Abstract
Purpose
Laboratory reference intervals (RIs) play a key role in clinical pharmacology trials, both in the screening process and in evaluating drug safety. However, RIs tend to be confined to the general population, and data about RIs for the trial population are limited. The purpose of this study was to determine appropriate RIs for use when screening a defined special subgroup of a healthy Chinese population in clinical pharmacology trials.
Methods
A total of 773 healthy Chinese volunteers (552 men and 221 women) who sought to participate in clinical pharmacology trials were included in this study. Sixteen different biochemical analytes were measured by a Beckman Coulter Unicel DxC 800 automatic analyzer. RIs were partitioned by gender using Harris and Boyd’s method and calculated using a non-parametric method.
Results
The RIs of 16 biochemical analytes for healthy Chinese volunteers during the screening process in clinical pharmacology trials are reported in this study. Noticeable differences between the RIs in this study and RIs provided by our laboratory or existing literature were also observed. Compared to our institutional RIs, the newly established RIs were more applicable to the current trial population.
Conclusions
The RIs in this study can serve as a powerful clinical tool during the screening process in clinical pharmacology trials. However, these RIs should be re-verified if any condition changes. The results also emphasize the importance re-establishing RIs which are more applicable to local trial populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reference intervals (RIs) are frequently used as a decision-making tool for clinicians to distinguish between healthy and diseased populations. In drug clinical trials, the accurate interpretation of laboratory data plays a critical role, for both trial inclusion decisions and post-intervention safety evaluation [1]. On the one hand, RIs are used during the screening process to exclude individuals with asymptomatic diseases. On the other hand, evaluating drug safety and maximum tolerated dose determination is conducted by a case-by-case analysis, sometimes using laboratory adverse events defined by RIs [2]. Therefore, inappropriate RIs for laboratory data not only may increase the workload and costs associated with the screening process, but could also lead to a misjudgment in clinical pharmacology trials. Given this fact, establishing accurate laboratory RIs should be an important consideration in clinical pharmacology trials.
Generally speaking, RIs are reported as population-based values comprising 95% of the healthy population in direct studies for RI estimation [3, 4]. RIs are typically derived from historical research values, instrument manufacturers, or suitable statistical analysis of patient data, all of which may result in sampling bias [5, 6]. They are usually affected by individual and laboratory factors, such as differences in age, gender, ethnicity, region, environment, genetic factors, laboratory methods, and instruments [7,8,9,10]. Therefore, each laboratory should periodically reevaluate its own RIs [11, 12]. However, RI formulation is a major challenge for laboratories. In particular, the recruitment of a reference population of healthy volunteers often poses considerable challenges [5, 13]. As with most clinical laboratories in China, our laboratory uses the RIs provided by manufacturers, which are usually derived from the data on European and American populations [9], and thus may be unsuitable for our local population. Furthermore, the demographic characteristics of healthy volunteers in clinical pharmacology trials, who tend to be young men, low-income, and unemployed, are thought to be a special subgroup of the general population [14, 15]. In addition, age dependence seems evident for several biochemical analytes including albumin, cholesterol, and urea, and special attention should be given to age stratification for RIs [8]. Accordingly, new laboratory RIs should be established which are more applicable to the trial population. So far, however, there has been little discussion about laboratory RIs for healthy volunteers in clinical trials [1, 15]. Therefore, there is an urgent need to determine the laboratory RIs which are more applicable to the local trial population. To the best of our knowledge, there are no reports on laboratory RIs for healthy Chinese volunteers in clinical pharmacology trials. Thus, this study aimed to determine appropriate RIs of biochemical analytes for use when screening a defined special subgroup of a healthy Chinese population in clinical pharmacology trials.
Materials and methods
Study population
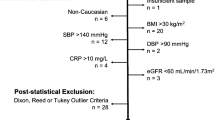
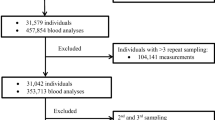
From September 20, 2018, to June 25, 2019, asymptomatic adult subjects who sought to participate in clinical pharmacology trials were recruited for this study. They were all from the BE and Phase I Clinical Trial Center at the First Affiliated Hospital of Xiamen University. The reference population started with 1042 asymptomatic adult individuals from both cities and villages in southern China. Age ranged from 18 to 55 years. The health status of each participant was strictly evaluated by physicians during the screening assessments, and the recommended information on participants was collected [16]. The health status of participants was checked by a physical examination, vital signs, and clinical laboratory tests including hematology, biochemistry, coagulation, and urinalysis. HIV, treponema pallidum (TP), hepatitis B or C serologies, and human chorionic gonadotropin (for women) were also included. Alcohol testing, drug abuse assessed by a urine test, and electrocardiogram (ECG) were applied as well. The exclusion criteria for selecting subjects were as follows:
-
1.
Abnormality found on physical examination or presence of acute or chronic disease requiring medical intervention
-
2.
Abnormal vital signs, including systolic blood pressure ≥ 140 mmHg or < 90 mmHg, diastolic blood pressure ≥ 90 mmHg; heart rate > 100 beats per minute or < 50 beats per minute; body temperature > 37.2 °C
-
3.
Obese (body mass index ≥28 kg/m2) or low weight [<50 kg (for men) or < 45 kg (for women)]
-
4.
History of severe allergy to drugs or allergic constitution
-
5.
Having taken any drugs within 2 weeks, and blood transfusion or blood donation within 3 months before screening
-
6.
Heavy smokers (more than five cigarettes per day) or excessive drinking (more than 36 g per day or positive alcohol testing)
-
7.
Women who were pregnant or postpartum for less than 1 year before
-
8.
Drug abuse assessed by a positive urine test
-
9.
Withdrawal of consent before specimen collection
-
10.
Positive HIV, treponema pallidum (TP), or hepatitis B or C serology
-
11.
Abnormal laboratory tests [17] including fasting blood glucose >7.0 mmol/L, AST or ALT >2 × upper limit of institutional RIs, hemoglobin <110 g/L (for women) or < 120 g/L (for men), cholesterol >8.04 mmol/L, and other irregular laboratory tests allowing diagnosis of current disease by physicians
-
12.
Significant ECG abnormality
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University. Written informed consent was obtained from each participant.
Specimen collection and handling
For blood sampling, volunteers were required to fast overnight for at least 10 h before the day of sampling, to maintain normal life habits, and to refrain from strenuous exercise 3 days before specimen collection. The venous blood samples for hematology were collected in 5 mL K2-EDTA tubes (BD Company, USA). Serum samples for clinical chemistry were collected in 5 mL gel separator tubes (Golden Company, China), and were left at room temperature for 30 min to clot, then centrifuged for 10 min at 1200×g (USTC Chuangxin Co., Ltd. ZonKia Branch, China). The plasma specimens for coagulation analysis were collected in the tubes containing a 1:9 volume of 109 mmol/L trisodium citrate (BD Company, USA), and were centrifuged at 3000 rpm for 10 min (USTC Chuangxin Co., Ltd.) at room temperature. All samples were analyzed by the same laboratory auto-analyzers within 4 h (within 2 h for coagulation). Clean midstream urine was collected as well.
Instrumentation and assays
A calibrated Beckman Coulter Unicel DxC 800 automatic analyzer (CA, USA) was applied to measure the biochemical analytes in serum. Samples were calibrated (Beckman Coulter, Inc., USA) prior to analysis. All reagents, including calibrators and controls, were provided by Beckman Coulter, Inc. (CA, USA). The following 16 analytes were detected: albumin (ALB), globulin (GLO), total protein (TP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (NBIL), urea (UR), creatinine (Cr), uric acid (UA), potassium (K), sodium (Na), chlorine (Cl), calcium (Ca). HIV, Treponema pallidum (TP), and hepatitis C serologies were detected by the chemiluminescence method, and hepatitis B serology was tested by the manual ELISA method. An Aution Max AX-4030 urine analyzer (Kyoto, Japan) was applied to measure the urine samples. Hematology and coagulation tests were measured by Sysmex XN-10 and Sysmex Cs-2000i automated analyzers (Kobe, Japan). All assays were carried out according to the manufacturers' instructions. To minimize errors, the pre-analysis, analysis, and post-analysis processes were performed according to standard operating procedures recommended by the National Committee for Clinical Laboratory Standards (NCCLS). For quality control, two samples of known analyte concentrations, PreciNorm U (normal) and PreciPath U (abnormal), were tested with each batch of analyses. Analytical performance for precision and accuracy was demonstrated according to the EP 15A standard recommended by the NCCLS. PreciNorm U and PreciPath U were used to estimate precision. The within-run coefficient of variation (CV) and total CV were calculated. The estimated total coefficient of variation and the percent bias were all less than one-half of CLIA ’88.
Statistical analyses
All statistical data were analyzed with SPSS version 23.0 software (IBM, USA). The determination of RIs in this study was performed according to the CLSI C28-A3 guideline [16]. Dixon-Reed’s outlier method was used to exclude outliers. After excluding outliers, a non-parametric method was used to calculate the RIs, and the lower and upper reference limits were assumed to indicate the estimated 2.5th and 97.5th percentiles. The 90% confidence intervals (CI) for the lower limit and upper limit were predicted by the bootstrap method conducted with SPSS. The subjects were divided into two subgroups according to gender. Age-partitioning was also applied in men. Whether separate intervals would be desired for a defined subclass of subjects was tested by Harris and Boyd’s method (Z-test). Stem-and-leaf plots and normal Q-Q plots were used for the data distribution. If the original data were highly skewed, then a simple transformation, such as the log transform, was used to produce a distribution of values much closer to the Gaussian form.
The out-of-range (OOR) values were calculated by RIs provided by our laboratory (institutional RIs) to examine the degree of difference between RIs for identifying healthy individuals enrolled in this study. The institutional RIs were from the reagent inserts (Beckman Coulter, Inc., USA). A Pearson chi-square or Fisher’s exact test was used to comparing the differences in OOR value between the male and female groups. Correlations between age and biochemical analytes were tested by Pearson correlation analyses.
Results
Screening assessment of healthy volunteers in clinical pharmacology trials
A total of 269 subjects were excluded and 773 healthy individuals (552 male and 221 female) were ultimately included in the study. The detailed screening assessment of healthy volunteers in clinical pharmacology trials is shown in Supplementary File 1, available online at the European Journal of Clinical Pharmacology.
Demographic characteristics of the study population
The main characteristics of the participants in the study are described in Table 1. Among the 773 screened participants, 552 participant (71.41%) were male, and 221 (28.59%) were female. These participants were predominantly young adults, and more than half were under 25 years old. The average age of this population was 26.57 (±7.38) years.
RIs of 16 biochemical analytes for healthy Chinese volunteers during the screening process in clinical pharmacology trials
Table 2 shows an overview of the RIs of 16 biochemical analytes for healthy Chinese volunteers during the screening process in clinical pharmacology trials. The results for albumin, ALT, urea, creatinine, uric acid, total bilirubin, direct bilirubin, indirect bilirubin, potassium, and calcium appeared generally higher in men than in women (P < 0.001, and Z > Z*). Conversely, women had markedly higher levels of globulin and chlorine (P < 0.001, and Z > Z*) than men. In the other analytes, no significant differences were found between the two groups. The RIs of albumin were partitioned by age in men, while other biochemistry analytes were not available for partitioning by age. Compared with the institutional RIs, our RIs had higher upper limits of ALT, uric acid, total bilirubin, direct bilirubin, and indirect bilirubin, but smaller lower limits of albumin (for women), urea, creatinine, and potassium. Comparisons between RIs established in the present study and RIs reported in the existing literature [12, 17,18,19,20,21] are shown in Supplementary File 2, available online at the European Journal of Clinical Pharmacology. The RIs for biochemical analytes established in this study were quite different from those reported in the existing literature, especially for albumin, ALT, urea, creatinine, uric acid, total bilirubin, direct bilirubin, indirect bilirubin, and potassium.
Out-of-range values calculated by institutional RIs
Out-of-range values calculated by institutional RIs are also shown in Table 2. Using the institutional RIs, the proportion of results with abnormal amylase levels exceeded 5% for seven analytes, including urea (30.79%), creatinine (5.44% for men and 29.86% for women), uric acid (6.52% for men), total bilirubin (12.29%), indirect bilirubin (12.81%), and potassium (6.60%). The proportion of results higher than institutional RIs for total bilirubin and indirect bilirubin was generally greater in men than in women (P < 0.05), and the proportion of results lower than institutional RIs for AST was also generally greater in men than in women (P < 0.05). Women had a significantly greater proportion of results lower than institutional RIs for albumin, urea, creatinine, and calcium (P < 0.05) compared with men.
Correlations between age and biochemical analytes
Correlations between age and biochemical analytes are shown in Table 3. Significant correlations were observed between age and albumin, total protein, ALT, AST, uric acid, total bilirubin, indirect bilirubin, calcium, and glucose in men. Meanwhile, significant correlations were also observed between age and albumin, total protein, uric acid, and glucose in women.
Discussion
Healthy volunteers in clinical pharmacology trials tend to be a special subgroup of the general population because of their unique demographic characteristics. Several reports [14, 22,23,24] have shown that these volunteers are predominantly young men, with low income, poor education, and high rates of unemployment, and who are easily influenced by the offer of financial compensation. However, RIs tend to be confined to the general population, and RIs for this special subgroup are limited. The main advantage of this study is that the establishment of RIs used direct sampling techniques, which ensured their high accuracy. Moreover, adequate reference individuals who completely meet the definition of “healthy” are optimal for the establishment of RIs. Fortunately, the physical condition of healthy volunteers is strictly assessed in clinical pharmacology trials. Fewer individuals with latent disease and more healthy individuals could be ideally included in this study. Therefore, the sampling bias was greatly reduced, and it is also one of the advantages of our study. The large sample size of volunteers recruited is another strength of our study.
Partitioning certain RIs by gender has generally been used in most clinical laboratories. It is interesting to note that gender-specific differences in albumin, globulin, bilirubin, urea, potassium, calcium, and chlorine were found in this study. Gender-specific differences in out-of-range values for most of these analytes were also observed. Similar to our results, Yang et al. [21] reported that gender differences in bilirubin and urea existed in a Chinese Han population. Moreover, the majority of the current trial population was young individuals, 53.17% of whom were under 25 years old. Given this result, the gender differences may be partly explained by this demographic characteristic. For instance, gender differences in bilirubin and albumin have been reported in several studies which included young adults only [25,26,27]. In addition, gender-specific differences were observed for the 12- to 29-year-old age group for chloride and the 20- to 39-year-old age group for calcium in a nationwide survey [28]. Therefore, these gender-specific differences may be due to the specific RIs from a relatively young, healthy Chinese population. Partitioning by age also plays a vital role in the establishment of RIs. In agreement with Adeli et al.’s study [28], our results showed that the RIs of albumin should be partitioned by age in men, and individuals aged <25 years had higher levels of albumin. This is further proof that reevaluating the RIs in this population is important. Of note, other analytes were not available for partitioning by age in men. Typically, 45 or 50 years of age is used as the boundary for age-partitioning in establishing RIs for adults [12, 18, 20, 28]; therefore, this result may be attributed to the narrow age range in this target population, almost 97.5% of whom were aged ≤45 years.
In addition to gender-specific differences, noticeable differences between the newly established RIs and RIs provided by our laboratory or existing literature were also observed, especially for albumin, ALT, urea, creatinine, uric acid, bilirubin, and potassium. In accordance with previous studies in Chinese populations [9, 21], the upper limits of RIs for bilirubin and ALT were much higher than those of institutional RIs. Similarly, the RIs of AST, creatinine, bilirubin, and potassium showed a great difference compared with those from the same reagent inserts in a multicenter study in China [20]. Hence, the fact that the reference population differed from those of reagent inserts may be an important contributor to our findings. There are, however, other possible explanations. First, given that over 60% of individuals in the present study who had abnormal levels of serum bilirubin were men aged <25 years (data not shown), the prevalence of Gilbert’s syndrome might be relatively high in these individuals, as Gilbert’s syndrome usually induces elevated indirect bilirubin in young asymptomatic individuals [29, 30]. Second, our results also showed that the levels of bilirubin and ALT were negative with age in men. This means that young male individuals have higher levels of bilirubin and ALT, and this result could be another contributor. Conversely, the lower limit of RIs for urea and creatinine (especially in women) was much lower than those of institutional RIs. The RIs of urea in women were thought to have an age-related change and should be partitioned by age [12, 20], and a similar low RI with 1.9-5.1 mmol/L for urea in women aged <45 years was reported in another study using the same analyzer [18]. Furthermore, as we have shown, women had lower RIs (38-50 g/L) of albumin and a greater proportion (7.69%) of results lower than institutional RIs due to low socioeconomic status, and an inadequate protein intake may also lead to lower urea or creatinine [31]. More interestingly, the lower RIs of potassium and a high proportion (6.60%) of results lower than institutional RIs were found in our study, especially in women. These findings seem to be consistent with other research [32]. Another prior study demonstrated that socioeconomic disadvantage is associated with unfavorable aspects of dietary potassium intake in young women [33], indicating that the depressed lower limits of potassium may be related to the lower potassium intake due to a low socioeconomic status in this trial population.
According to the present results, we can conclude that our RIs are more applicable to the current trial population than our institutional RIs, which is a meaningful finding for the screening process in clinical pharmacology trials. It was suggested that a high number of false positives existed in healthy volunteers in clinical trials due to an a priori chance of disease in the trial population [2, 15]. When the institutional RIs were used as the inclusion and exclusion criteria during the screening process, over 40% of healthy volunteers in the present study were excluded owing to laboratory abnormalities, the majority of which were thought to be false-positive results (data not shown). Similarly, data from several studies suggest that over half of the volunteers would be screened out of a trial if RIs not derived from the local population were used [26, 27]. The problem of false-positive results in laboratory tests may be attributed in part to the use of RIs which are not applicable to the trial population. Thus, the use of improper laboratory RIs may falsely exclude eligible healthy volunteers from participating in drug clinical trials, increasing the workload and cost associated with the screening process [34]. Given this view, RIs with three standard deviations or slight elevation (10-20% of the upper limit) are recommended for the purpose of screening healthy volunteers for clinical trials [2, 15]. If the RIs in this study are used, few volunteers will be falsely excluded. Therefore, the newly established RIs can serve as a powerful clinical tool during the screening process in clinical pharmacology trials. Even so, the problem of false negatives should also be considered, especially when these RIs are applied in evaluating drug safety and maximum tolerated dose. A combination of clinical decision limits and these RIs should be considered as a much more reliable way to avoid this problem.
Despite the intriguing findings of our study, several important limitations should be taken into account. First, the imbalance in sample size between genders may have a potential impact on the establishment of the RIs. Second, the small size of the female sample did not allow RIs to be partitioned by age in that group. Third, a secondary exclusion after recruitment was applied in this study according to the suggestions provided by the IFCC [35], which led to a narrower reference range. Last but not least, the newly established RIs were based on a relatively young, healthy population, which was also considered to be free of disease after medical examination. These RIs should only be applied to a trial population (aged ≤45 years optimally) under the study conditions and using the same instruments. If any condition changes (e.g. population, instrument), these RIs should be reverified.
Conclusions
In summary, for the first time in China, this study reports RIs of 16 biochemical analytes for use when screening a defined special subgroup of a healthy Chinese population in clinical pharmacology trials. Since this was a special subgroup of the general population, noticeable differences between the newly established RIs and RIs provided by our laboratory or existing literature were also observed. Compared with our institutional RIs, the newly established RIs are more applicable to the current trial population. Hence, the RIs in this study can serve as a powerful clinical tool during the screening process in clinical pharmacology trials. Nevertheless, these RIs should be reverified if any condition changes. The results also emphasize the importance of reestablishing RIs which are more applicable to local trial populations.
References
Sibille M, Deigat N, Durieu I et al (1999) Laboratory data in healthy volunteers: reference values, reference changes screening and laboratory adverse event limits in phase I clinical trials. Eur J Clin Pharmacol 55:13–19. https://doi.org/10.1007/s002280050586
Breithaupt-Groegler K, Coch C, Coenen M et al (2017) Who is a ‘healthy subject’?-consensus results on pivotal eligibility criteria for clinical trials. Eur J Clin Pharmacol 73:409–416. https://doi.org/10.1177/1740774517722130
Miller WG, Horowitz GL, Ceriotti F et al (2016) Reference intervals: strengths, weaknesses, and challenges. Clin Chem 62:916–923. https://doi.org/10.1373/clinchem.2016.256511
Ozarda Y (2016) Reference intervals: current status, recent developments and future considerations. Biochem Med 26:5–16. https://doi.org/10.11613/BM.2016.001
Friedberg RC, Souers R, Wagar EA et al (2007) The origin of reference intervals. Arch Pathol Lab Med 131:348–357. https://doi.org/10.1043/1543-2165(2007)131[348:TOORI]2.0.CO;2
Shine B (2008) Use of routine clinical laboratory data to define reference intervals. Ann Clin Biochem 45:467–475. https://doi.org/10.1258/acb.2008.008028
Mekonnen Z, Amuamuta A, Mulu W et al (2017) Clinical chemistry reference intervals of healthy adult populations in Gojjam zones of Amhara National Regional State, Northwest Ethiopia. PLoS One 12:e0184665. https://doi.org/10.1371/journal.pone.0184665
Rustad P, Felding P, Lahti A et al (2004) Descriptive analytical data and consequences for calculation of common reference intervals in the Nordic reference interval project 2000. Scand J Clin Lab Invest 64:343–370. https://doi.org/10.1080/00365510410006306
Mu R, Chen W, Pan B et al (2013) First definition of reference intervals of liver function tests in China: a large-population-based multi Center study about healthy adults. PLoS One 8:e72916. https://doi.org/10.1371/journal.pone.0072916
Lee SG, Lee W, Kim JH et al (2012) Gender-specific reference intervals for serum total bilirubin in healthy Korean adults. Clin Biochem 45:1257–1259. https://doi.org/10.1016/j.clinbiochem.2012.06.033
International Organization for Standardization. ISO 15189:2012 Medical laboratories- Requirements for quality and competence, 3rd ed., 2012
Ozarda Y, Ichihara K, Aslan D et al (2014) A multicenter nationwide reference intervals study for common biochemical analytes in Turkey using Abbott analyzers. Clin Chem Lab Med 52:1823–1833. https://doi.org/10.1515/cclm-2014-0228
Jones GRD, Haeckel R, Loh TP et al (2018) Indirect methods for reference interval determination-review and recommendations. Clin Chem Lab Med 57:20–29. https://doi.org/10.1515/cclm-2018-0073
Grady C, Bedarida G, Sinaii N et al (2017) Motivations, enrollment decisions, and socio-demographic characteristics of healthy volunteers in phase 1 research. Clin Trials 14:526–536. https://doi.org/10.1177/1740774517722130
den Elzen WP, van Gerven J, Schenk PW et al (2017) How to define reference intervals to rule in healthy individuals for clinical trials? Clin Chem Lab Med 55:e59–e61. https://doi.org/10.1515/cclm-2016-0307
Clinical and Laboratory Standards Institute (CLSI) (2010) Defifining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline, CLSI document C28-A3, Third edition
Ichihara K, Ceriotti F, Tam TH, S et al (2013) The Asian project for collaborative derivation of reference intervals: (1) strategy and major results of standardized analytes. Clin Chem Lab Med 51: 1429-1442. https://doi.org/10.1515/cclm-2012-0421
Shah SAV, Ichihara K, Dherai AJ et al (2018) Reference intervals for 33 biochemical analytes in healthy Indian population: C-RIDL IFCC initiative. Clin Chem Lab Med 56:2093–2103. https://doi.org/10.1515/cclm-2018-0152
Borai A, Ichihara K, Al Masaud A et al (2016) Establishment of reference intervals of clinical chemistry analytes for adult population in Saudi Arabia: a study conducted as a part of the IFCC global study on reference values. Clin Chem Lab Med 54:843–855. https://doi.org/10.1515/cclm-2015-0490
Xia L, Chen M, Liu M et al (2016) Nationwide Multicenter reference interval study for 28 common biochemical Analytes in China. Medicine 95:e2915. https://doi.org/10.1097/MD.0000000000002915
Yang S, Qiao R, Li Z et al (2012) Establishment of reference intervals of 24 chemistries in apparently healthy adult Han population of northern China. Clin Biochem 45:1213–1218. https://doi.org/10.1016/j.clinbiochem.2012.06.022
Iltis AS (2009) Payments to normal healthy volunteers in phase 1 trials: avoiding undue influence while distributing fairly the burdens of research participation. J Med Philos 34:68–90. https://doi.org/10.1093/jmp/jhn036
Almeida L, Azevedo B, Nunes T et al (2007) Why healthy subjects volunteer for phase I studies and how they perceive their participation. Eur J Clin Pharmacol 63:1085–1094. https://doi.org/10.1007/s00228-007-0368-3
Stones M, McMillan J (2010) Payment for participation in research: a pursuit for the poor? J Med Ethics 36:34–36. https://doi.org/10.1136/jme.2009.030965
Abebe M, Melku M, Enawgaw B et al (2018) Reference intervals of routine clinical chemistry parameters among apparently healthy young adults in Amhara National Regional State, Ethiopia. PLoS One 13:e0201782. https://doi.org/10.1371/journal.pone.0201782
Zeh C, Amornkul PN, Inzaule S et al (2011) Population-based biochemistry, immunologic and hematological reference values for adolescents and young adults in a rural population in Western Kenya. PLoS One 6:e21040. https://doi.org/10.1371/journal.pone.0021040
Tembe N, Joaquim O, Alfai E et al (2014) Reference values for clinical laboratory parameters in young adults in Maputo, Mozambique. PLoS One 9:e97391. https://doi.org/10.1371/journal.pone.0097391
Adeli K, Higgins V, Nieuwesteeg M et al (2015) Biochemical marker reference values across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian health measures survey. Clin Chem 61:1049–1062. https://doi.org/10.1373/clinchem.2015.240515
Claridge LC, Armstrong MJ, Booth C et al (2011) Gilbert's syndrome. BMJ 342:d2293. https://doi.org/10.1136/bmj.d2293
Horsfall LJ, Nazareth I, Pereira SP et al (2013) Gilbert's syndrome and the risk of death: a population-based cohort study. J Gastroenterol Hepatol 28:1643–1647. https://doi.org/10.1111/jgh.12279
Cirillo M, Lombardi C, Chiricone D et al (2014) Protein intake and kidney function in the middle-age population: contrast between cross-sectional and longitudinal data. Nephrol Dial Transplant 29:1733–1740. https://doi.org/10.1093/ndt/gfu056
Rowland R, O'Hara GA, Hamill M et al (2013) Determining the validity of hospital laboratory reference intervals for healthy young adults participating in early clinical trials of candidate vaccines. Hum Vaccin Immunother 9:1741–1751. https://doi.org/10.4161/hv.24998
Murakami K, Sasaki S, Takahashi Y et al (2009) Neighborhood socioeconomic disadvantage is associated with higher ratio of 24-hour urinary sodium to potassium in young Japanese women. J Am Diet Assoc 109:1606–1611. https://doi.org/10.1016/j.jada.2009.06.391
Eller LA, Eller MA, Ouma B et al (2008) Reference intervals in healthy adult Ugandan blood donors and their impact on conducting international vaccine trials. PLoS One 3:e3919. https://doi.org/10.1371/journal.pone.0003919
Ichihara K (2014) Statistical considerations for harmonization of the global multicenter study on reference values. Clin Chim Acta 432:108–118. https://doi.org/10.1016/j.cca.2014.01.025
Acknowledgments
The authors are very grateful to Dr. Wenling Zhu for her dedicated contribution to the assessment of the health status of participants in this study. In addition, the authors are very grateful to Dr. Songxue Guo for his dedicated contribution to the English language editing on the manuscript.
Funding
This work was supported by the following grants: National Natural Science Foundation of China (NSFC) Grants 81671909, 81901958 and Zhejiang Provincial Natural Science Foundation of China Grants LY18H150004, LY19H150004, LY20H150010.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: PZ, XL. Performed the experiments: PZ, HC, XL JL. Analyzed the data: PZ, HC. Wrote the paper: PZ, XL. Initiated and organized this project: PZ. All authors reviewed and edited the manuscript and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, P., Chen, H., Luo, X. et al. Establishment of reference intervals of biochemical analytes for healthy Chinese volunteers during the screening process in clinical pharmacology trials. Eur J Clin Pharmacol 76, 1227–1235 (2020). https://doi.org/10.1007/s00228-020-02912-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02912-1