Abstract
Aims
Investigations on the possible effect of the Nordic diet (ND) on the glycemic control and the risk of diabetes have led to inconsistent results. The present study tried to determine the effect of the ND on the markers of blood glucose control using a systematic review and meta-analysis of randomized controlled clinical trials (RCTs).
Methods
Predefined keywords were used to search PubMed, ISI Web of Science, Scopus and Google Scholar up to April 2019. The random effects model was used to compute the overall estimates.
Results
In total, six RCTs with 618 participants (6–26 weeks of follow-up period) were included in the present study. The meta-analysis revealed that the ND might not have a considerable effect on fasting blood glucose levels [weighted mean difference (WMD) = −0.05 mmol/l, 95% CI − 0.13, 0.01, P = 0.112]. In contrast, the analyses showed that the ND significantly reduces serum insulin concentrations (WMD = −1.12 mU/l, 95% CI − 1.84, − 0.39, P = 0.002) and the homeostasis model assessment for insulin resistance (HOMA-IR) (WMD = − 0.34, 95% CI − 0.53, − 0.14, P = 0.001) compared to control diets. The effect on serum insulin levels was sensitive to one of the included studies. This dietary pattern did not significantly affect 2-h post-prandial blood glucose and Matsuda index.
Conclusions
Adherence to the ND might improve serum insulin and HOMA-IR levels; however, this effect was not confirmed for other markers of blood glucose control. Future well-designed and long-term clinical trials are highly recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that about 415 million adults (8.8%) are affected by type 2 diabetes mellitus (T2DM) globally, and according to the prediction of international diabetes federation (IDF), it would increase up to 642 million in 2040 [1]. T2DM is associated with an increased risk of micro- and macro-vascular complications such as cardiovascular disease, retinopathy, nephropathy, and neuropathy [2]. Therefore, because of its fast growth in prevalence, complications, and burden on the economy, T2DM has become a major public health concern in different countries around the world [3].
Several genetic, environmental, and lifestyle-related factors (e.g., dietary intake, smoking, and physical activity) might directly or indirectly play a role in the pathogenesis of T2DM [4, 5]. Lifestyle modification particularly adherence to a healthy diet and physical activity is regarded as important not only for the prevention but also for the treatment of the disease [6,7,8]. It is revealed that adherence to healthy dietary patterns which contain high amounts of healthy food groups like fruits, vegetables, whole grains, nuts, and legumes, and less amounts of unhealthy foods like processed meats, saturated and trans fats and sugar-sweetened beverages like the dietary approaches to stop hypertension, and the Mediterranean diet might improve blood glucose control [9, 10].
The Nordic diet is another dietary pattern which is regarded as healthy. This diet is characterized by a decreased intake of meat and sugar-sweetened beverages and increased consumption of fruits (berries), vegetables, potatoes, whole grains, nuts, seafood (fish and shellfish), organic foods from the wild countryside (typically used for northern European regions). This diet also emphasizes the consumption of low-fat dairy products [11, 12]. It is proposed by a number of researchers that this dietary pattern might beneficially affect human health [13,14,15]. Recent systematic reviews and meta-analyses revealed that this diet significantly improves total and LDL cholesterol, as well as, systolic and diastolic blood pressure [16]; however, the diet might not affect inflammatory markers including C-reactive protein (CRP), tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) [17]. The Nordic diet contains high amounts of anti-oxidants, vitamins, polyphenols, unsaturated fatty acids, and fiber; therefore, a number of studies suggested that this diet might beneficially affect the markers of blood glucose control [18, 19]; however, other publications could not replicate the same results [12, 20]. Although the previous studies have led to inconsistent results, we are not aware of any systematic review trying to summarize the evidence in this regard. Therefore, the present systematic review and meta-analysis aimed to summarize the randomized controlled clinical trials (RCTs) which examined the effect of adherence to the Nordic dietary pattern on the markers of blood glucose control and if possible, to quantify the overall effects, using meta-analysis.
Materials and method
The study protocol was registered in PROSPERO database (http://www.crd.york.ac.uk/PROSPERO, registration no: CRD42017058954) [21]. We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to report the present study [22].
Search strategy
The online search was accomplished using PubMed, ISI Web of Science, Scopus, and Google Scholar. The international registry of clinical trials (clinicaltrials.gov) was also searched to find any additional study related to the subject. No restrictions on the language, publication date or other filters were applied. The following strategies were used to find the related evidence: (nordic AND diet*) OR (nordiet) OR (“baltic sea” AND diet*). Full details on our search strategy for all databases are shown in Supplementary Table 1. The search was updated up to April 2019.
Eligibility criteria
We included parallel or crossover RCTs which examined the effect of the Nordic diet on the markers of blood glucose control in adults (aged 18 years or more). We excluded the studies if: RCTs reported duplicated data, examined the effect of only one component of the Nordic diet (not the whole dietary pattern), did not report sufficient data on glycemic control indices. To find the eligible studies, all titles and abstracts were independently screened by two authors (MM, NRJ) and when the titles/abstracts were inconclusive, the full-texts were evaluated. Furthermore, studies identified from reference lists of the related pieces of literature were added. A third reviewer (ASA) rechecked the retrieved articles to ensure that they meet the inclusion criteria.
Data extraction
The following data were extracted from studies included in the present review: surname of the first author, publication year, the country in which the study was performed, study design, sample size, sex and age of the participants, follow-up duration, details of the dietary strategy administered to the intervention and the control groups, and the study results. The data were independently extracted by two investigators (MM, NRJ) and rechecked by (AZ) to diminish possible errors, and the lead author (ASA) made the final decision in case of disagreements.
Risk of bias assessment
The risk of bias in the individual RCTs was assessed using the Cochrane collaboration’s tool, by two reviewers (AZ, MM). Random sequence generation, allocation concealment, blinding of the participants and outcome data, incomplete outcome data, and selective reporting were considered to evaluate the quality of studies [23]. As blinding of the participants is almost impossible in the trials which assess the effect of dietary patterns, the other five domains were considered to assess the overall quality of the included RCTs. These domains were classified as low risk of bias, high risk of bias and unclear [23]. The overall quality of the investigations was stratified as good if they were low risk of bias in more than two domains, fair when they were low risk of bias in two domains and weak when they were low risk of bias in less than two domains.
Statistical analysis
The mean change [and standard deviation (SD)] in outcome variables including fasting blood glucose (FBG), circulating insulin, 2-h post-prandial blood glucose (2 h-PBG), homeostasis model assessment for insulin resistance (HOMA-IR), and Matsuda index for the Nordic diet and control groups were extracted from the included studies. We considered the correlation coefficient of 0.5 to compute the SD for mean change values. All analyses were replicated assuming 0.1 and 0.9 as correlation coefficients for calculating the change values, to check the sensitivity of meta-analyses to selected correlation coefficient (r = 0.5). The raw difference in mean and its standard error (SE) was calculated using the change values calculated for the Nordic diet and control groups and then used as effect sizes for meta-analysis.
The random effects model was used to compute the weighted mean differences (WMD) and their corresponding 95% confidence intervals (CI) [24]. The between-study heterogeneity was assessed using Cochran’s Q test and I-squared (I2) [25]. Subgroup analyses based on the type of control diet (typical diet/average Danish diet) and duration of the intervention (≤ 3 months/> 3 months) were done to evaluate the potential sources of heterogeneity [26]. Sensitivity analysis was done by omitting included studies one by one from the meta-analyses to assess if a study might affect the overall estimates. To check the publication bias, funnel plots were visually inspected [27]. We performed all analysis using STATA, version 11.2 (Stata Corp, College Station, TX) and considered P values < 0.05 as statistically significant.
Results
Study selection and characteristics
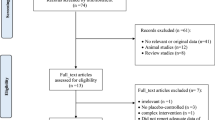
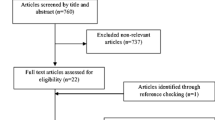
In total, 2859 articles were retrieved using electronic database search and 2288 publications remained after the duplicates were removed. These studies were screened by reading their title and abstract and 47 relevant full-texts were selected for further consideration. Finally 41 full-texts were excluded because of the following reasons: 10 studies were performed in children [28,29,30,31,32,33,34,35,36,37]; 18 studies did not consider the outcomes of interest [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]; 6 studies did not prescribe the Nordic diet and only provided advice to the participants for following the Nordic nutrition recommendations [56,57,58,59,60,61]; 4 studies evaluated the effect of a single food item rather than the whole dietary pattern [62,63,64,65]; 2 studies represented duplicate reports [19, 66]; and 1 study was before-after in design [67]. Therefore, six RCTs met the eligibility criteria and were included in the present systematic review [12, 18, 20, 68,69,70]. We had two papers that originated [68, 69] from two main studies [12, 70]. We included all of the studies in the systematic review because different outcomes were reported in these papers and the data were extracted from all of them. It should be noted that we considered these studies in the systematic review but we included one effect size from each paper in the meta-analysis. Figure 1 shows the flow diagram of the study selection process.
The publication date of eligible articles ranged from 2011 to 2017. Three included studies were carried out in Finland [68,69,70], one in Denmark [18], one in Sweden [20], and one in different regions in Europe [12]. The Nordic diet was provided for the intervention groups, and other diets (e.g., typical diets or average Danish diet) had been also recommended for control individuals. In all of the included studies, the Nordic diets provided 46–52% of the total calorie intake from carbohydrate, 27–32% from fat and 17–19% from protein, and there was no considerable difference in energy intake between prescribed diets (1968–2186 kcal/day). Altogether, the total intake of whole grains, fatty fishes, bilberries and salt are the main differences between the Nordic diets in comparison with the control diets (Table 1).
All studies used a parallel design [12, 18, 20, 68,69,70], and the participants aged 20–70 years. Duration of the studies ranged from 42 to 182 days and all of included studies were conducted in both genders. Moreover, the baseline health status of participants was as follows: patients with metabolic syndrome [68,69,70], individuals with mild hypercholesterolemia [20], and subjects with obesity [12, 18]. The characteristics of the included studies are demonstrated in Table 1.
Assessment of the risk of bias
All studies were low risk in at least three domains of the Cochrane collaboration’s tool for assessing the risk of bias and were classified as good quality [12, 18, 20, 68,69,70]. All studies except one [18] did not report the allocation concealment, all of the included studies were low risk in incomplete outcome data and selective reporting, and three of them explained the method of random sequence generation [18, 20, 68]. Blinding of outcome assessors was explained in the included studies [12, 69, 70]. None of the eligible studies described the blinding of participants or personnel. The detailed results for the risk of bias assessment are provided in Supplementary Table 2.
Meta-analysis
Fasting blood glucose (FBG)
Four studies with 492 participants assessed the effect of the Nordic dietary pattern on FBG levels [12, 18, 20, 68]. The overall results demonstrated that adherence to the Nordic diet had no significant effect on FBG levels (WMD = −0.05 mmol/l, 95% CI − 0.13, 0.01, P = 0.112; Fig. 2), and there was no evidence of between-study heterogeneity (Cochran’s Q test, Q statistic = 1.32, P = 0.726, I2 = 0%). The subgroup analysis revealed that the Nordic diet does not significantly reduce FBG levels in all subgroups. The overall effect of the Nordic diet on FPG levels and the effect based on several subgroup analyses are presented in Table 2.
Serum insulin levels
Four studies (including 359 participants) evaluated the effect of the Nordic diet on serum insulin concentrations and were included in the meta-analysis [18, 20, 68, 69]. The analysis showed that serum insulin levels were significantly reduced in participants who followed the Nordic diet when compared to the control diets (WMD = −1.12 mU/l, 95% CI − 1.84, − 0.39, P = 0.002; Fig. 3) and no heterogeneity was observed between included studies (Cochran’s Q test, Q statistic = 1.92, P = 0.589, I2 = 0%).
Subgroup analysis also demonstrated that the reducing effect was significant when the Nordic diet was compared with either typical diets (WMD = −1.04 mU/l, 95% CI − 1.93, − 0.15, P = 0.022) or the average Danish diet (WMD = −1.28 mU/l, 95% CI − 2.51, − 0.04, P = 0.043). Moreover, the reduction of insulin levels was observed in studies with more than 3 months of intervention (WMD = −1.07 mU/l, 95% CI − 2.10, − 0.05, P = 0.039), while the effect was not significant when the studies with lower than 3 months of follow-up duration were separately analyzed (WMD = −0.88 mU/l, 95% CI − 2.61, 0.84, P = 0.315) (Table 2).
The effect of Nordic diet on other markers of blood glucose control
Two eligible studies reported data on HOMA-IR levels [18, 20]. The meta-analysis revealed that adherence to the Nordic dietary pattern significantly reduces the HOMA-IR levels [WMD = −0.34, 95% CI − 0.53, − 0.14, P = 0.001]. However, the meta-analyses of the two studies which examined the effect of the Nordic diet on 2 h-PBG levels [12, 68] and the three studies which provided data for Matsuda index [12, 18, 70] did not show a significant effect (WMD = −0.03, 95% CI − 0.96, 0.88, P = 0.936, for 2 h-PBG levels and WMD = 0.006, 95% CI − 0.57, 0.58, P = 0.984, for Matsuda index).
Sensitivity analysis and publication bias
The results of sensitivity analysis showed that the meta-analysis of serum insulin levels was sensitive to one study carried out by Adamsson et al. [20] which its removal changed the overall effect to nonsignificant (WMD = −0.89, 95% CI − 1.86, 0.07) (Supplementary Figure 1A). The omission of any individual studies did not alter the other observed pooled effects.
Moreover, the overall effect of the Nordic diet on serum insulin levels changed to nonsignificant when the meta-analysis was conducted on effect estimates calculated based on correlation r of 0.9. Other meta-analyses were not sensitive to the correlation coefficients selected for meta-analysis.
No evidence of asymmetry was detected after inspecting Begg’s funnel plots for the meta-analysis of FBG (Fig. 4a) and serum insulin (Fig. 4b) levels.
Discussion
This was the first comprehensive review and meta-analysis which considered the effect of the Nordic dietary pattern on blood glucose control. The results demonstrated that adherence to the Nordic diet could significantly decrease serum insulin levels and HOMA-IR index. However, the diet might not affect the FBG, 2 h-PBG, and Matsuda index.
The present study also revealed that serum insulin levels significantly decreased in trials with 3 months of follow-up or more. The previous investigations also proposed that the effects of lifestyle interventions on health status can be achieved in long-term interventions; this is especially true in chronic diseases [71, 72].
The Nordic diet focuses on the intake of dietary fiber, anti-oxidants, polyphenols, and polyunsaturated fatty acids which are provided by the consumption of whole grains, fruits, vegetables, berries, fish and other seafoods. It also recommends decreased consumption of sugar-sweetened beverages and meat products simultaneously and choosing low-fat dairy products [11].
The high content of dietary fiber of the Nordic diet might improve insulin sensitivity and capacity of pancreatic β-cell secretion, and also might decrease glucose and cholesterol absorption to promote weight reduction, which results to a reduced risk of T2DM [73]. Indeed, weight reduction plays a major role in the improvement of glycemic control [74, 75], and it has been observed that the Nordic diet might improve weight loss. Fiber-rich foods have a large amount of water which results in gastric distention enhancement which is associated with satiety [76]. Moreover, consuming nuts which are high in dietary fiber, fat and protein are associated with satiety and also a reduced risk of T2DM especially in individuals with overweight and impaired glucose tolerance [77,78,79]. Notably, a high amount of proteins in the Nordic diet might change the appetite-regulating hormones which in turn results in better weight control [80, 81]. On the other hand, seafoods which contain polyunsaturated fatty acids (PUFAs) are associated with improved glucose metabolism and stimulating G-protein-coupled receptors (GPCR) such as GPR40 and GPR120 [82,83,84]. GPR40 is especially expressed in pancreatic β-cells which has a direct effect on insulin secretion. In addition, GPR120 is expressed in adipose tissue, gastrointestinal tract and pro-inflammatory macrophages [82]. When omega 3-PUFAs are bonded to these receptors, insulin secretion will be stimulated by enhancing glucagon-like peptide-1 (GLP-1) release [82, 83]. In addition, glucose transporter type 4 (GLUT-4) concentrations are reduced in T2DM individuals [85]. When PUFAs bind to GPR120, increased translocation of GLUT-4 might lead to improved uptake of glucose in adipocytes [83]. Expression of GLUT-4 in the muscle cells plasma membrane and translocation of intracellular vesicles, containing GLUT-4, can be promoted by insulin [85]. Therefore, glucose uptake via GLUT-4 in adipocytes and insulin secretion, directly and indirectly, can be increased following consumption of PUFAs [83].
Berries are also another component of the Nordic diet and contain high amounts of anti-oxidants and polyphenols which might have beneficial effects on insulin resistance by reducing oxidative stress [86]. Polyphenols, in particular flavonoids, have been inversely associated not only with the risk of diabetes [87] but also mortality [88], cardiovascular disease [89] and several cancers [90]. Indeed, flavonoids have various beneficial effects such as modulation of the enzymatic activity, inhibition of cellular proliferation, and anti-oxidant or anti-inflammatory features [91,92,93]. Also, increased magnesium intake by consuming vegetables, nuts and legumes might have favorable effects on insulin sensitivity [94]. Indeed, it has been observed that either dietary or plasma magnesium insufficiency, are contributed to the development of glucose intolerance and diabetes [95,96,97], and also magnesium intake has an inverse association with insulin resistance and incidence of T2DM [98].
Previous meta-analyses have also shown that the adherence to the DASH and the Mediterranean diets were associated with improvement in insulin sensitivity and glycemic control in a long-term intervention. Indeed, the Nordic diet has some similar features with other healthy dietary patterns like Mediterranean and DASH diets including recommendations for a higher intake of whole grains, vegetables, fruits, nuts and low intake of red meat and sweets [9, 99].
The previous population-based studies have led to inconsistent results regarding the association between the healthy Nordic diet and the development of T2DM [100, 101]. One study indicated an inverse relationship between the Nordic diet score and its components such as oatmeal and root vegetables and the development of T2DM [100]. However, the other study could not support the reducing effects of adherence to the Nordic diet on the risk of T2DM [101].
The current meta-analysis has some limitations. A limited number of studies were included in the meta-analyses. In addition, although we found that the Nordic diet has a significant reducing effect on serum insulin, this effect was sensitive to one of the included studies and the correlation coefficient selected to calculate the effect sizes. These two limitations show that more high-quality trials still are needed to confirm these results. All of the included studies were performed in north European countries which might limit the generalizability of the results to other populations. The included studies had diverse methodologies; however, there was no heterogeneity among studies.
In conclusion, the present systematic review and meta-analysis revealed that following the Nordic diet might lead to an improvement in serum insulin and HOMA-IR levels. Future high-quality long-term intervention studies should be carried out to confirm our results. Also, interventions in other countries can be done to recognize the possible effects of the Nordic diet on other populations.
References
International Diabetes Federation (2016) IDF seventh edition. http://www.diabetesatlas.org/. Accessed May 2019
WHO (2016) Fact sheet no. 312: diabetes. WHO, Geneva
Zandbergen AA, Sijbrands EJ, Lamberts SW, Bootsma AH (2006) Normotensive women with type 2 diabetes and microalbuminuria are at high risk for macrovascular disease. Diabetes Care 29(8):1851–1855. https://doi.org/10.2337/dc06-0287
Mahajan A, Go MJ, Zhang W et al (2014) Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet 46(3):234–244. https://doi.org/10.1038/ng.2897
Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344(18):1343–1350. https://doi.org/10.1056/nejm200105033441801
American Diabetes Association (2017) 4. Lifestyle management. Diabetes Care 40(Suppl 1):S33–S43. https://doi.org/10.2337/dc17-s007
American Diabetes Association (2017) 9. Cardiovascular disease and risk management. Diabetes Care 40(Suppl 1):S75–S87. https://doi.org/10.2337/dc17-s012
Brug J, Oenema A (2006) Healthful nutrition promotion in Europe: goals, target populations, and strategies. Patient Educ Couns 63(1–2):255–257
Shirani F, Salehi-Abargouei A, Azadbakht L (2013) Effects of dietary approaches to stop hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition 29(7–8):939–947. https://doi.org/10.1016/j.nut.2012.12.021
Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB (2011) The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol 57(11):1299–1313. https://doi.org/10.1016/j.jacc.2010.09.073
Mithril C, Dragsted LO, Meyer C, Blauert E, Holt MK, Astrup A (2012) Guidelines for the New Nordic Diet. Public Health Nutr 15(10):1941–1947. https://doi.org/10.1017/s136898001100351x
Uusitupa M, Hermansen K, Savolainen MJ et al (2013) Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome—a randomized study (SYSDIET). J Intern Med 274(1):52–66. https://doi.org/10.1111/joim.12044
Sofi F, Cesari F, Abbate R, Gensini GF, Casini A (2008) Adherence to Mediterranean diet and health status: meta-analysis. BMJ (Clin Res Ed) 337:a1344. https://doi.org/10.1136/bmj.a1344
Sacks FM, Svetkey LP, Vollmer WM et al (2001) Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344(1):3–10. https://doi.org/10.1056/nejm200101043440101
Whelton PK, He J, Appel LJ et al (2002) Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 288(15):1882–1888
Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A (2018) The effect of healthy Nordic diet on cardio-metabolic markers: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Nutr. https://doi.org/10.1007/s00394-018-1804-0
Sakhaei R, Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A (2019) The healthy Nordic dietary pattern has no effect on inflammatory markers: a systematic review and meta-analysis of randomized controlled clinical trials. Nutrition 58:140–148. https://doi.org/10.1016/j.nut.2018.06.020
Poulsen SK, Due A, Jordy AB et al (2014) Health effect of the New Nordic Diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am J Clin Nutr 99(1):35–45. https://doi.org/10.3945/ajcn.113.069393
Fritzen AM, Lundsgaard AM, Jordy AB et al (2015) New Nordic Diet—induced weight loss is accompanied by changes in metabolism and AMPK signaling in adipose tissue. J Clin Endocrinol Metab 100(9):3509–3519. https://doi.org/10.1210/jc.2015-2079
Adamsson V, Reumark A, Fredriksson IB et al (2011) Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: a randomized controlled trial (NORDIET). J Intern Med 269(2):150–159. https://doi.org/10.1111/j.1365-2796.2010.02290.x
Salehi-abargouei A, Zimorovat A, Mohammadi M, Ramezani-Jolfaie N (2017) Effects of Nordic diet on glycemic control in adults: a systematic review and meta-analysis of controlled clinical trials. PROSPERO. CRD42017058954. https://www.crd.york.ac.uk/PROSPERO/myprospero.php. Cited 3 Apr 2017
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
Higgins JP, Green S (2011) Cochrane handbook for systematic reviews of interventions, vol 4. Wiley, New York
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
Egger M, Davey-Smith G, Altman D (2008) Systematic reviews in health care: meta-analysis in context. Wiley, New York
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed) 315(7109):629–634
Andersen R, Biltoft-Jensen A, Andersen EW et al (2015) Effects of school meals based on the New Nordic Diet on intake of signature foods: a randomised controlled trial. The OPUS School Meal Study. Br J Nutr 114(5):772–779. https://doi.org/10.1017/S0007114515002299
Biltoft-Jensen A, Damsgaard CT, Andersen R et al (2015) Accuracy of self-reported intake of signature foods in a school meal intervention study: comparison between control and intervention period. Br J Nutr 114(4):635–644. https://doi.org/10.1017/S0007114515002020
Damsgaard CT, Dalskov SM, Laursen RP et al (2014) Provision of healthy school meals does not affect the metabolic syndrome score in 8–11-year-old children, but reduces cardiometabolic risk markers despite increasing waist circumference. Br J Nutr 112(11):1826–1836. https://doi.org/10.1017/s0007114514003043
Damsgaard CT, Dalskov SM, Petersen RA et al (2012) Design of the OPUS School Meal Study: a randomised controlled trial assessing the impact of serving school meals based on the New Nordic Diet. Scand J Public Health 40(8):693–703. https://doi.org/10.1177/1403494812463173
Damsgaard CT, Ritz C, Dalskov SM et al (2016) Associations between school meal-induced dietary changes and metabolic syndrome markers in 8–11-year-old Danish children. Eur J Nutr 55(5):1973–1984. https://doi.org/10.1007/s00394-015-1013-z
Petersen RA, Damsgaard CT, Dalskov SM et al (2015) Effects of school meals with weekly fish servings on vitamin D status in Danish children: secondary outcomes from the OPUS (Optimal well-being, development and health for Danish children through a healthy New Nordic Diet) School Meal Study. J Nutr Sci. https://doi.org/10.1017/jns.2015.15
Sorensen LB, Damsgaard CT, Dalskov SM et al (2015) Diet-induced changes in iron and n-3 fatty acid status and associations with cognitive performance in 8–11-year-old Danish children: secondary analyses of the Optimal Well-Being, Development and Health for Danish Children through a Healthy New Nordic Diet School Meal Study. Br J Nutr 114(10):1623–1637. https://doi.org/10.1017/S0007114515003323
Sorensen LB, Dyssegaard CB, Damsgaard CT et al (2015) The effects of Nordic school meals on concentration and school performance in 8- to 11-year-old children in the OPUS School Meal Study: a cluster-randomised, controlled, cross-over trial. Br J Nutr 113(8):1280–1291. https://doi.org/10.1017/S0007114515000033
Thorsen AV, Lassen AD, Andersen EW et al (2015) Plate waste and intake of school lunch based on the new Nordic diet and on packed lunches: a randomised controlled trial in 8- to 11-year-old Danish children. J Nutr Sci. https://doi.org/10.1017/jns.2015.3
Andersen R, Biltoft-Jensen A, Christensen T et al (2014) Dietary effects of introducing school meals based on the New Nordic Diet—a randomised controlled trial in Danish children. The OPUS School Meal Study. Br J Nutr 111(11):1967–1976. https://doi.org/10.1017/S0007114514000634
Adamsson V, Cederholm T, Vessby B, Riserus U (2014) Influence of a healthy Nordic diet on serum fatty acid composition and associations with blood lipoproteins—results from the NORDIET study. Food Nutr Res. https://doi.org/10.3402/fnr.v58.24114
Andersen MBS, Rinnan A, Manach C et al (2014) Untargeted metabolomics as a screening tool for estimating compliance to a dietary pattern. J Proteome Res 13(3):1405–1418. https://doi.org/10.1021/pr400964s
Brader L, Rejnmark L, Carlberg C et al (2014) Effects of a healthy Nordic diet on plasma 25-hydroxyvitamin D concentration in subjects with metabolic syndrome: a randomized, placebo-controlled trial (SYSDIET). Eur J Nutr 53(4):1123–1134. https://doi.org/10.1007/s00394-014-0674-3
Brader L, Uusitupa M, Dragsted LO, Hermansen K (2014) Effects of an isocaloric healthy Nordic diet on ambulatory blood pressure in metabolic syndrome: a randomized SYSDIET sub-study. Eur J Clin Nutr 68(1):57–63. https://doi.org/10.1038/ejcn.2013.192
Cuparencu CS, Andersen MBS, Gürdeniz G et al (2016) Identification of urinary biomarkers after consumption of sea buckthorn and strawberry, by untargeted LC–MS metabolomics: a meal study in adult men. Metabolomics 12(2):1–20. https://doi.org/10.1007/s11306-015-0934-0
Hanhineva K, Lankinen MA, Pedret A et al (2015) Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J Nutr 145(1):7–17. https://doi.org/10.3945/jn.114.196840
Huseinovic E, Bertz F, Agelii ML, Johansson EH, Winkvist A, Brekke HK (2016) Effectiveness of a weight loss intervention in postpartum women: results from a randomized controlled trial in primary health care. Am J Clin Nutr 104(2):362–370. https://doi.org/10.3945/ajcn.116.135673
Jobs E, Adamsson V, Larsson A et al (2014) Influence of a prudent diet on circulating cathepsin S in humans. Nutr J. https://doi.org/10.1186/1475-2891-13-84
Khakimov B, Poulsen SK, Savorani F et al (2016) New Nordic diet versus average Danish diet: a randomized controlled trial revealed healthy long-term effects of the new Nordic diet by GC–MS blood plasma metabolomics. J Proteome Res 15(6):1939–1954. https://doi.org/10.1021/acs.jproteome.6b00109
Lankinen M, Schwab U, Kolehmainen M et al (2016) A healthy Nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J Nutr 146(4):662–672. https://doi.org/10.3945/jn.115.220459
Leder L, Kolehmainen M, Narverud I et al (2016) Effects of a healthy Nordic diet on gene expression changes in peripheral blood mononuclear cells in response to an oral glucose tolerance test in subjects with metabolic syndrome: a SYSDIET sub-study. Genes Nutr. https://doi.org/10.1186/s12263-016-0521-4
Magnusdottir OK, Landberg R, Gunnarsdottir I et al (2013) Plasma alkylresorcinols reflect important whole-grain components of a healthy Nordic diet. J Nutr 143(9):1383–1390. https://doi.org/10.3945/jn.113.175588
Marckmann P, Sandstrom B, Jespersen J (1995) Food intake of Danes and cardiac risk factors. Ugeskr Laeger 157(12):1667–1671
Marckmann P, Sandström B, Jespersen J (1994) Low-fat, high-fiber diet favorably affects several independent risk markers of ischemic heart disease: observations on blood lipids, coagulation, and fibrinolysis from a trial of middle-aged Danes. Am J Clin Nutr 59(4):935–939
Poulsen S, Frost S, Rasmussen L, Astrup A, Larsen T (2011) Weight loss after 12 weeks with new Nordic diet vs. average Danish diet provided ad libitum—a randomized controlled trial using the shop model. Ann Nutr Metab 58:289
Roager HM, Licht TR, Poulsen SK, Larsen TM, Bahl MI (2014) Microbial enterotypes, inferred by the prevotella-to-bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the New Nordic Diet. Appl Environ Microbiol 80(3):1142–1149. https://doi.org/10.1128/aem.03549-13
Salomo L, Poulsen SK, Rix M, Kamper AL, Larsen TM, Astrup A (2016) The New Nordic Diet: phosphorus content and absorption. Eur J Nutr 55(3):991–996. https://doi.org/10.1007/s00394-015-0913-2
Sandstrom B, Marckmann P, Bindslev N (1992) An eight-month controlled study of a low-fat high-fibre diet: effects on blood lipids and blood pressure in healthy young subjects. Eur J Clin Nutr 46(2):95–109
Andersson J, Mellberg C, Otten J et al (2016) Left ventricular remodelling changes without concomitant loss of myocardial fat after long-term dietary intervention. Int J Cardiol 216:92–96. https://doi.org/10.1016/j.ijcard.2016.04.050
Blomquist C, Alvehus M, Buren J et al (2017) Attenuated low-grade inflammation following long-term dietary intervention in postmenopausal women with obesity. Obesity 25(5):892–900. https://doi.org/10.1002/oby.21815
Boraxbekk CJ, Stomby A, Ryberg M et al (2015) Diet-induced weight loss alters functional brain responses during an episodic memory task. Obes Facts 8:261–272. https://doi.org/10.1159/000437157
Chorell E, Ryberg M, Larsson C et al (2016) Plasma metabolomic response to postmenopausal weight loss induced by different diets. Metabolomics. https://doi.org/10.1007/s11306-016-1013-x
Mellberg C, Sandberg S, Ryberg M et al (2014) Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr 68(3):350–357. https://doi.org/10.1038/ejcn.2013.290
Otten J, Mellberg C, Ryberg M et al (2016) Strong and persistent effect on liver fat with a Paleolithic diet during a two-year intervention. Int J Obes 40(5):747–753. https://doi.org/10.1038/ijo.2016.4
Adamsson V, Reumark A, Marklund M, Larsson A, Riserus U (2015) Role of a prudent breakfast in improving cardiometabolic risk factors in subjects with hypercholesterolemia: a randomized controlled trial. Clin Nutr 34(1):20–26. https://doi.org/10.1016/j.clnu.2014.04.009
Magnusdottir OK, Landberg R, Gunnarsdottir I et al (2014) Plasma alkylresorcinols C17:0/C21:0 ratio, a biomarker of relative whole-grain rye intake, is associated to insulin sensitivity: a randomized study. Eur J Clin Nutr 68(4):453–458. https://doi.org/10.1038/ejcn.2014.12
Magnusdottir OK, Landberg R, Gunnarsdottir I et al (2014) Whole grain rye intake, reflected by a biomarker, is associated with favorable blood lipid outcomes in subjects with the metabolic syndrome—a randomized study. PLoS ONE 9(10):e110827. https://doi.org/10.1371/journal.pone.0110827
Ulven SM, Leder L, Elind E et al (2016) Exchanging a few commercial, regularly consumed food items with improved fat quality reduces total cholesterol and LDL-cholesterol: a double-blind, randomised controlled trial. Br J Nutr. https://doi.org/10.1017/S0007114516003445
Marklund M, Magnusdottir OK, Rosqvist F et al (2014) A dietary biomarker approach captures compliance and cardiometabolic effects of a healthy Nordic diet in individuals with metabolic syndrome. J Nutr 144(10):1642–1649. https://doi.org/10.3945/jn.114.193771
Darwiche G, Höglund P, Roth B et al (2016) An Okinawan-based Nordic diet improves anthropometry, metabolic control, and health-related quality of life in Scandinavian patients with type 2 diabetes: a pilot trial. Food Nutr Res. https://doi.org/10.3402/fnr.v60.32594
Lankinen M, Kolehmainen M, Jaaskelainen T et al (2014) Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: a randomized trial (Sysdimet). PLoS ONE 9(2):e90352. https://doi.org/10.1371/journal.pone.0090352
Kolehmainen M, Ulven SM, Paananen J et al (2015) Healthy Nordic diet downregulates the expression of genes involved in inflammation in subcutaneous adipose tissue in individuals with features of the metabolic syndrome. Am J Clin Nutr 101(1):228–239. https://doi.org/10.3945/ajcn.114.092783
de Mello VD, Schwab U, Kolehmainen M et al (2011) A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia 54(11):2755–2767. https://doi.org/10.1007/s00125-011-2285-3
Li G, Zhang P, Wang J et al (2008) The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371(9626):1783–1789. https://doi.org/10.1016/s0140-6736(08)60766-7
Knowler WC, Fowler SE, Hamman RF et al (2009) 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374(9702):1677–1686. https://doi.org/10.1016/s0140-6736(09)61457-4
Cho SS, Qi L, Fahey GC Jr, Klurfeld DM (2013) Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr 98(2):594–619. https://doi.org/10.3945/ajcn.113.067629
Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ (2015) Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet 115(9):1447–1463. https://doi.org/10.1016/j.jand.2015.02.031
Rock CL, Flatt SW, Pakiz B et al (2014) Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care 37(6):1573–1580. https://doi.org/10.2337/dc13-2900
Burton-Freeman B (2000) Dietary fiber and energy regulation. J Nutr 130(2S Suppl):272s–275s. https://doi.org/10.1093/jn/130.2.272S
Overby NC, Sonestedt E, Laaksonen DE, Birgisdottir BE (2013) Dietary fiber and the glycemic index: a background paper for the Nordic Nutrition Recommendations 2012. Food Nutr Res. https://doi.org/10.3402/fnr.v57i0.20709
Blaak EE, Antoine JM, Benton D et al (2012) Impact of postprandial glycaemia on health and prevention of disease. Obes Rev 13(10):923–984. https://doi.org/10.1111/j.1467-789X.2012.01011.x
Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ (2000) Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 342(19):1392–1398. https://doi.org/10.1056/nejm200005113421903
Santesso N, Akl EA, Bianchi M et al (2012) Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr 66(7):780–788. https://doi.org/10.1038/ejcn.2012.37
Weigle DS, Breen PA, Matthys CC et al (2005) A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 82(1):41–48. https://doi.org/10.1093/ajcn.82.1.41
Hirasawa A, Tsumaya K, Awaji T et al (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11(1):90–94. https://doi.org/10.1038/nm1168
Oh DY, Talukdar S, Bae EJ et al (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142(5):687–698. https://doi.org/10.1016/j.cell.2010.07.041
Briscoe CP, Tadayyon M, Andrews JL et al (2003) The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278(13):11303–11311. https://doi.org/10.1074/jbc.M211495200
Gannon NP, Conn CA, Vaughan RA (2015) Dietary stimulators of GLUT4 expression and translocation in skeletal muscle: a mini-review. Mol Nutr Food Res 59(1):48–64. https://doi.org/10.1002/mnfr.201400414
Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PI Jr (2016) Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 473(24):4527–4550. https://doi.org/10.1042/bcj20160503c
Xu H, Luo J, Huang J, Wen Q (2018) Flavonoids intake and risk of type 2 diabetes mellitus: a meta-analysis of prospective cohort studies. Medicine 97(19):e0686. https://doi.org/10.1097/md.0000000000010686
Grosso G, Micek A, Godos J et al (2017) Dietary flavonoid and lignan intake and mortality in prospective cohort studies: systematic review and dose-response meta-analysis. Am J Epidemiol 185(12):1304–1316. https://doi.org/10.1093/aje/kww207
Wang X, Ouyang YY, Liu J, Zhao G (2014) Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr 111(1):1–11. https://doi.org/10.1017/s000711451300278x
Grosso G, Godos J, Lamuela-Raventos R et al (2017) A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: level of evidence and limitations. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201600930
Williamson G, Manach C (2005) Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 81(1 Suppl):243s–255s. https://doi.org/10.1093/ajcn/81.1.243S
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 22:19–34. https://doi.org/10.1146/annurev.nutr.22.111401.144957
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56(11):317–333
Kim DJ, Xun P, Liu K et al (2010) Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care 33(12):2604–2610. https://doi.org/10.2337/dc10-0994
Song Y, Manson JE, Buring JE, Liu S (2004) Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 27(1):59–65
Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL (1999) Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med 159(18):2151–2159
Humphries S, Kushner H, Falkner B (1999) Low dietary magnesium is associated with insulin resistance in a sample of young, nondiabetic Black Americans. Am J Hypertens 12(8 Pt 1):747–756
Dong JY, Xun P, He K, Qin LQ (2011) Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diab Care 34(9):2116–2122. https://doi.org/10.2337/dc11-0518
Huo R, Du T, Xu Y et al (2015) Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr 69(11):1200–1208. https://doi.org/10.1038/ejcn.2014.243
Lacoppidan SA, Kyro C, Loft S et al (2015) Adherence to a healthy Nordic food index is associated with a lower risk of type-2 diabetes—the Danish diet, Cancer and Health Cohort Study. Nutrients 7(10):8633–8644. https://doi.org/10.3390/nu7105418
Kanerva N, Rissanen H, Knekt P, Havulinna AS, Eriksson JG, Mannisto S (2014) The healthy Nordic diet and incidence of type 2 diabetes—10-year follow-up. Diabetes Res Clin Pract 106(2):e34–e37. https://doi.org/10.1016/j.diabres.2014.08.016
Funding
The study was funded by Nutrition and Food Security research center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Grant No. 5961).
Author information
Authors and Affiliations
Contributions
The authors’ contribution was as follows: ASA, MM, and NRJ conceived and designed the research; MM and NRJ conducted the systematic research and study selection; MM, NRJ, and AZ extracted data; ASA and MM analyzed data; AZ, MM and ASA wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to report for the present study.
Ethical standard
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Not applicable.
Additional information
Managed by Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zimorovat, A., Mohammadi, M., Ramezani-Jolfaie, N. et al. The healthy Nordic diet for blood glucose control: a systematic review and meta-analysis of randomized controlled clinical trials. Acta Diabetol 57, 1–12 (2020). https://doi.org/10.1007/s00592-019-01369-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01369-8