Abstract
Purpose
Idiopathic nephrotic syndrome (INS) is the most frequent form of childhood nephrotic syndrome. Steroids represent the best therapeutic option; however, inter-individual differences in their efficacy and side effects have been reported. To date, there is no way to predict patients’ resistance and/or dependence. Alterations in the cytokine profile of INS patients might contribute to proteinuria and glomerular damage and affect drug sensitivity.
Methods
The cytokine plasma levels were measured in 21 INS children at diagnosis to investigate the association among cytokines pattern and clinical response. Patients were selected on the basis of their clinical response: 7 steroid sensitive (SS), 7 dependent (SD), and 7 resistant (SR). Significant results were then analyzed in 41 additional pediatric INS patients.
Results
Within the 48 cytokines analyzed, macrophage migration inhibitory factor (MIF) was a good predictor of steroid response. Indeed, SR patients showed significantly higher MIF plasma levels compared with all others (p = 0.022; OR = 4.3, 95%CI = 1.2–25.4): a cutoff concentration of MIF > 501 pg/ml significantly discriminated SR patients (sensitivity = 85.7%, specificity = 71.4%). On the contrary, SD patients showed lower MIF plasma levels compared with others (p = 0.010; OR = 0.12, 95%CI = 9.2 × 10−3–6.7 × 10−1). Significant results were confirmed in the entire cohort.
Conclusions
Our comprehensive cytokine analysis indicates that assessing MIF plasma levels at diagnosis could predict response to glucocorticoids in children with INS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic nephrotic syndrome (INS) is the most common pediatric primary glomerular disease, affecting 16–17 per 100,000 children between the ages of 2 and 8 years, with a peak of incidence between 3 and 5 years. INS is characterized by an increase in permeability of the capillary walls of glomeruli, leading to proteinuria.
Glucocorticoids (GCs) are the first-choice drugs and induce remission in 85–90% of children with this disease; however, 10–15% of patients are steroid resistant (SR) [1,2,3]. Moreover, despite initial complete remission, almost 50% of patients show recurrence of the proteinuria and are classified as steroid dependent (SD): these patients, after a prolonged steroid therapy, with the possibility of severe adverse effects, often need to switch to other immunomodulating or immunosuppressive drugs. Responsiveness to steroids is the most important prognostic factor and patients that do not respond to therapy are subjected to aggressive treatments and often develop several complications and side effects. The mechanisms involved in GC dependence and resistance are scarcely understood and studies considering GC pharmacodynamics and pharmacogenetics have been performed without conclusive results [4,5,6].
Various studies have demonstrated the involvement of cytokines in the occurrence of proteinuria that characterizes INS [4, 7, 8]. Relapses are quite frequent in this disease, and are often triggered by viral infections, which result in the release of cytokines, causing immunoregulatory imbalances [9]. Cytokines levels and other markers of immune activation have been used in the diagnosis of different diseases, also for prognostic purposes [1, 10]. However, in INS patients, changes in various plasma cytokine profiles prior to and after steroid treatment have not been extensively examined.
In the present study, we have investigated the plasma levels of a panel of cytokines in patients with INS undergoing steroid treatment, in order to elucidate whether there is any specific cytokine that could serve as biomarker to predict treatment efficacy.
Methods
Study design and population
One hundred eighty-four pediatric patients were enrolled between August 2011 and February 2014 in the prospective multicenter trial for INS treatment from the Italian pediatric nephrology network NEFROKID (ClinicalTrials.gov identifier: NCT01386957). Approval was obtained from the ethics committees of all the participating centers and parents gave written informed consent before enrollment in the study. One hundred twenty two patients were excluded because of the following reasons: non-adherence to the protocol (5 patients), written informed consent for the biological part of the study could not be obtained (1 patient), onset of the disease at weekends or holidays and blood samples could not be sent to the collecting center in Trieste (52 patients), the sample volumes were insufficient or not correctly shipped (32 patients), other reasons (32 patients). Therefore, 62 patients were enrolled in the present study; this group of patients was representative of the whole cohort with regard to demographical and clinical characteristics [11].
All patients were treated with a common therapeutic protocol consisting of prednisone at a dose of 60 mg/m2/day for either 4 or 6 weeks, depending on whether time to remission was < or ≥ 10 days, respectively, and tapering of steroids over 16 weeks. Remission was defined as the disappearance of proteinuria for at least 3 consecutive days. Total prednisone dosage was 2828 mg/m2 in patients who went into remission within 10 days and 3668 mg/m2 in the others. Patients were divided into 3 groups, defined as in the therapeutic protocol of the clinical trial NCT01386957, according to their clinical response: absence of remission despite steroid therapy (steroid resistant SR), steroid dependent (SD) patients, presenting two relapses during treatment or within 14 days of discontinuation of therapy or presenting two or more relapses within 6 months of initial response or four or more relapses in any 12-month period and steroid sensitive (SS), with less than two relapses within 6 months of initial response.
The first 7 consecutive patients for each group of steroid response (SR, SD and SS) were characterized for their plasma cytokine levels. Significant results were then investigated in the entire cohort of 62 patients and analyzed also in a subgroup of healthy pediatric subjects without any acute or chronic infectious disease, any clinically significant disorder, and any medication with known influence on immunological factors.
Sample and cytokine measurements
Peripheral venous blood, anticoagulated with EDTA, was collected at the onset of the disease and sent refrigerated within 24 h to the Department of Life Sciences at the University of Trieste; plasma was separated as described [11] and stored at − 80 °C for measurement of 48 cytokines. Each sample (20 μl) was analyzed by magnetic beads suspension array using the Bio-Plex Pro Human Cytokine 21- and 27-plex panels (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. The 21-plex panel and 27-plex panels are described in the Supplementary Materials. A Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, CA, USA), with Bio-Plex Manager 6.0 software (Bio-Rad Laboratories, Hercules, CA, USA) was used. Detection ranges for each cytokine, with the lowest concentration of analyte that can be detected (LOD), and the uppermost and lowest quantifiable concentration (respectively ULOQ and LLOQ) are reported in the Supplementary materials. The method was validated in the laboratory and tested for recovery.
Genetic analysis
For the most significant cytokine associated in this study with steroid response, MIF, a common genetic variant G-173C (rs755622) was examined. Total genomic DNA was isolated from peripheral blood by using a commercial kit (Gene Elute Blood Genomic DNA kit, Sigma Aldrich, Milan, Italy) and the MIF polymorphism was determined by TaqMan® SNP genotyping technologies (Applied Biosystems, Bedford, UK) on an ABI7900 HT sequence detection system device.
Statistical analysis
For statistical purposes, for cytokines with concentration out of the detection ranges (Supplementary Table 1), an arbitrary value was defined, corresponding to half of the minimum or double of the maximum detectable concentration, according to whether the samples were below or above the sensitivity range of the assay.
Initially, 7 patients for each response group were analyzed, selected on the basis of sample availability for resistant patients, and each resistant case was matched with an SS and SD patient. Statistical comparisons were done considering each response group against the other two grouped together, to increase statistical power. This approach provided a statistical power sufficient to identify an effect of large magnitude (non-overlap between the two distributions of at least 65%) with a p value threshold of 0.05 and a power of 0.80 [12].
Univariate analysis was performed by logistic regression models testing any possible association between cytokine levels, MIF polymorphism and clinical response. Multivariate analyses were performed by logistic regression models combining independent variables significant in the univariate analysis. The best cutoff value to determinate patients’ response using cytokine concentration was identified by receiver operating characteristic (ROC) curves. Sensitivity and specificity of the selected cutoffs were analyzed. Fisher’s exact test was applied to support the significance of these cutoff values. Statistical analyses were performed using the software R version 3.2.4.
P values lower than 0.05 were considered statistically significant. Odds ratio (OR) and 95% confidence interval (95% CI) were calculated for all the analyses.
Results
Patients
Demographical characteristics of the 62 enrolled patients are reported in Table 1. A statistically significant difference in age was observed between SR and SS+SD patients, the former group being older (univariate logistic regression, p value = 0.024, OR = 1.33, 95% CI = 1.03–1.92) while SD patients and SS were not different compared with other patients; gender distribution was not different among patients’ groups. The first analyses were performed in a group consisting of 21 patients that were the first 7 consecutively enrolled subjects for each group of steroid response (SR, SD and SS); this group was representative of the entire group of patients in terms of age and sex distribution.
Plasma cytokine measurements
Using the Bioplex assays, in 21 patients (7 SS, 7 SD and 7 SR), we measured the concentration of 48 soluble plasma immune mediators at baseline, before initiation of steroid treatment. Cytokine concentrations are presented in Supplementary Table 1. No measurable value for MCP-3, IL-15, IL-12p40, IL-3, IL-1α, TNF-β, and MCP-1 was obtained; therefore, these cytokines were excluded from data analyses.
Baseline plasma cytokines in SR patients
Given the clinical interest in recognizing SR patients, the association between cytokine levels and clinical response was analyzed by comparing SR with the other patients. Univariate logistic regression analysis showed significantly elevated concentration of MIF (mean concentration 759.7 pg/ml in SR vs 414.1 pg/ml in SD + SS, p = 0.022, OR = 4.3, 95% CI = 1.2–25.4) and SCGF-β (mean 33.5 pg/ml in SR vs 21.2 pg/ml in SD + SS, p = 0.034, OR = 5.5, 95% CI = 1.1–56.4) (Fig. 1).
Boxplot comparing cytokine concentrations and clinical response between SR and SS+SD. Cytokine concentrations are plotted in logarithmic scale. The bold horizontal line represents the distribution median. Statistical significance was assessed by logistic regression analysis. A significant association was found for a MIF (p = 0.022, OR = 4.3, 95% CI = 1.2–25.4) and for b SCGF-β (p = 0.034, OR = 5.5, 95% CI = 1.1–56.4)
However, when multivariate logistic regression analysis was performed considering all significant variables (MIF, SCGF-β and age), only MIF (p = 0.022) and age at diagnosis (p = 0.025) were able to distinguish the two groups.
Baseline plasma cytokines to identify SD patients
Treatment of SD patients is a serious challenge for clinicians; therefore, we analyzed the differences in cytokine levels between SD and all other patients. Univariate logistic regression analysis identified 5 cytokines as differentially expressed between SD and all other patients (SR+SS; Fig. 2). Significantly lower concentrations of IL-18 (mean concentration 36.8 pg/ml in SD vs 74.4 pg/ml in SR+SS, p = 0.0003, OR = 0.01, 95% CI = 4.5 × 10−5–2.4 × 10−1, Fig. 2a), MIF (mean concentration 339.6 pg/ml in SD vs 619.4 pg/ml in SR + SS, p = 0.010, OR = 0.12, 95% CI = 9.2 × 10−3–6.7 × 10−1, Fig. 2b), and SCGF-β (mean concentration 17.8 pg/ml in SD vs 29.1 pg/ml in SR+SS, p = 0.030, OR = 0.21, 95% CI = 2.9 × 10−2–8.8 × 10−1, Fig. 2c) and significantly higher concentrations of IL-17 (mean concentration 102.5 pg/ml in SD vs 60.7 pg/ml in SR+SS, p = 0.031, OR = 4.1, 95% CI = 1.1–23.2, Fig. 2d) and G-CSF (mean concentration 58.4 pg/ml in SD vs 39.3 pg/ml in SR+SS, p = 0.019, OR = 11.2, 95% CI = 1.4–255.6, Fig. 2e).
Boxplot comparing cytokine concentrations and clinical response between SD and SS+SR. Cytokine concentrations are plotted in logarithmic scale. The bold horizontal line represents the distribution median. Statistical significance was assessed by logistic regression analysis. A significant association was found for a IL-18 (p = 0.0003, OR = 0.01, 95% CI = 4.5 × 10−5–2.4 × 10−1), b MIF (p = 0.010, OR = 0.12, 95% CI = 9.2 × 10−3–6.7 × 10−1), c SCGF-β (p = 0.030, OR = 0.21, 95% CI = 2.9 × 10−2–8.8 × 10−1), d IL-17 (p = 0.031, OR = 4.1, 95% CI = 1.1–23.2) and e G-CSF (p = 0.019, OR = 11.2, 95% CI = 1.4–255.6)
When multivariate analysis was performed, only MIF, IL-18, and SCGF-β were able to significantly distinguish the two groups (p = 0.028, p = 0.00033 and p = 0.0056, respectively).
Confirmation of results in the entire cohort: IL-18, SCGF-β, and MIF
To confirm the results obtained, we investigated the cytokines significantly associated with clinical response (IL-18, MIF and SCGF-β) in the entire cohort of patients. Considering all 62 patients, univariate logistic regression models showed a significant difference only for MIF plasma levels between SR patients and SD+SS ones (mean concentration 683 pg/ml in SR vs 436.8 pg/ml in SD + SS, p = 0.039; OR = 0.4, 95% CI = 0.2–0.9) and between SD patients and SR+SS (mean concentration 375.5 pg/ml in SD vs 534.3 pg/ml in SD+SS, p = 0.014; OR = 2.3, 95% CI = 1.2–5.5).
Identification of a MIF cutoff to distinguish SR patients
Since after the multivariate analysis MIF was the only cytokine able to distinguish SR patients, we performed ROC curves to identify cutoff values for MIF levels significantly associated with steroid resistance: a unique cutoff of 501 pg/ml was found. Area under the ROC curve (AUC) was 76.0% (Fig. 3). The test had high sensitivity (85.7%) and specificity (71.4%). Fisher’s exact test confirmed a higher proportion of SR patients among those who reached the optimal cutoff point (p value = 0.024, OR = 7.8 × 10−2, 95% CI = 1.3 × 10−3–9.4 × 10−1) in comparison to those who did not.
Areas under the ROC curves of MIF plasma level in SR (panel a) or SD (panel b) vs all other patients among 7 SS, 7 SD, and 7 SR patients. ROC, receiver operating characteristic. Optimal cutoff value for SR patients was 501 pg/ml (sensitivity 85.7%, specificity 71.4%, panel a, while for SD patients was 355 pg/ml was found (sensitivity = 85.7%, specificity = 78.6%; panel b
For SD patients, a cutoff of 355 pg/ml was found (AUC = 83.2%, sensitivity = 85.7%, specificity = 78.6%; Fig. 3). Fisher’s exact test confirmed higher proportion of SD patients among those who did not reach the cutoff point (p = 0.016, OR = 18.1, 95% CI = 1.4–1.1 × 103) in comparison with those who reach it.
The cutoff value found was investigated in the extended cohort of 62 patients. Fisher’s exact test analysis confirmed a higher proportion of SR patients among those who reached the optimal cutoff point (MIF > 501 pg/ml; p = 0.02; OR = 0.14, 95% CI = 0.01–0.8) in comparison with those who did not.
For SD patients, the cutoff value of 355 pg/ml, found in the discovery cohort, was not confirmed in the entire cohort of patients.
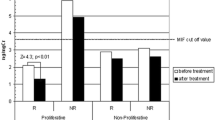
Finally, we evaluated the differences in MIF plasma levels between our cohort of INS patients and 11 pediatric controls; control subjects were similar in terms of age compared with our cohort (median 6.0; range 1–11 years). Univariate logistic regression model shows a significantly higher concentration of MIF in patients with INS, in comparison with healthy controls (MIF mean concentration 466.1 pg/ml in patients with INS vs 124.5 pg/ml in healthy controls, p < 0.0001; Fig. 4).
MIF level correlation with MIF G-173C polymorphism
Elevated circulating serum MIF levels have been related to MIF gene polymorphisms, although with controversial results [6]. The 62 patients involved in the study were therefore genotyped for MIF polymorphism G-173C (rs755622). Genetic results were available for 59 patients and the genotype distribution was in Hardy-Weinberg equilibrium (p = 0.28); 44 patients were wild type (74.6%; 22 SS, 15 SD and 7 SR), 15 were heterozygous (25.4%; 5 SS, 8 SD and 2 SR), while none of the patients showed a mutated genotype. We evaluated the possible correlation between MIF G-173C polymorphism and MIF plasma levels without finding any significant association (Supplementary Fig. 1). Moreover, there was no genotype effect on clinical response.
Discussion
In this study, we evaluated the plasma concentration of 48 cytokines in patients at the first episode of INS who underwent steroid treatment, with the final aim of finding a biomarker useful to predict their clinical response. The study was at first conducted in a group of 21 patients. Patients were selected from an Italian cohort of pediatric patients, clinically characterized and treated with a common protocol. Significant results were then considered including additional 41 patients treated with the same therapeutic protocol.
INS is the most frequent primary glomerular disease in children [3, 13]. The physiopathologic mechanisms of the disease are still not clear; however, the disease is triggered by an increase in glomerular permeability caused by an abnormal immunologic response, resulting in an alteration of the capillary structure and of the integrity of the glomerular membrane [3].
Since the 1950s, steroid treatment is the first line therapy for INS [14], but response to these drugs is variable. Steroids are potent inhibitors of cytokines production in immune and non-immune cells and are able to induce remission in about 85–90% of subjects; however, variable degrees of steroid responsiveness and different patterns of disease relapse have been observed [15]. Response to steroid treatment is a key index of outcome; indeed, patients with steroid resistant disease represent a difficult therapeutic challenge for clinicians; moreover, to date, approximately 40–50% of responding patients presents frequent relapses or steroid dependence when therapy is discontinued; these patients are at high risk of treatment related side effects [3, 16]. There is still no way of predicting this pattern of ineffective therapy and steroid dependence.
In this study, we showed that, within the 48 cytokines analyzed, MIF is the best predictor of steroid response before any treatment in children with INS. Indeed, patients non-responsive to GCs show significantly higher MIF plasma levels compared with steroid sensitive ones. These results are supported also by Wang et al. [17] in patients with systemic lupus erythematosus; these authors demonstrated that MIF serum levels were correlated with steroid resistance. In the present study, for the first time, a cutoff value for MIF plasma level could be identified at 501 pg/ml to distinguish resistant and sensitive patients, with a high sensitivity and specificity (respectively 85.7% and 71.4%). Considering patients achieving this cutoff, almost all (7/9) of SR patients could be identified. This finding could be useful for the early identification of patients who will not respond to steroids, avoiding ineffective treatments. Furthermore, MIF plasma levels were also able to distinguish INS patients from healthy subjects that show lower levels of the cytokine.
Moreover, in this study we focused our attention also on SD patients, who show very low plasma MIF secretion as compared with all other patients. This is the first study, to our knowledge, which investigates cytokine plasma levels in patients who show steroid dependence. These patients represent almost 40–50% of INS patients and may require a more aggressive treatment with increased risk of adverse events and disease related complications. Plasma MIF level in these patients was shown to be lower than in all other patients; however, a cutoff value able to distinguish SD patients could not be confirmed in our extended cohort of patients. This finding of low plasma MIF levels in SD patients was quite unexpected, even if previous studies have shown that these patients do not have a pharmacological phenotype intermediate between SS and SR [11]; more studies are needed to shed light on the contribution of MIF plasma levels as a biomarker for this clinically challenging group of patients.
MIF is a pleiotropic cytokine with pro-inflammatory activity, which appears to be due to effects on macrophages and T cells. Steroids reduce the production of inflammatory molecules; however, they increase the release of MIF from macrophages [18] and T cells [19]. In turn, MIF counter-regulates the inhibitory activity of steroids on pro-inflammatory cytokines [18, 20]. It is not clear yet how MIF can exert this effect; it has been suggested that, in inflammatory conditions, MIF interferes with GC activity on cytokine transcription, mediated by nuclear factor κB (NFκB) [21]. Under basal conditions, NFκB, complexed with IκBα (inhibitor of NFκB) is located in the cytosol. Inflammatory stimuli activate IκBα kinase (IκK) that phosphorylates and degrades IκBα. NFκB can therefore enter into the nucleus, inducing transcription. GCs induce the synthesis of IκBα, that binds to NFκB in the cytosol, preventing its nuclear localization. MIF, on the contrary reduces IκBα cytosolic levels, increasing NFκB migration in the nucleus and transcription and counteracts GC effects [21] (Fig. 5). Moreover, MIF strongly stimulates the extracellular-signal-regulated kinase (ERK)-1 and ERK-2 pathways; the cytoplasmic isoform of phospholipase A2 (PLA2) is activated and arachidonic acid is released [22]. GCs are well-known inhibitors of PLA2 activation, and this effect is counteracted by MIF. In addition to repressing the transcription of immune genes, GCs are also able to increase the degradation of mRNAs of pro-inflammatory genes [23]; also, this phenomenon has been shown to be related to MIF inhibitory effect on GC activity [24](Fig. 5). Although insufficient to explain all the pro-inflammatory activities of MIF, the mechanisms described can add to explain its antagonism on GC-mediated immunosuppression.
Pro-inflammatory mechanism of action of MIF [24]. GC, glucocorticoid; GR, glucocorticoid receptor; PLA2, phospholipase A2; IkBα, inhibitor of NFκB; NFkB, nuclear factor κB; ERK1/2, extracellular-signal-regulated kinase 1 and 2; AA, arachidonic acid
MIF has been already investigated and proved to be involved in diseases such as systemic lupus erythematous [25], rheumatoid arthritis [26], and chronic kidney disease [27]; however, to our knowledge, this is the first work that found a cutoff value for MIF plasma level able to distinguish different clinical response.
Several studies in INS patients have shown that specific MIF polymorphisms are associated with GC response. The most studied and correlated polymorphism in these patients is the MIF G-173C (rs755622), although literature studies show controversial results [6]. Berdeli et al. [28] showed a correlation of the MIF–173C allele with INS and steroid resistance in Turkish children and Vivarelli et al. [29] obtained similar results in an Italian population. However, these findings are not in agreement with Choi’s study that could not find an association between the polymorphism and steroid responsiveness [30]. In our study, MIF G-173C did not affect MIF plasma level measured in the patients (Supplementary Fig. 1), as recently demonstrated also by Ramayani et al. [31]; moreover, no significant correlation was found between this SNP and clinical response.
This study has some limitations, in particular the number of patients enrolled is small and there is a significant difference in age between GC resistant and dependent / sensitive patients. Therefore, it would be important to extend similar studies to larger patients’ groups.
In conclusion, the results of the present study suggest that, within the 48 cytokines considered, increased MIF plasma levels could be used to clinically identify patients at high risk of steroid resistance at diagnosis; if these results are confirmed in a larger cohort of patients, MIF plasma levels could be considered for alternative treatments avoiding useless steroid administration and subsequent side effects.
References
Barnes PJ (2010) Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 120:76–85. https://doi.org/10.1016/j.jsbmb.2010.02.018
Barnes PJ, Adcock IM (2009) Glucocorticoid resistance in inflammatory diseases. Lancet 373:1905–1917. https://doi.org/10.1016/S0140-6736(09)60326-3
Eddy AA, Symons JM (2003) Nephrotic syndrome in childhood. Lancet 362:629–639. https://doi.org/10.1016/S0140-6736(03)14184-0
Carlotti AP, Franco PB, Elias LL, Facincani I, Costa EL, Foss N et al (2004) Glucocorticoid receptors, in vitro steroid sensitivity, and cytokine secretion in idiopathic nephrotic syndrome. Kidney Int 65:403–408. https://doi.org/10.1111/j.1523-1755.2004.00392.x
Cuzzoni E, De Iudicibus S, Bartoli F, Ventura A, Decorti G (2012) Association between BclI polymorphism in the NR3C1 gene and in vitro individual variations in lymphocyte responses to methylprednisolone. Br J Clin Pharmacol 73:651–655. https://doi.org/10.1111/j.1365-2125.2011.04130.x
Cuzzoni E, De Iudicibus S, Franca R, Stocco G, Lucafò M, Pelin M et al (2015) Glucocorticoid pharmacogenetics in pediatric idiopathic nephrotic syndrome. Pharmacogenomics 16:1631–1648. https://doi.org/10.2217/pgs.15.101
Araya CE, Wasserfall CH, Brusko TM, Mu W, Segal MS, Johnson RJ, Garin EH (2006) A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol 21:603–610. https://doi.org/10.1007/s00467-006-0026-5
Garin EH (2000) Circulating mediators of proteinuria in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol 14:872–878
Schnaper HW (1989) The immune system in minimal change nephrotic syndrome. Pediatr Nephrol 3:101–110
Farrell RJ, Kelleher D (2003) Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol 178:339–346
Cuzzoni E, De Iudicibus S, Stocco G, Favretto D, Pelin M, Messina G et al (2016) In vitro sensitivity to methyl-prednisolone is associated with clinical response in pediatric idiopathic nephrotic syndrome. Clin Pharmacol Ther 100:268–274. https://doi.org/10.1002/cpt.372
Becker LA. Effect size calculators [home page on the internet] UCCS University of Colorado. 03/021/00 [2018 July 6]. Available from: http://www.uccs.edu/lbecker/effect-size
Gipson DS, Massengill SF, Yao L, Nagaraj S, Smoyer WE, Mahan JD, Wigfall D, Miles P, Powell L, Lin JJ, Trachtman H, Greenbaum LA (2009) Management of childhood onset nephrotic syndrome. Pediatrics 124:747–757. https://doi.org/10.1542/peds.2008-1559
Hodson EM, Knight JF, Willis NS, Craig JC (2000) Corticosteroid therapy in nephrotic syndrome: a meta-analysis of randomised controlled trials. Arch Dis Child 83:45–51
Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J (2000) Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the national kidney foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105:1242–1249
Hahn D, Hodson EM, Willis NS, Craig JC (2007) Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 3:CD001533. https://doi.org/10.1002/14651858.CD002290.pub3
Wang FF, Zhu LA, Zou YQ, Zheng H, Wilson A, Yang CD, Shen N, Wallace DJ, Weisman MH, Chen SL, Lu LJ (2012) New insights into the role and mechanism of macrophage migration inhibitory factor in steroid-resistant patients with systemic lupus erythematosus. Arthritis Res Ther 14:R103. https://doi.org/10.1186/ar3828
Calandra T, Roger T (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3:791–800. https://doi.org/10.1038/nri1200
Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R (1996) An essential regulatory role for macrophage migration inhibitory factor in t-cell activation. Proc Natl Acad Sci U S A 93:7849–7854
Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377:68–71. https://doi.org/10.1038/377068a0
Daun JM, Cannon JG (2000) Macrophage migration inhibitory factor antagonizes hydrocortisone-induced increases in cytosolic IkappaBalpha. Am J Physiol Regul Integr Comp Physiol 279:R1043–R1049. https://doi.org/10.1152/ajpregu.2000.279.3.R1043
Mitchell RA, Metz CN, Peng T, Bucala R (1999) Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase a2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J Biol Chem 274:18100–18106
Bevilacqua A, Ceriani MC, Capaccioli S, Nicolin A (2003) Post-transcriptional regulation of gene expression by degradation of messenger RNAs. J Cell Physiol 195:356–372. https://doi.org/10.1002/jcp.10272
Roger T, Chanson AL, Knaup-Reymond M, Calandra T (2005) Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur J Immunol 35:3405–3413. https://doi.org/10.1002/eji.200535413
Santos LL, Morand EF (2009) Macrophage migration inhibitory factor: a key cytokine in ra, sle and atherosclerosis. Clin Chim Acta 399:1–7. https://doi.org/10.1016/j.cca.2008.09.014
Leech M, Metz C, Hall P, Hutchinson P, Gianis K, Smith M, Weedon H, Holdsworth SR, Bucala R, Morand EF (1999) Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum 42:1601–1608. https://doi.org/10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B
Bruchfeld A, Carrero JJ, Qureshi Lindholm B, Barany P, Heimburger O, Hu M et al (2009) Elevated serum macrophage migration inhibitory factor (mif) concentrations in chronic kidney disease (CKD) are associated with markers of oxidative stress and endothelial activation. Mol Med 15:70–75. https://doi.org/10.2119/molmed.2008.00109
Berdeli A, Mir S, Ozkayin N, Serdaroglu E, Tabel Y, Cura A (2005) Association of macrophage migration inhibitory factor -173C allele polymorphism with steroid resistance in children with nephrotic syndrome. Pediatr Nephrol 20:1566–1571. https://doi.org/10.1007/s00467-005-1930-9
Vivarelli M, D’Urbano LE, Stringini G, Ghiggeri GM, Caridi G, Donn R et al (2008) Association of the macrophage migration inhibitory factor -173*C allele with childhood nephrotic syndrome. Pediatr Nephrol 23:743–748. https://doi.org/10.1007/s00467-007-0729-2
Choi HJ, Cho HY, Ro H, Lee SH, Han KH, Lee H, Kang HG, Ha IS, Choi Y, Cheong HI (2011) Polymorphisms of the MDR1 and MIF genes in children with nephrotic syndrome. Pediatr Nephrol 26:1981–1988. https://doi.org/10.1007/s00467-011-1903-0
Ramayani OR, Sekarwana N, Trihono PP, Sadewa AH, Lelo A (2016) A genetic study of steroid-resistant nephrotic syndrome: relationship between polymorphism −173 G to C in the MIF gene and serum level MIF in children. J Dev Orig Health Dis 7:102–107. https://doi.org/10.1017/S2040174415007850
Acknowledgments
We thank all the members of Nefrokid Study Group, whose collaboration was essential in order to perform the study. The following institutions participated in the study: Ospedale della Gruccia Montevarchi (Arezzo); Ospedale San Donato Arezzo; Ospedale di Asola - Asola (MN); Policlinico-ospedale Giovanni XXIII, Bari; AUO di Bologna, Policlinico S.Orsola-Malpighi; Ospedale dei Bambini di Brescia; AUSL Modena Presidio Ospedaliero Carpi, Ospedale Ramazzini; AUSL di Cesena Ospedale Maurizio Bufalini; Ospedale di Circolo di Desio (MI); Ospedale di Esine (BS); A.O.Universitaria di Ferrara, Arcispedale S. Anna; Azienda Ospedaliera Universitaria Meyer Firenze; Ospedale Gaslini, Genova; Ospedale Santa Maria della Scaletta, AUSL di Imola; Ospedale “C. Poma” - Mantova; Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico - Milano; Ospedale dei Bambini “Vittore Buzzi” - Milano; Azienda Ospedaliero-Universitaria Policlinico di Modena; Fondazione MBBM, S. Gerardo - Monza; ARNAS Civico, Di Cristina e Fatebenefratelli, Palermo; AO San Salvatore Pesaro; Ospedale Guglielmo da Saliceto, Piacenza; Ospedale Santa Maria degli Angeli, Pordenone; AO. Santa Maria Nuova di Reggio Emilia; Ospedale degli Infermi Rimini; Ospedale Generale Provinciale di Saronno (VA); Nuovo Ospedale Civile Sassuolo; Ospedale di Circolo Galmarini - Tradate (VA); Ospedale Pediatrico “Burlo Garofalo,” Trieste; Ospedale “Filippo Del Ponte” - Varese; Ospedale di Vimercate (MI).
Funding
EC was supported by the Italian Society of Pharmacology grant SIF-MSD Italy 2015. We would also thank the charity organization “Il Sogno di Stefano” and The Nando Peretti Foundation for their generous financial support.
Author information
Authors and Affiliations
Contributions
Eva Cuzzoni designed the research, wrote the manuscript, planned and performed experiments, and analyzed data; Raffaella Franca wrote and revised the manuscript; Sara De Iudicibus designed the research and analyzed data; Annalisa Marcuzzi performed experiments and analyzed data; Marianna Lucafò performed experiments and analyzed data; Marco Pelin performed experiments; Diego Favretto performed experiments; William Morello enrolled patients and revised the manuscript, Luciana Ghio enrolled patients and revised the manuscript, Claudio La Scola enrolled patients and revised the manuscript, Francesca Mencarelli enrolled patients and revised the manuscript, Andrea Pasini designed the research, enrolled patients and revised the manuscript; Giovanni Montini designed the research, enrolled patients and revised the manuscript; Giuliana Decorti analyzed data and revised the manuscript; Gabriele Stocco analyzed data, wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cuzzoni, E., Franca, R., De Iudicibus, S. et al. MIF plasma level as a possible tool to predict steroid responsiveness in children with idiopathic nephrotic syndrome. Eur J Clin Pharmacol 75, 1675–1683 (2019). https://doi.org/10.1007/s00228-019-02749-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02749-3