroquine group versus 7 into the pioglitazone group. Nonetheless, the overall effect of this antimalarial significantly decreased HbA1c values.
Interestingly, hydroxychloroquine treatment did not affect insulin levels and HOMA-β. However, it is important to note that this finding is based on the analysis of only two arms for each parameter; therefore, it is not possible to draw definitive conclusions on the effect of hydroxychloroquine on beta-cell function. In this context, only one study reported the impact of hydroxychloroquine on insulin resistance through HOMA [19], and no significant changes were observed; hence, this parameter was not analyzed in our meta-analysis. Thus, further clinical trials investigating the impact of hydroxychloroquine on surrogate markers of insulin resistance and beta-cell function are needed to clarify the potential benefits of this drug as antidiabetic.
Hydroxychloroquine, a drug extensively used for the treatment of malaria and autoimmune diseases, has immunomodulatory and anti-inflammatory properties [24, 25]. However, the mechanisms involved in improving glycemic control are not well-established. In this regard, it has been suggested that hydroxychloroquine may decrease the dissociation of the insulin-receptor complex, increasing its half-life and enhancing insulin action [26]. Furthermore, an experimental study reported that hydroxychloroquine reduces the lysosomal degradation of insulin, leading to an increase in insulin concentration and decreased glucose levels [27]. Also, hydroxychloroquine inhibits the insulin degrading enzyme by increasing intracellular pH and consequently improves the biological action of insulin [28]. Moreover, it has been observed that chloroquine, a hydroxychloroquine analog, induces glucose uptake and glycogen synthase via Akt activation [29].
There were some limitations in our study. Some clinical trials included a small sample size. Besides, only two arms were analyzed for insulin levels and HOMA-β, which is insufficient to draw definitive conclusions on the effects of hydroxychloroquine on beta-cell function. In this regard, it is important to note that C-peptide is a more reliable marker of beta-cell function. Also, the included studies were heterogeneous in terms of target population including patients with type 2 diabetes, primary dyslipidemia, prediabetes, rheumatoid arthritis, and non-diabetic individuals. Additionally, the present meta-analysis was limited to this study design; however, this study design exhibits the highest level of evidence among clinical trials. Due to the heterogeneity of the included clinical trials, our findings should be treated with caution. Finally, the quality of some studies was unclear; therefore, this could have introduced a potential source of bias.
Conclusion
According to our findings, hydroxychloroquine treatment improves glucose control through reduction of fasting glucose, 2-h postprandial glucose, and HbA1c values. However, additional studies are still needed to determine the potential hypoglycemic properties of this drug, as well as clarify the molecular mechanisms involved.
References
Xu G, Liu B, Sun Y et al (2018) Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ 362:k1497. https://doi.org/10.1136/bmj.k1497
Saeedi P, Petersohn I, Salpea P, et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.2019.107843
Defronzo RA (2009) Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58(4):773–795. https://doi.org/10.2337/db09-9028
Stein SA, Lamos EM, Davis SN (2013) A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf 12(2):153–175. https://doi.org/10.1517/14740338.2013.752813
Shippey EA, Wagler VD, Collamer AN (2018) Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med 85(6):459–467. https://doi.org/10.3949/ccjm.85a.17034
Wasko MC, McClure CK, Kelsey SF, Huber K, Orchard T, Toledo FG (2015) Antidiabetogenic effects of hydroxychloroquine on insulin sensitivity and beta cell function: a randomised trial. Diabetologia 58(10):2336–2343. https://doi.org/10.1007/s00125-015-3689-2
Sheikhbahaie F, Amini M, Gharipour M, Aminoroaya A, Taheri N (2016) The effect of hydroxychloroquine on glucose control and insulin resistance in the prediabetes condition. Adv Biomed Res 5:145. https://doi.org/10.4103/2277-9175.187401
Bajaj S (2018) RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017. Int J Diabetes Dev Ctries 38(Suppl 1):1–115. https://doi.org/10.1007/s13410-018-0604-7
Das AK, Kalra S, Tiwaskar M, Bajaj S et al (2019) Expert Group Consensus Opinion: Role of Anti-inflammatory Agents in the Management of Type-2 Diabetes (T2D). J Assoc Physicians India 67(12):65–74
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Baidya A, Ahmed R (2018) Effect of early addition of hydroxychloroquine in type 2 diabetic patients inadequately controlled on metformin and sulfonylurea combination therapy. Int J Res Med Sci 6:2626–2632
Baidya A, Kumar M, Kumar Pathak S, Ahmed R (2018) Study of comparative effect of hydroxychloroquine and vildagliptin on glycaemic efficacy and HbA1c in type 2 diabetes patients who were inadequately controlled with metformin and glimepiride dual therapy. JMSCR 6:409–415
Chakravarti HN, Nag A (2020) Efficacy and safety of hydroxychloroquine as add-on therapy in uncontrolled type 2 diabetes patients who were using two oral antidiabetic drugs. J Endocrinol Invest 1–12 https://doi.org/10.1007/s40618-020-01330-5
Hsia SH, Duran P, Lee ML, Davidson MB (2020) Randomized controlled trial comparing hydroxychloroquine with pioglitazone as third-line agents in type 2 diabetic patients failing metformin plus a sulfonylurea: a pilot study. J Diabetes 12(1):91–94. https://doi.org/10.1111/1753-0407.12989
Kumar V, Singh MP, Singh AP et al (2018) Efficacy and safety of hydroxychloroquine when added to stable insulin therapy in combination with metformin and glimepiride in patients with type 2 diabetes compare to sitagliptin. Int J Basic Clin Pharmacol 7:1959–1964 https://doi.org/10.18203/2319-2003.ijbcp20183930
Pareek A, Chandurkar N, Thomas N et al (2014) Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr Med Res Opin 30(7):1257–1266. https://doi.org/10.1185/03007995.2014.909393
Pareek A, Chandurkar N, Thulaseedharan NK et al (2015) Efficacy and safety of fixed dose combination of atorvastatin and hydroxychloroquine: a randomized, double-blind comparison with atorvastatin alone among Indian patients with dyslipidemia. Curr Med Res Opin 31(11):2105–2117. https://doi.org/10.1185/03007995.2015.1087989
Ranjan P, Ahsan S, Bhushan R, KumarTushar BGA et al (2018) Comparison of efficacy and safety of hydroxychloroquine and teneligliptin in type 2 diabetes patients who are inadequately controlled with glimepiride, metformin and insulin therapy: a randomized controlled trial with parallel group design. Ann Clin Endocrinol Metabol 2:033–040. https://doi.org/10.29328/journal.acem.1001009
Solomon DH, Garg R, Lu B et al (2014) Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthritis Care Res (Hoboken) 66(8):1246–1251. https://doi.org/10.1002/acr.22285
Zannah S, Islam M, Rahman A et al (2014) Antidiabetic drugs in combination with hydroxychloroquine improve glycemic control in alloxan induced diabetic rats. Pharmacol Pharm 5:725–735. https://doi.org/10.4236/pp.2014.57082
Singh UP, Baidya A, Singla M et al (2018) Efficacy and safety of substituting teneligliptin with hydroxychloroquine in inade- quately controlled type II diabetes subjects with combination therapy of teneligliptin, metformin, and glimepiride with or without other antidiabetic therapy: the TENE-HYQ SHIFT Study. Clin Diabetol 7:209–214
Singh UP, Jain S, Singla M et al (2018) Comparison between the clinical efficacy and safety of hydroxychloroquine and sitagliptin added to inadequately controlled with glimepiride and metformin in Indian patients with type 2 diabetes mellitus: a real-world observational study. EC Endocrinol Metab Res 3:147–155
Wondafrash DZ, Desalegn TZ, Yimer EM, Tsige AG, Adamu BA, Zewdie KA (2020) Potential effect of hydroxychloroquine in diabetes mellitus: a systematic review on preclinical and clinical trial studies. J Diabetes Res 2020:5214751. https://doi.org/10.1155/2020/5214751
Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF (2015) Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 23(5):231–269. https://doi.org/10.1007/s10787-015-0239-y
Infante M, Ricordi C, Fabbri A (2020) Antihyperglycemic properties of hydroxychloroquine in patients with diabetes: risks and benefits at the time of COVID-19 pandemic. J Diabetes 12(9):659–667. https://doi.org/10.1111/1753-0407.13053
Paul H (2018) Managing uncontrolled type 2 diabetes: role of hydroxychloroquine in therapy as AD on antidiabetic agent: a case study. EC Endocrinol Metabol Res 3:84–88
Emami J, Gerstein HC, Pasutto FM, Jamali F (1999) Insulin-sparing effect of hydroxychloroquine in diabetic rats is concentration dependent. Can J Physiol Pharmacol 77(2):118–123
Chakravorty S, Purkait I, Pareek A, Talware A (2017) Hydroxychloroquine: looking into the future. Rom J Diabetes Nutr Metab Dis 24:369–375
Halaby MJ, Kastein BK, Yang DQ (2013) Chloroquine stimulates glucose uptake and glycogen synthase in muscle cells through activation of Akt. Biochem Biophys Res Commun 435(4):708–713. https://doi.org/10.1016/j.bbrc.2013.05.047
Author information
Authors and Affiliations
Contributions
All authors have participated in the design and approved the final version of the manuscript, taking responsibility for the contents of the article. LESM and MSM equally analyzed and interpreted data and wrote the manuscript. ASG collected analyzed data, and reviewed and edited the manuscript. ELT collected analyzed data, and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
228_2021_3144_MOESM4_ESM.pdf
Supplementary file4 (PDF 65 KB) Figure S1. Funnel plot detailing publication bias in the studies reporting the impact of hydroxychloroquine on fasting glucose analysis for HbA1c.
Rights and permissions
About this article
Cite this article
Simental-Mendía, L.E., Simental-Mendía, M., Sánchez-García, A. et al. Effect of hydroxychloroquine on glucose control in patients with and without diabetes: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Clin Pharmacol 77, 1705–1712 (2021). https://doi.org/10.1007/s00228-021-03144-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03144-7