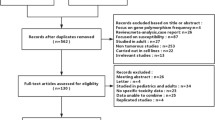
Abstract
Purpose
The most common cause of treatment failure in acute lymphoblastic leukaemia (ALL) is the relapse. Genetic polymorphisms of dihydrofolate reductase (DHFR) enzyme affect the response to methotrexate (MTX) treatment. Inter-individual variability exists in the distribution of DHFR variants, and they influence MTX treatment outcome. To the best of our knowledge, there are no genetic studies reported from India, which have explored the influence of DHFR variants on the outcome of MTX treatment. Therefore, we aim to study the influence of DHFR rs408626 (-317A>G) and rs442767 (-680C>A) variants on ALL outcome in South Indian patients.
Methods
A total of 70 ALL patients who were on MTX-based maintenance therapy were recruited for the study. DNA was extracted from leukocytes, and genotyping was done by real-time PCR.
Results
The DHFR-317GG genotype was associated with the increased risk of relapse in patients with ALL (relative risk 2.25, 95 % confidence interval (CI) 1.38 to 3.6, p = 0.02). DHFR-317AA and -680CA genotypes were found to be associated with severe leucopenia (p < 0.05). In Cox regression model, -317GG genotype was found to have lower relapse-free survival (hazard ratio (HR) 2.56, 95 % CI 1.06 to 6.19, p = 0.03) and overall survival (HR 3.72, 95 % CI 1.44 to 9.65, p = 0.007). Similarly, patients with white blood cell (WBC) count >50,000 cells/mm3 were also found to have lower relapse-free survival (HR 2.20, 95 % CI 1.10 to 4.79, p = 0.04) and overall survival (HR 3.30, 95 % CI 1.45 to 7.53, p = 0.004).
Conclusion
The GG genotype of DHFR-317A>G variant is associated with increased risk of ALL relapse and lower overall survival in South Indian population. Both variants of DHFR (-317 AA and -680 CA) are found to be associated with severe leucopenia caused by MTX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukaemia (ALL) is a haematological malignancy characterized by an uncontrolled proliferation of lymphoblasts. Accumulation of blasts hinders the production of cellular components of blood. Although it affects all age groups, it is the most frequent form of childhood cancers [1, 2]. A total number of 5730 new ALL cases were reported from the USA in 2011, and around 1420 deaths occur per year in the USA that are due to ALL [3]. Population-based data from the Indian cancer registries suggest that approximately 10,000 new cases are being diagnosed each year [4]. Since the introduction of systemic chemotherapy, cure rates of childhood ALL have improved from less than 10 % in the 1960s to 80 % today in western countries [5, 6]. Despite high cure rates, the annual number of children relapsing after initial therapy remains greater than new cases of various childhood cancers [7]. Moreover, relapsed ALL is the fourth most common childhood malignancy [8]. The relapse rate in North and South India were found to be 30 and 40 %, respectively [4, 9]. Observational and clinical studies indicate 6-mercatopurine/methotrexate (MTX) maintenance therapy to be superior to other drug combinations [10, 11]. A systematic review of 42 randomized studies with 12,000 childhood ALL cases indicated that longer maintenance therapy was associated with a slightly lower risk of relapse [11]. Data from our regional cancer registries suggest that despite following 6MP/MTX combination during maintenance therapy at Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), the percentage of relapse cases are high (40 %) during maintenance therapy even after achieving complete remission during the induction and consolidation phases of ALL treatment. MTX, an essential component of ALL treatment protocols, exerts its anticancer activity by inhibiting the enzyme dihydrofolate reductase (DHFR). In both experimental and clinical settings, altered levels of DHFR or decreased DHFR–MTX complex formation are found to be associated with relapse and MTX resistance [12–15]. Many drugs metabolizing enzymes are known to be genetically polymorphic, which leads to inter-individual variations in drug response [16, 17]. Hence, polymorphisms in genes coding for folate-metabolizing enzymes may serve as predictors of MTX response or toxicity in ALL. Among the folate-metabolizing enzymes, the polymorphisms of genes involved in DNA synthesis such as DHFR (target of MTX) and thymidylate synthase can potentially affect MTX action.
Functional/regulatory polymorphisms in the DHFR gene can affect its function or expression and subsequently can alter sensitivity to MTX [18, 19]. To the best of our knowledge, only three studies exist in the literature where the influences of DHFR promoter polymorphisms on outcome of ALL have been studied. Interestingly, the results of all these studies were contradictory to each other [18, 20, 21]. Discordant results of DHFR promoter variants could be due to a difference in the distribution of DHFR variant alleles, different treatment protocols with different doses of MTX, end points used and sample size. In the previous studies, patients across different phases of ALL treatment protocol (induction, consolidation and maintenance) were considered. There might be an effect of other drugs on the outcome which was given along with MTX during these phases. The high-dose MTX was given across different phases of ALL protocol that might confound the influence of genotype on the outcome of MTX therapy. Since the genotype effects may be more evident when drugs are given at lower doses, only patients receiving low-dose MTX orally during maintenance therapy were considered in the present study. Moreover, the end point of the previous studies was an event, which was defined as either relapse or death or induction failure. In the present study, the end point is the only relapse of patients who achieved complete remission. Besides, South Indian population represents a genetically distinct group [22]. As the difference in the distribution of alleles could confound genotype-phenotype association studies and are the primary reasons for the conflicting results, it is important to explore the influence of DHFR variants in each population. Therefore, we aimed to evaluate the association of DHFR gene variants rs408626 (-317A>G) and rs442767 (-680C>A) with ALL outcome during MTX-based maintenance therapy in South Indian patients.
Materials and methods
Study population
A prospective cohort study with a sample size of 70 patients of either gender who were on MTX-based maintenance therapy was conducted in the Department of Medical Oncology, JIPMER, from September 2010 to September 2013. We calculated the sample size to be 70 patients with ALL considering a power of 90 %, 5 % type-I error rate, a relative risk (RR) of 2.5, relapse rate of 30 % and minor allelic frequency of 10 % using power and sample size software (version 3.0, 2009). All patients were followed up from the start of maintenance therapy until the occurrence of relapse or death or till the last date of analysis (June 18th, 2015). Patients who were less than 1 year of age, pregnant women, patients with hepatic or renal dysfunction and patients with other malignancies were excluded from the study. The study was approved by the Institute Ethics Committee of JIPMER, Puducherry, India. Written informed consent was obtained from all study participants, and in the case of children, consent was obtained from their legally accepted representatives.
Treatment protocol and end points in the analysis
Patients below 25 years of age were treated using Multicentric protocol-841(MCP) I2A and above 25 years of age were treated with modified GMALL-84 protocols. MTX dose did not differ across both the protocols during maintenance therapy. All patients received 15–20 mg/m2/week dose of MTX during maintenance therapy for 2 to 3 years. Relapse was defined as the presence of >5 % of malignant blast cells in the bone marrow or on histological or cytological documentation of blasts in extramedullary sites. Relapse-free survival (RFS) was the duration between the start of maintenance therapy and the date of occurrence of relapse or last date of analysis. Overall survival (OS) was the time between the date of diagnosis and date of death or till the last date of analysis. Toxicities were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE-version 4) recommended by the National Cancer Institute [23] and included haematopoietic toxicity, as well as non-haematopoietic toxicity (hepatic and renal toxicity). Maintenance phase consisted of 6 cycles and each cycle lasted for 3 months. The highest grade of toxicity during each cycle was recorded. All toxicities recorded during the first three cycles of maintenance phase were considered as early phase toxicity.
DNA extraction and genotyping
Five millilitres of the venous blood was collected in tubes containing 100 μL of 10 % ethylenediamine tetra-acetic acid (EDTA) and centrifuged for 5 min at 2500g. Plasma was discarded and the pellets containing red blood corpuscles with the buffy coat were stored at −20 °C until DNA extraction. Genomic DNA was extracted from the peripheral leucocytes by the phenol–chloroform method. The extracted DNA was analysed qualitatively and quantitatively using biophotometer plus (Eppendorf AG 22331, Hamburg, Germany). Each DNA sample was diluted to an optimal concentration of 50 ng/μL suitable for further downstream analysis and stored in aliquots at 4 °C. Genotyping of DHFR variants was carried out by real-time PCR (7300 Applied Biosystems; Life Technologies Corporation, Carlsbad, CA, USA) using TaqMan SNP genotyping assays [rsid 408626 (-317A>G) and rsid 442767 (-680 C>A) Online source- 1] according to manufacturer instructions. The version 1.4 of 7300 sequence detection software (SDS) was used for allelic discrimination. All samples were analysed in duplicates and the results were found to be 100 % concordant.
Statistical analysis
The observed genotype frequencies were tested for Hardy–Weinberg Equilibrium (HWE) using the chi-square test. The proportions of relapse between different genotypes as well as various prognostic factors were compared using the Fisher’s exact test and the relative risk (RR) with 95 % confidence interval (CI) is presented. Comparison of early phase and late phase toxicities were done by Fisher’s exact test. The association between genotype and toxicity in each cycle of maintenance phase was tested using Fisher’s exact test. Univariate binary logistic regression was done to estimate the odds ratio (OR) and CI. Factors or variables whose p value was less than 0.25 in univariate analysis were considered for multivariate binary logistic regression analysis to estimate the percentage of variability in the clinical outcome because of genetic/non-genetic factors. Kaplan–Meier curves were plotted for RFS and OS among different genotypes and other prognostic factors of ALL. Log-rank test was used to test for statistical significance of the above parameters. Univariate Cox regression analysis was used to estimate the hazard ratio (HR) with 95 % CI; multivariate Cox regression was used to assess the impact of genotypes on clinical outcomes in the presence of other covariates. Statistical analysis of data was performed using GraphPad Instat 3 (GraphPad Software Inc., San Diego, CA, USA) and SPSS software (version 16). The level of statistical significance was set at p < 0.05.
Results
Characteristics of the ALL patients
Out of the 70 patients, 28 relapsed. There were 25 females and 45 males in the study. The age (mean ± SD) of relapsers was 14.75 ± 11.13 and that of non-relapsers was 15 ± 12.14. The demographic and clinical characteristics were not significantly different between relapse and non-relapse groups (Table 1). Patients with white blood cell (WBC) count >50,000 cells/mm3 at the time of diagnosis were at a twofold increased risk of death (RR 1.89, 95 % CI = 1.09 to 3.26, p = 0.03; Table 1).
The genotype distribution and allele frequencies of DHFR –317A>G and -680C>A variants in patients with ALL
The percentages of A and G alleles of the DHFR-317 variant were 67.1 and 32.9, respectively, in the study population. The percentages of DHFR-317A>G variant genotypes were as follows: AA = 47.1 %, AG = 40.1 %, GG = 12.8 %, and these were concordant with HWE. The percentage of C and A alleles of -680 variant were 39 and 61, respectively. The CC genotype was found to be absent in our population, and the percentages of AC and AA are 78.6 and 21.4, respectively. DHFR-680C>A variant genotype frequencies were discordant with HWE.
Association between genotype and outcome were tested in different genetic models [24] using Fisher’s exact test. The allele and genotype frequencies of DHFR-317A>G variant were significantly different between patients with and without relapse and similarly between dead and alive groups in recessive genetic model (RR = 2.25; 95 % CI = 1.38 to 3.6; p = 0.02; Table 2). DHFR-680C>A variant was not associated with either relapse or death in ALL patients (Table 2).
In a multivariate regression model that included covariates such as WBC, haemoglobin and hepatosplenomegaly, -317GG genotype remained associated with relapse (Table 3). The model with genotype and non-genetic covariates explained 11 to 21 % of the variability in relapse with DHFR-317GG genotype contributing to 11.4 % of this variability. Similarly -317A>G variant and WBC count remained significantly associated with death in the presence of other known prognostic factors of ALL (Table 3) which explained 11 to 26 % of the variability. DHFR-317GG genotype and WBC contributed 11 and 9 % to this variability, respectively.
Association of toxicity between early and late phases of maintenance therapy
A total of 64, 57, 51, 45, 42 and 40 patients were considered for the assessment of haematological toxicity in 1, 2, 3, 4, 5 and 6 cycles of maintenance therapy, respectively. We found that grade 3 and 4 haematopoietic toxicities were more in the early phase of maintenance therapy (p < 0.001, Table 4).
There was no difference in the liver toxicity between early and late phases of maintenance therapy. Renal toxicity was not observed in the studied population.
Association between DHFR variants and MTX toxicity
We compared the different grades of toxicity between genotypes in each cycle of maintenance therapy. We found that patients with DHFR-317AA genotype are at increased risk of developing grade 3 or 4 toxicity in the second cycle (a part of early phase toxicity) of maintenance phase (RR 3.09, 95 % CI 1.30 to 7.29, p = 0.005). Similarly DHFR-680AC genotype was also associated with grade 3 or 4 toxicity in cycle 2 of maintenance treatment (Table 5).
Association of DHFR-317A>G polymorphism with RFS and OS in patients with ALL
Patients with homozygous mutant genotype (GG) had a median RFS time and OS time of 14 and 24 months, respectively. The RFS (HR = 2.56, 95 % CI = 1.08 to 6.08, p = 0.02) and OS (HR = 2.51, 95 % CI = 1.06 to 5.95, p = 0.02) were found to be lower in patients with GG genotype (Fig. 1a, b).
Kaplan–Meier curves showing RFS and OS for patients with ALL according to DHFR variants (-317A>G and -680C>A) and WBC count. a Association of DHFR-317A>G variant with RFS. b Association of DHFR-317A>G variant with OS. c Association of DHFR-680C>A variant with RFS. d Association of DHFR-680C>A variant with OS. e Association of WBC count with RFS. f Association of WBC count with OS. The number of patients in each curve representing the patients with indicated variable, number of individuals with an event (in the parenthesis), as well as the p value, estimated by log-rank test for the survival differences between the patients’ groups, are indicated in the plot
There was no significant difference in RFS and OS time between -680AA carriers and CC carriers (Fig. 1c, d, p = 0.65). Patients who had WBC count of >50,000 cells/mm3 at the time of diagnosis had a lower RFS (Fig. 1e, HR = 2.35, CI = 1.09 to 5.0, p = 0.02) and OS (Fig. 1f, HR = 2.5, CI = 1.22 to 5.46, p = 0.02). Genotype and WBC retained their significance in co-regression, when other prognostic factors were taken into account (Table 6).
Discussion
To the best of our knowledge, this is the first study from India to provide an insight into the influence of DHFR promoter polymorphisms (-317A>G and -680C>A) on outcome of MTX-based maintenance therapy in a South Indian population. DHFR plays a crucial role in folate metabolism, and it is an important target of MTX. Mutations in the DHFR gene were found to influence susceptibility to various diseases and drug response [19, 25–29]. There is a paucity of the literature on the influence of DHFR promoter polymorphisms on ALL outcome. An unpublished data from our lab showed that frequencies of DHFR-317A>G and -680C>A polymorphisms were comparable to those of South Indian ALL patients. The absence of CC genotype of -680C>A variant resulted in discordant Hardy–Weinberg equilibrium (DHWE). Population stratification as a cause of discordance is unlikely in our subjects given the high prevalence of endogamy [30].
In the present study, we found that DHFR-317GG genotype confers two fold increased risk of ALL relapse (p = 0.02, RR = 2.25, 95 % CI = 1.38 to 3.6; Table 2.). Our study results are in agreement with the Gomez-Gomez et al. study on Mexican ALL patients (N = 70; OR = 8.55, 95 % CI 1.84–39.70; p = 0.006) [20] but contradictory to Dulucq et al. study where -317 polymorphism was not associated with either relapse or death [18]. DHFR-680C>A had no influence either on relapse or death of patients with ALL, similar to the findings of Dulucq et al. [18]. We hypothesize that patients with GG genotype had altered levels of DHFR expression that might have affected the response to MTX treatment. Transcription factor (TF) binding sites for growth factor independence 1 zinc finger protein (Gfi-1b) was predicted in the region spanning rs 408626 (DHFR-317A>G) using MatInspector, Genomatix, Munich, Germany, http://www.genomatix.d [31]. Gfi-1b is abundant in blood cells and bone marrow tissues [32] and is an important regulator of blood cell production [33–36]. Therefore, Gfi-1b may play a major role in haematological malignancies. However, the impact of the DHFR-317A>G SNPs on the binding pattern of Gfi-1b has not been studied. Further functional studies must be conducted to investigate the binding pattern and its relation to the expression of DHFR.
Age, gender and WBC count were the known prognostic factors of ALL in the western population, but it may vary across developing countries due to various reasons [4].
Patients with ≤9 years of age are known to have a better prognosis than patients who are above 9 years [37]. But in the present study, we did not find any difference between these two groups similar to other studies conducted in India [4, 38]. Initially, we thought this might be due to the inclusion of patients who are older than >24 years of age (13 patients were treated with G-MALL protocol). Hence, we reanalysed the data excluding them from the analysis even then we did not find any association between the groups (Online resource 2, p = 0.59). In the present study, the number of males were almost twice that of females similar to the other study from India [38] but in contrast to the figures reported from developed countries [39, 40]. This might be due to the neglect of female children in India and probably the special treatment of male siblings. It has been reported in various studies [24, 26] that females have better survival than males of the same age with ALL. However, we did not observe such a gender-based outcome (p > 0.05) (Table 3), similar to Dulucq et al., Kim et al. and Dervieux et al. [18, 41]. This could be due to the small sample size of the female group (n = 25/70) in our study. In univariate analysis, the presence of WBC count >50,000 cells/mm3 showed a trend towards significance in conferring a possible risk of relapse (p = 0.09, OR 2.4, 95 % CI 0.85 to 6.73). Failure to attain significance may be due to the exclusion of patients with advanced stage of the disease (induction and consolidation failures) and also the small sample size of our study. In univariate logistic regression analysis, we found that -317GG genotype is associated with both relapse and death (OR = 6.7, CI = 1.27 to 34.9, p = 0.02). WBC count was found to be associated with death (OR = 3.17, 95 % CI = 1.1 to 9, p = 0.03) which is in accordance with Dulucq et al. study [18]. DHFR-680C>A did not have any effect on the ALL outcome similar to the other studies [18, 21]. Multiple logistic regression analysis showed that -317GG genotype contributes to 11 % of the variability in relapse compared with non-genetic factors alone (9 %). WBC count and -317GG genotype were found to be associated with death contributing to 11 and 9 % of the variability, respectively. WBC and genotype together contributed to 21 % of the variability in death. Owing to the technical constraints and similar diet habits of the population residing in the area of sample collection, we could not include folate levels as a covariate in the multivariate analysis.
We observed that grade 3 or 4 haematological toxicity was more in the early phase of maintenance therapy than in the later phase. This could be due to the adjustment of MTX dose in the early phase, leading to reduced toxicity in the later phase of maintenance therapy. When we tested the association of genotypes with toxicity in each cycle of maintenance, -317AA and -680CA carriers were found to have severe leucopenia (grade 3 or 4) compared to other genotype carriers in the second oral cycle of maintenance therapy.
A study conducted by Salazar et al. on ALL patients who were on consolidation treatment reported that -317A>G and 19-bp ins/del polymorphisms in DHFR gene were in linkage disequilibrium (r 2 = 0.956). Allele A of -317 variant contained an insertion while G residue contained a deletion in the 19-bp ins/del polymorphism. It was reported that patients with 19-bp ins/ins, who were also the carrier of -317AA genotype, experienced severe thrombocytopenia (HR 4.7, 95 % CI 1.8 to 12.6, p = 0.002). The same study reported that CC genotype of -680C>A was associated with neutropenia (HR 2.9, 95 % CI 1.3 to 7.1, p = 0.01) [21]. In contrast to our results, Dulucq et al. did not find any association between -317A>G and -680C>A variants of DHFR and toxicity in ALL [18]. This discrepancy could be due to the use of different doses of MTX across induction, consolidation (high-dose MTX) and maintenance phases (low-dose MTX) of ALL treatment. Interestingly, in the present study, the occurrence of relapse was found to be more in the third cycle of maintenance therapy. Intermittent discontinuation of chemotherapy or the repetitive adjustment of dose during the second cycle might have reduced the efficacy of MTX, resulting in higher relapse during the third cycle of maintenance. A study has shown that the degree of myelosuppression correlates with relapse risk [10]. Therefore, it would be better to adjust the dose of MTX based on its levels in the body rather than absolute neutrophil count.
RFS and OS were lower in patients with WBC count >50,000 cells/mm3 at the time of diagnosis (p < 0.05), similar to other studies [18, 20, 42, 43]. In co-regression model, -317GG genotype was associated with lower RFS and OS which is discordant with Dulucq et al. and Salazar et al. study reports. Dulucq et al. study reported AA genotype remained significant with event-free survival (EFS) (event is either relapse or death or induction failure) in multivariate regression model [18] whereas the later study did not find any association between -317A>G variant EFS [21]. WBC count was also significantly associated with lower RFS and OS in regression model similar to Dulucq et al. study [18] but dissimilar to Salazar et al. findings [21].
In spite of the small sample size, the present study is sufficiently powered to show a statistically significant difference in the risk of relapse (statistical power of the risk of association >85 %). However, a large multicentric study needs to be carried out to further strengthen our study findings.
Conclusion and future directions
The GG genotype of DHFR-317A>G variant is associated with increased risk of ALL relapse and lower overall survival in South Indian population. Both variants of DHFR (-317AA and -680CA) are found to be associated with severe leucopenia caused by MTX. The results of the present study could further be strengthened by measuring the MTX levels and DHFR mRNA levels to establish their role in predicting the outcome of MTX treatment. In addition, screening for other SNPs in DHFR gene will help us in understanding the complex genetic interactions and in identifying the risk haplotypes.
References
Pui C-H, Robison LL, Look AT (2008) Acute lymphoblastic leukaemia. Lancet 371:1030–1043. doi:10.1016/S0140-6736(08)60457-2
Hunger SP, Lu X, Devidas M, et al. (2012) Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 30:1663–1669. doi:10.1200/JCO.2011.37.8018
Cancer Facts & Figures 2011 - acspc-029771.pdf [Internet]. [cited 2015 July 14]. Available from: http://www.cancer.org/acs/groups/content@epidemiologysurveilance/documents/document/acspc-029771.pdf
Magrath I, Shanta V, Advani S, et al. (2005) Treatment of acute lymphoblastic leukaemia in countries with limited resources; lessons from use of a single protocol in India over a twenty year period [corrected]. Eur J Cancer 41:1570–1583. doi:10.1016/j.ejca.2004.11.004
Gaynon PS (2005) Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol 131:579–587. doi:10.1111/j.1365-2141.2005.05773.x
Eden T (2002) Translation of cure for acute lymphoblastic leukaemia to all children. Br J Haematol 118:945–951
Chessells JM, Veys P, Kempski H, et al. (2003) Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol 123:396–405
Locatelli F, Schrappe M, Bernardo ME, Rutella S (2012) How I treat relapsed childhood acute lymphoblastic leukemia. Blood 120:2807–2816. doi:10.1182/blood-2012-02-265884
Rajalekshmy KR, Abitha AR, Anuratha N, Sagar TG (2011) Time trend in frequency of occurrence of major immunophenotypes in paediatric acute lymphoblastic leukemia cases as experienced by Cancer Institute, Chennai, south India during the period 1989-2009. Indian J Cancer 48:310–315. doi:10.4103/0019-509X.84932
Schmiegelow K, Heyman M, Gustafsson G, et al. (2010) The degree of myelosuppression during maintenance therapy of adolescents with B-lineage intermediate risk acute lymphoblastic leukemia predicts risk of relapse. Leukemia 24:715–720. doi:10.1038/leu.2009.303
Schmiegelow K, Heyman M, Kristinsson J, et al. (2009) Oral methotrexate/6-mercaptopurine may be superior to a multidrug LSA2L2 maintenance therapy for higher risk childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. J Pediatr Hematol Oncol 31:385–392. doi:10.1097/MPH.0b013e3181a6e171
Matherly LH, Taub JW, Ravindranath Y, et al. (1995) Elevated dihydrofolate reductase and impaired methotrexate transport as elements in methotrexate resistance in childhood acute lymphoblastic leukemia. Blood 85:500–509
Serra M, Reverter-Branchat G, Maurici D, et al. (2004) Analysis of dihydrofolate reductase and reduced folate carrier gene status in relation to methotrexate resistance in osteosarcoma cells. Ann Oncol 15:151–160
Göker E, Waltham M, Kheradpour A, et al. (1995) Amplification of the dihydrofolate reductase gene is a mechanism of acquired resistance to methotrexate in patients with acute lymphoblastic leukemia and is correlated with p53 gene mutations. Blood 86:677–684
Galbiatti ALS, Castro R, Caldas HC, et al. (2013) Alterations in the expression pattern of MTHFR, DHFR, TYMS, and SLC19A1 genes after treatment of laryngeal cancer cells with high and low doses of methotrexate. Tumour Biol 34:3765–3771. doi:10.1007/s13277-013-0960-3
Relling MV, Dervieux T (2001) Pharmacogenetics and cancer therapy. Nat Rev Cancer 1:99–108. doi:10.1038/35101056
Cheok MH, Evans WE (2006) Acute lymphoblastic leukaemia: a model for the pharmacogenomics of cancer therapy. Nat Rev Cancer 6:117–129. doi:10.1038/nrc1800
Dulucq S, St-Onge G, Gagné V, et al. (2008) DNA variants in the dihydrofolate reductase gene and outcome in childhood ALL. Blood 111:3692–3700. doi:10.1182/blood-2007-09-110593
Al-Shakfa F, Dulucq S, Brukner I, et al. (2009) DNA variants in region for noncoding interfering transcript of dihydrofolate reductase gene and outcome in childhood acute lymphoblastic leukemia. Clin Cancer Res 15:6931–6938. doi:10.1158/1078-0432.CCR-09-0641
Gómez-Gómez Y, Organista-Nava J, Saavedra-Herrera MV, et al. (2012) Survival and risk of relapse of acute lymphoblastic leukemia in a Mexican population is affected by dihydrofolate reductase gene polymorphisms. Exp Ther Med 3:665–672. doi:10.3892/etm.2012.447
Salazar J, Altés A, del Río E, et al. (2012) Methotrexate consolidation treatment according to pharmacogenetics of MTHFR ameliorates event-free survival in childhood acute lymphoblastic leukaemia. Pharmacogenomics J 12:379–385. doi:10.1038/tpj.2011.25
Tamang R, Singh L, Thangaraj K (2012) Complex genetic origin of Indian populations and its implications. J Biosci 37:911–919
CTCAE_4.03_2010–06-14_QuickReference_5x7.pdf.
Lewis CM (2002) Genetic association studies: design, analysis and interpretation. Brief Bioinformatics 3:146–153
Wang B, Liu M, Yan W, et al. (2013) Association of SNPs in genes involved in folate metabolism with the risk of congenital heart disease. J Matern Fetal Neonatal Med 26:1768–1777. doi:10.3109/14767058.2013.799648
Martinelli M, Girardi A, Cura F, et al. (2014) Evidence of the involvement of the DHFR gene in nonsyndromic cleft lip with or without cleft palate. Eur J Med Genet 57:1–4. doi:10.1016/j.ejmg.2013.12.002
Orjuela MA, Cabrera-Muñoz L, Paul L, et al. (2012) Risk of retinoblastoma is associated with a maternal polymorphism in dihydrofolatereductase (DHFR) and prenatal folic acid intake. Cancer 118:5912–5919. doi:10.1002/cncr.27621
Owen SA, Hider SL, Martin P, et al. (2013) Genetic polymorphisms in key methotrexate pathway genes are associated with response to treatment in rheumatoid arthritis patients. Pharmacogenomics J 13:227–234. doi:10.1038/tpj.2012.7
Sharma S, Das M, Kumar A, et al. (2009) Purine biosynthetic pathway genes and methotrexate response in rheumatoid arthritis patients among north Indians. Pharmacogenet Genomics 19:823–828
Sebro R, Lange C, Laird NM, et al. (2012) Differentiating population stratification from genotyping error using family data. Ann Hum Genet 76:42–52. doi:10.1111/j.1469-1809.2011.00689.x
Cartharius K, Frech K, Grote K, et al. (2005) MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933–2942. doi:10.1093/bioinformatics/bti473
Elmaagacli AH, Koldehoff M, Zakrzewski JL, et al. (2007) Growth factor-independent 1B gene (GFI1B) is overexpressed in erythropoietic and megakaryocytic malignancies and increases their proliferation rate. Br J Haematol 136:212–219. doi:10.1111/j.1365-2141.2006.06407.x
Garçon L, Lacout C, Svinartchouk F, et al. (2005) Gfi-1B plays a critical role in terminal differentiation of normal and transformed erythroid progenitor cells. Blood 105:1448–1455. doi:10.1182/blood-2003-11-4068
Saleque S, Cameron S, Orkin SH (2002) The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages. Genes Dev 16:301–306. doi:10.1101/gad.959102
Randrianarison-Huetz V, Laurent B, Bardet V, et al. (2010) Gfi-1B controls human erythroid and megakaryocytic differentiation by regulating TGF-beta signaling at the bipotent erythro-megakaryocytic progenitor stage. Blood 115:2784–2795. doi:10.1182/blood-2009-09-241752
Laurent B, Randrianarison-Huetz V, Frisan E, et al. (2012) A short Gfi-1B isoform controls erythroid differentiation by recruiting the LSD1-CoREST complex through the dimethylation of its SNAG domain. J Cell Sci 125:993–1002. doi:10.1242/jcs.095877
Möricke A, Zimmermann M, Reiter A, et al. (2005) Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. Klin Padiatr 217:310–320. doi:10.1055/s-2005-872515
Arya LS, Kotikanyadanam SP, Bhargava M, et al. (2010) Pattern of relapse in childhood ALL: challenges and lessons from a uniform treatment protocol. J Pediatr Hematol Oncol 32:370–375. doi:10.1097/MPH.0b013e3181d7ae0d
Wojtuszkiewicz A, Peters GJ, van Woerden NL, et al. (2015) Methotrexate resistance in relation to treatment outcome in childhood acute lymphoblastic leukemia. J Hematol Oncol 8:61. doi:10.1186/s13045-015-0158-9
Radtke S, Zolk O, Renner B, et al. (2013) Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood 121:5145–5153. doi:10.1182/blood-2013-01-480335
Dervieux T, Greenstein N, Kremer J (2006) Pharmacogenomic and metabolic biomarkers in the folate pathway and their association with methotrexate effects during dosage escalation in rheumatoid arthritis. Arthritis Rheum 54:3095–3103. doi:10.1002/art.22129
Yanada M, Jinnai I, Takeuchi J, et al. (2007) Clinical features and outcome of T-lineage acute lymphoblastic leukemia in adults: a low initial white blood cell count, as well as a high count predict decreased survival rates. Leuk Res 31:907–914. doi:10.1016/j.leukres.2006.08.004
Rujkijyanont P, Kaewinsang S, Monsereenusorn C, Traivaree C (2014) Pediatric acute leukemia: the effect of prognostic factors on clinical outcomes at Phramongkutklao Hospital, Bangkok, Thailand. J Med Assoc Thai 97(Suppl 2):S188–S195
Acknowledgments
We thank the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India, for providing intramural fund to conduct the study. We also thank Mr. Ravi Prasad for assisting us in the laboratory work.
Conflict of interest
The authors declare that they have no competing interests.
Compliance with Ethical Standards
All procedures performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The study was supported by an intramural grant from JIPMER.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Online source 1
(DOCX 11 kb)
Online source 2
(DOCX 10 kb)
Rights and permissions
About this article
Cite this article
Kodidela, S., Pradhan, S.C., Dubashi, B. et al. Influence of dihydrofolate reductase gene polymorphisms rs408626 (-317A>G) and rs442767 (-680C>A) on the outcome of methotrexate-based maintenance therapy in South Indian patients with acute lymphoblastic leukemia. Eur J Clin Pharmacol 71, 1349–1358 (2015). https://doi.org/10.1007/s00228-015-1930-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1930-z