Abstract
Background
Acute lymphoblastic leukemia (ALL) is one of the major malignancies affecting children in Jordan. Methotrexate (MTX) is the cornerstone of chemotherapy for ALL, and works by targeting enzymes involved in the folate pathway. We hypothesize that genetic polymorphisms of the folate pathway are associated with MTX toxicity in children with ALL.
Methods
A total of 64 children with ALL were included in this study; 31 (48.4%) boys and 33 (51.6%) girls aged 2–16 years. The folate pathway genes were genotyped using polymerase chain reaction followed by sequencing and studying the association between genetic polymorphisms and MTX toxicity.
Results
The immunophenotype was B-lineage in 55 patients (85.9%) and T-lineage in nine patients (14.1%). All genetic polymorphisms, except for dihydropyrimidine dehydrogenase polymorphisms, were associated with hematological toxicities and did not appear to precipitate any non-hematological adverse events. Patients with ALL carrying dominant alleles of methylene tetrahydrofolate (MTHFR) C677T and dihydrofolate reductase 19 bp deletion were at a higher risk of developing severe leucopenia [OR (95% CI) = 4.5 (1.2–17), p = 0.03; 5.4 (1.6–17.8); p = 0.006] while minor allele carriers of MTHFR A1298C were more likely to develop neutropenia [OR (95% CI) = 6.1 (1.3–29.5); 0.04]. Furthermore, dominant allele carriers of thymidylate synthase 1494 del6 were at a higher risk of developing neutropenia [OR (95% CI) = 6 (1.2–31.1); p = 0.04].
Conclusion
Genetic polymorphisms of the folate pathway may modulate MTX-induced toxicity in childhood ALL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukemia (ALL) is the eleventh most common cancer worldwide. In Jordan, ALL is the most common cancer in childhood accounting for almost 30% of childhood cancers [1, 2]. Treatment of ALL usually consists of 3 phases: (a) induction phase, which usually includes glucocorticoids, vincristine, l-asparaginase and anthracyclines; (b) consolidation phase, which lasts for 8 weeks, where high doses of methotrexate (MTX) along with 6-mercaptopurine (6-MP) are given, and (c) maintenance phase, which lasts for 2–3 years [3]. In recent years, cure rates of ALL have increased to approximately 80% due to the use of multi-chemotherapeutic agents regimen, However, treatment-related toxicities still present a challenge for clinicians and may lead to interruption or discontinuation of treatment [4, 5]. One of the chemotherapeutic agents used that leads to treatment-related toxicity which in some cases could be life-threatening is MTX [6].
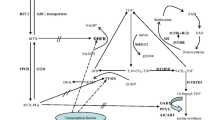
MTX is an anti-metabolite, specifically, a folate antagonist. Its direct target is dihydrofolate reductase (DHFR) enzyme which is responsible for the conversion of dihydrofolate into tetrahydrofolate that plays a crucial role in cell growth and cellular metabolism [7]. DHFR enzyme is encoded by the DHFR gene that is located on chromosome no. 5 and spans 30 kb, inhibition of DHFR by MTX raises plasma homocysteine levels by 50%, which reflects a low folate status, this is particularly observed in patients with 19 bp deletion in intron 1 of DHFR [8].
Inhibition of DHFR also affects methylene tetrahydrofolate reductase enzyme (MTHFR) which converts 5,10 methylene tetrahydrofolate into 5 methylene tetrahydrofolate; the latter being the main circulatory form of folate which acts as a co-substrate for the conversion of homocysteine to methionine. Afterwards, methionine is converted to S-adenosylmethionine which serves as a methyl donor for many biological compounds such as nucleic acids and proteins [9]. More commonly studied polymorphisms of MTHFR gene are C677T and A1298C which are both known missense polymorphisms associated with decreased enzyme activity and have been linked to MTX toxicity not only in ALL but also in rheumatoid arthritis, osteosarcoma, and lymphoma [10,11,12]. C677T transition results in substitution of alanine to valine while A1298C transversion leads to the substitution of alanine to glutamate [13, 14].
Thymidylate synthase enzyme is another target of the anti-folate, MTX. It is responsible for the initial step in DNA synthesis, in which deoxythymidine monophosphate, a precursor needed for DNA synthesis, is produced from deoxyuridine monophosphate, resulting in the oxidation of methylene tetrahydrofolate to dihydrofolate [15]. This enzyme is coded by the gene thymidylate synthase (TYMS), located on chromosome 18p11.32 and is composed of six introns with sizes ranging between 507 and 6271 bp, and seven exons with sizes ranging between 72 and 250 bp [16]. One of the polymorphisms seen in TYMS is the 6-bp deletion at nucleotide 1494 of the TYMS mRNA which has been associated with decreased mRNA stability in vitro and lower TYMS expression in vivo [17]. Another gene involved in the folate pathway is the dihydropyrimidine dehydrogenase (DPYD) which is located on the short arm of chromosome number one (1p22), and is known to be highly polymorphic with approximately 40 different polymorphisms, of these, are T85C and T496C [18]. DPYD T496C is a missense mutation, in which valine substitutes methionine in the protein product at position 166, while in DPYD T85C cysteine is substituted for arginine [19].
As multiple genes affect MTX treatment outcomes in patients with ALL, the aim of this study was to assess the relationship between folate pathway gene polymorphisms and high-dose MTX (HDMTX) toxicity in children with ALL.
Materials and methods
Study population
This is a cross-sectional observational study with toxicity data of 50 patients collected retrospectively while toxicity data of the remaining 14 patients were collected prospectively over a period of 8 weeks of consolidation. It involved 64 children with ALL recruited from the Royal Medical Services (RMS)/Amman that were diagnosed between the year 2013 and 2017 and treated according to modified St. Jude total XIII protocol [20]. Patients were eligible for recruitment if they fulfilled the following criteria: (1) younger than 18 years, (2) diagnosed with ALL as their primary disease by an oncologist, and (3) lacking other malignancies. Patients were recruited between March, 2017 and December, 2017. The research was approved by the ethics committee in RMS (institutional review board, TF3/1/IRB/1762 on February 14th, 2017) in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration (IRB no. 1762, 14/2/2017). Informed consent was obtained from all participants and their parents or legal guardians before inclusion into the study.
Treatment plan
Patients were stratified according to the modified St. Jude protocol into two groups: low and high risk, where patients in the high-risk group received 5 g/m2 MTX and patients in the low-risk group received 2.5 g/m2 during the consolidation phase. The consolidation phase consisted of 8 weeks; during this period MTX was given 4 times with 2-week intervals in addition to daily doses of 6-MP 50 mg/m2.
Data collection
All patients’ guardians in this study were interviewed, and a detailed data collection sheet was filled. Information gathered from the patients or their caregivers included age, gender, clinical history of the patient and his or her family history. The date of starting induction, consolidation, and maintenance treatments, immunologic subtype, risk classification, the total dose of MTX, body weight, and body surface area (BSA) were collected from the patients’ medical files without further confirmation.
At the beginning and after each of the four cycles of MTX therapy, the following parameters were collected when available: total dose of MTX, measured MTX concentrations at 48 h after MTX infusion, kidney function test, including; blood urea nitrogen (BUN) and serum creatinine (Scr), liver function test, including transaminase enzymes, and blood test, including WBCs, absolute neutrophil count (ANC), platelets, and hemoglobin values. Other toxicity parameters such as a delay in MTX cycle, incidence of infections, and number of “packed RBCs given” were also recorded.
Genotyping
Three milliliters of peripheral venous blood were collected from patients in K3EDTA-coated tubes and DNA extraction was performed by Promega Wizard® DNA purification kit (Promega Corporation, USA) according to manufacturer’s instructions. Polymerase chain reaction (PCR) technique was conducted using a thermal cycler (Bio-Rad®, USA).
DHFR 19 bp deletion, MTHFR C677T, MTHFR A1298C, DPYD T496C, DPYD T85C and TYMS 1494del6 polymorphisms were analyzed using PCR amplification followed by sequencing [21]. Primers used in the amplification process are shown in Table 1. All primers were synthesized by Princess Haya Biotechnology Center (Irbid, Jordan). Sequences were analyzed using the sequencing analysis software (Chromas Lite, version 2.1.1).
Statistical analysis
Data were analyzed using SPSS software version 22 (SPSS® Inc, Chicago, USA). Descriptive data were expressed as count and percentage for categorical variables or as mean ± SD for continuous data. Lowest values of hematological toxicities during the consolidation phase were graded according to the National Cancer Institute criteria version 3 [22]. Presence of hepatic toxicity was considered significant when aspartate transaminase (AST) or alanine transaminase (ALT) levels were three times the upper normal limit. Published research represents toxicities related to MTX utilizing different methods, and we will adopt two of these methods. First method used is the worst toxicity during the four MTX cycles [23], whilst the second one is total MTX courses taken by the patients [24]. Discrete variables were compared using Chi-square or Fisher exact tests as appropriate. Common odds ratios (OR) and 95% confidence intervals (95% CI) were calculated as a measurement of association among discrete variables. All tests were two-tailed and p value < 0.05 was considered to be statistically significant.
Haplotype analysis
The interaction between genetic polymorphisms at the two loci was assessed by evaluating the combined genotype effects and haplotype analysis. Haplotype frequencies were calculated using Multiallelic Interallelic Disequilibrium Analysis Software [(MIDAS®), University of Southampton, Highfield, Southampton, UK] [25] and linkage disequilibrium was represented by Lewontin’s coefficient (D′).
Results
Patient demographics
This study included 64 children with ALL aged 2–16 years (mean 7.7 ± 4 years); 31 boys and 33 girls. The most common immunophenotype was B-lineage (N = 55, 85.9%); however, few carried the T-lineage (N = 9, 14.1%). Most of the T-lineage carriers were observed in boys (N = 8, 89%). Patients’ characteristics and treatment related data are summarized in Table 2. We analyzed the distribution of genotypes according to different patient characteristics (gender, age, risk status) to ensure internal validity and there was no significant association between the studied polymorphisms and patients’ demographics (Supplementary Tables 1, 2, 3).
MTX-related toxicities
Hematologic toxicities were manifested the most, as 40 (62.5%) patients developed grade 3–4 neutropenia and 55 (85.9%) were neutropenic. Similarly, all patients had leucopenia with 20 (31.3%) patients had grade 3 /4. A fewer number of patients developed grade 3–4 thrombocytopenia [7 (11.1%)], and grade 3 anemia [5 (8.1%)]. Regarding non-hematologic toxicities, 22 (40%) and 11 (19%) patients had AST and ALT levels three times higher than the upper normal limit, respectively. Data reflecting renal toxicity (Scr and BUN) were also collected but no reading was above normal values.
Genotype, allele, diplotype and haplotype frequencies
Genotype and allele frequencies are described in Table 3. The minor allele frequencies (MAF) for DHFR 19 bp deletion, MTHFR C677T, MTHFR A1298C, DPYD T85C, DPYD T496C and TYMS, were 0.336, 0.414, 0.313, 0.198, 0.103, and 0.46, respectively. The most common genotype in MTHFR C677T, MTHFR A1298C and TYMS 1494del6 was heterozygous [N = 29 (45.3%), N = 30 (46.9%), and 29 (45.3%), respectively]. On the other hand, the most common genotype in DHFR 19 bp deletion, DPYD T85C, DPYD T496C and TYMS 1494del6 was homozygous dominant [N = 32 (50%), N = 43 (68.3%) and N = 50 (79.4%), respectively].
The most common MTHFR diplotype was CT/AC [N = 19 (29.7%)] followed by TT/AA [N = 11 (17.2%)] while the least common MTHFR diplotype was TT/CC [N = 0 (0%)] followed by TT/AC and CT/CC with equal percentages (N = 1; 1.6%). The diplotype TT/CC was not encountered in this study. There was a strong linkage disequilibrium (D′ = 0.8, p = 0.0003) between C677T and A1298C, where the distance between the two single nucleotide polymorphisms (SNPs) is 1092 bp. All four haplotypes were observed, with T_A haplotype having the highest frequency (N = 50, 39%) and T_C haplotype having the lowest frequency (N = 3, 2.3%), which is consistent with the diplotype analysis. MTHFR haplotype frequencies are summarized in Table 4.
All four haplotypes of DPYD were observed, with T_T haplotype having the highest frequency (N = 95; 75.4%) and the C_C haplotype having the lowest frequency (N = 6; 4.8%). Relatively moderate strength linkage disequilibrium was observed (D′ = 0.37, p = 0.04). It should be noted that the distance between the two DPYD SNPs is 183,794 bp, still there was significant linkage disequilibrium. There were only 6 diplotypes of DPYD because of the lack of a homozygous minor genotype of DPYD T496C. The most common DPYD diplotype was TT/TT (N = 38; 59.4%) and the least common diplotype was CC/TT (N = 2; 3.2%) and there was no CC/CC (0; 0%). Diplotype analysis was consistent with haplotype data. DPYD haplotypes are summarized in Table 5.
Association of polymorphisms with MTX toxicity
Analysis of the effect of genetic polymorphisms on different MTX toxicities during the consolidation phase showed that a significant association exists between DHFR 19 bp deletion, MTHFR C677T and leucopenia (Table 6). Carriers of the homozygous dominant genotype of both genes are at an increased risk of developing the more serious grades of leucopenia. Carriers of insertion/insertion (Ins/Ins) genotype of DHFR are 5.4 times more likely to develop grade 3 and grade 4 leucopenia compared to minor allele carriers [Ins/Ins: 50% vs. Ins/Del + Del/Del: 15.6%; OR (95% CI) = 5.4 (1.6–17.8); p = 0.006] while carriers of one C allele of MTHFR C677T are 4.5 times more likely to develop grade 2 and higher leucopenia compared to TT carriers [CC + CT: 84% vs.TT: 53.8%; OR (95% CI) = 4.5 (1.2–17); p = 0.03] (Table 6). Regarding MTHFR A1298C, carriers of the minor allele C are at an increased risk of developing neutropenia [AC + CC: 97% vs. AA: 78.6%, OR (95% CI) = 6.1 (1.3–29.5); p = 0.04]. Furthermore, carriers of the deletion allele of TYMS 1494del6 are at risk of developing neutropenia compared to the insertion genotype carriers [Del/Del + Del/Ins: 94% vs. Ins/Ins: 71.4%, respectively. OR (95% CI) = 6 (1.2–31.1); p = 0.04].
When the results were analyzed in light of “total MTX courses” carriers of the dominant allele and dominant genotype of MTHFR C677T needed more frequent MTX cycles and showed grade 3 and grade 4 neutropenia [CC + CT: 41.6% vs. TT: 21.4%; OR (95% CI) = 2.6 (1–6.8); p = 0.054 and CC: 43.3% vs. TT: 21.4%; OR (95%): 2.5 (1.01–6.5); p = 0.046]. This observation comes in line with the findings of the first method in assessing toxicity. Similarly, carriers of the MTHFR 1298A dominant allele are at a higher risk of leucopenia [AA: 15 (21.4%) vs. AC: 8 (9.3%); OR (95% CI): 2.7 (1.05–6.7); p = 0.042 and AA vs. AC + CC: 11 (11.3%); OR (95% CI): 2.1 (0.91–5.0); p = 0.076].
Furthermore, a significant association was found between carriers of DHFR 19 bp insertion/insertion genotype and grade 3 and higher leucopenia compared to patients carrying the minor allele [Ins/Ins: 21.2% vs. Ins/Del + Del/Del: 10.3%; OR (95% CI): 2.3 (0.98–5.6); p = 0.052]. Additionally, there was a significant association between TYMS 1494 deletion allele carriers and the worst degrees of leucopenia as they were 2.8 times more likely to develop grade 3 and 4 leucopenia compared to the minor genotype carriers [Ins/Del + Del/Del: 24 (18.6%) vs. Ins/Ins = 2 (5%); OR (95% CI) = 2.8 (1.1–7.5); p = 0.044].
On the other hand, no significant association was found between DPYD genetic polymorphisms and MTX toxicities irrespective of assessment methods. Moreover, no significant association was found between any of the studied genetic polymorphisms and non-hematologic toxicities (hepatic, renal, incidence of infections, MTX level > 1 µl and delay in MTX cycle). Data on association of (MTHFR C677T and A1298C), (DHFR 19 bp deletion), (DPYD T85C and T496C), and (TYMS 1494del6) with severe (grade3/grade4) toxicity are detailed in Supplementary Tables 4–7.
Discussion
In this study, we evaluated the influence of genetic polymorphisms in the folate genes on HDMTX toxicity in children with ALL. Study findings showed that out of all the toxicities studied, hematologic toxicities were affected the most. All patients suffered from leucopenia and 85.9% had neutropenia indicating that WBCs are affected the most among all hematologic toxicities. The least observed hematologic toxicity was anemia, with 5 (8.1%) patients having grade 3 anemia. The severity of myelosuppression is affected by the life span of cells. Because RBCs survive 120 days, clinically significant anemia is least expected. Platelets survive around 10 days, and granulocytes survive approximately 6–8 h [26]. This explains the variability in severity of the observed hematologic toxicities. Similar trends were observed previously [23, 24]. Regarding genetic polymorphisms, we found that the MTHFR 677C>T polymorphism was associated with leucopenia. Specifically, ALL patients harboring the dominant allele exhibited a substantial increase in the incidence of worst degree leucopenia. This was consistent with the “total MTX courses” method, as carriers of the dominant allele were at an increased risk of neutropenia compared to TT carriers. As for MTHFR A1298C, we found an association between this polymorphism and neutropenia as well as leucopenia. This was the opposite with respect to MTHFR C677T. This can be explained by the strong linkage disequilibrium existing between the minor 677T allele and the dominant1298A allele (39%) (Table 4). Dominant alleles with genes involved in the folate pathway were associated with the worst outcome of adverse drug reactions (leucopenia and neutropenia), in contrast with a number of studies where the minor allele was the one associated [11, 23, 27,28,29,30,31]. On the other hand, similar trends were reported by four studies in German, Uruguayan, Canadian and Mexican children with ALL. In Germany, 34 children with ALL were included in a study that had interesting findings: the major genotype of C677T was associated with an increased risk of developing grade 3 and 4 leucopenia. Also, it was concluded that carriers of 1298CC had an increased incidence of anemia [24]. Similarly, 41 Uruguayan patients were studied, where carriers of the 677TT genotype had a protective effect against hematologic toxicities decreasing the risk by four times [32]. Additionally, the Canadian study showed that carriers of the minor allele of MTHFR C677T had lower rates of grade 3 leucopenia [33]. The Mexican studied the effect of MTHFR on the outcome of ALL in 109 patients and concluded that carriers of the dominant genotype 677CC had three times higher risk of developing mucositis compared to minor allele 677T [34].
The conflicting results reported to date are likely due to differences in MTX dosing, dose adjustments in the case of toxicity, and schedules. Recruited patients in different studies were treated by different protocols (St Jude’s Children Research Hospital protocols and their modifications [23, 27, 34]; Berlin–Frankfurt–Muenster protocols and their modifications [28, 29, 31]; Associazione Italiana Ematologia Oncologia Pediatrica [30]; SHOP [35]; HYPERCVAD–MA [35]; CODOX–M–IVAC [35]; MTX–ARAC [35]; GIMEMA ALL [36]; or multiple protocols [11, 24, 30,31,32,33, 35, 36]). Incomplete ascertainment of toxicity, especially in retrospective studies, is one of the reasons for conflicting findings (prospective studies [27, 28, 31,32,33, 35, 36] vs. retrospective studies [11, 23, 24, 30, 34]). Additionally, some studies evaluated toxicities during maintenance phase [28, 35] while others were during consolidation phase [11, 23, 24, 27, 30,31,32,33,34, 36].
A further confounding factor is the age of participants and cancer type. Although the majority of studies were conducted on pediatric subjects, some were evaluated in adults [32, 36]. Some studies included patients with malignant lymphoma [11] or non-Hodgkin lymphoma [32] besides ALL. Moreover, modest and heterogeneous sample sizes were recruited in different studies, which might impact their statistical power to detect clinically meaningful differences. Small sample sizes were reported (34 [24]; 40 [27, 28]; 41 [32, 36]; 47 [29]), while larger sizes were reported as well (109 [34]; 122 [36]; 126 [37]; 127 [23]; 151 [30]; 186 [33]; 286 [31]). Researchers utilized different methods in genotyping which contributes to the conflicting findings. Most of studies adopted polymerase chain reaction/restriction length polymorphisms [23, 27, 28, 30,31,32, 34, 36]. Other methods included TaqMan SNP genotyping assays [11], GeneProof [38] and in situ hybridization with subsequent enzymatic color reaction [24].
To the best of our knowledge, this is the second study that finds an association between DHFR 19 bp deletion and MTX toxicity in children with ALL. Carriers of the dominant genotype of DHFR are more likely to develop the worst degrees of leucopenia compared to the minor allele carriers. The same was observed when “total MTX courses” method was considered. On the other hand, Giletti et al. found no association between DHFR 19 bp deletion and MTX hematologic toxicity [32]. This lack of association was also observed in a study done on 122 adult Italian patients [36]. A recently published Spanish study revealed that carriers of at least one insertion allele had a lower median level of WBCs [35]. Regarding TYMS 1494del6, we found considerable results as patients with the deletion allele were at 6 times higher risk of developing neutropenia compared to patients with the minor genotype and 4.5 more likely to develop grade 3 and 4 leucopenia when “total MTX courses” method was used. On the other hand, another published study indicated a lack of association [23]. Concerning DPYD polymorphisms, none of the toxicities evaluated revealed association. None of the previously published studies (N = 13) evaluated DPYD genetic polymorphisms in children with ALL treated with HDMTX [27, 30, 39, 40].
We decided to utilize two different methods to assess toxicity. Out of 13 different studies reviewed, only two studies evaluated toxicities by “total MTX courses” methods. Generally, we observed the overall concordance between the two methods. However, the interpretations of current findings should be taken with caution because of a number of limitations;. one of them is the magnitude of missing data. It should be noted that such shortcoming was observed in previous studies [11, 23, 24]. Also the nature of the study should be taken into account as the data were collected retrospectively.
In conclusion, this pharmacogenetic study is the first to be carried out on a sample of Jordanian children with ALL. Results showed that the dominant alleles in three genes (DHFR, MTHFR, TYMS) were associated with hematologic toxicity, but none of them was associated with MTX level, hepatotoxicity or renal function.
References
Jordan national cancer registry (2013) Cancer incidence in Jordan. http://www.moh.gov.jo/Echobusv3.0/SystemAssets/ba7d2a38-c47f-4058-b779-57e23c06292b.pdf. Accessed 5 Jan 2018
Globocan (2012) Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 25 Dec 2017
Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. N Engl J Med 373(16):1541–1552. https://doi.org/10.1056/NEJMra1400972
Cooper SL, Brown PA (2015) Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin N Am 62(1):61
Kishi S, Cheng C, French D, Pei D, Das S, Cook EH, Hijiya N, Rizzari C, Rosner GL, Frudakis T, Pui CH, Evans WE, Relling MV (2007) Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood 109(10):4151–4157. https://doi.org/10.1182/blood-2006-10-054528
Cheng KK (2008) Association of plasma methotrexate, neutropenia, hepatic dysfunction, nausea/vomiting and oral mucositis in children with cancer. Eur J Cancer Care (Engl) 17(3):306–311. https://doi.org/10.1111/j.1365-2354.2007.00843.x
Tian H, Cronstein BN (2007) Understanding the mechanisms of action of methotrexate. Bull NYU Hosp Jt Dis 65(3):168–173
Gellekink H, Blom HJ, van der Linden IJ, den Heijer M (2007) Molecular genetic analysis of the human dihydrofolate reductase gene: relation with plasma total homocysteine, serum and red blood cell folate levels. Eur J Hum Genet 15(1):103–109. https://doi.org/10.1038/sj.ejhg.5201713
Shao W, Yuan Y, Li Y (2017) Association between MTHFR C677T polymorphism and methotrexate treatment outcome in rheumatoid arthritis patients: a systematic review and meta-analysis. Genet Test Mol Biomark 21(5):275–285
Qiu Q, Huang J, Lin Y, Shu X, Fan H, Tu Z, Zhou Y, Xiao C (2017) Polymorphisms and pharmacogenomics for the toxicity of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic review and meta-analysis. Medicine (Baltimore) 96(11):e6337. https://doi.org/10.1097/md.0000000000006337
Faganel Kotnik B, Grabnar I, Bohanec Grabar P, Dolzan V, Jazbec J (2011) Association of genetic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol 67(10):993–1006. https://doi.org/10.1007/s00228-011-1046-z
Lambrecht L, Sleurs C, Labarque V, Dhooge C, Laenen A, Sinnaeve F, Renard M, Uyttebroeck A (2017) The role of the MTHFR C677T polymorphism in methotrexate-induced toxicity in pediatric osteosarcoma patients. Pharmacogenomics 18(8):787–795. https://doi.org/10.2217/pgs-2017-0013
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10(1):111–113. https://doi.org/10.1038/ng0595-111
van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, van den Heuvel LP, Blom HJ (1998) A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet 62(5):1044–1051. https://doi.org/10.1086/301825
Hagner N, Joerger M (2010) Cancer chemotherapy: targeting folic acid synthesis. Cancer Manag Res 2:293
Lima A, Azevedo R, Sousa H, Seabra V, Medeiros R (2013) Current approaches for TYMS polymorphisms and their importance in molecular epidemiology and pharmacogenetics. Pharmacogenomics 14(11):1337–1351
Mandola MV, Stoehlmacher J, Zhang W, Groshen S, Mimi CY, Iqbal S, Lenz H-J, Ladner RD (2004) A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenet Genom 14(5):319–327
Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K (2001) dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 29(1):308–311
Kristensen M, Pedersen P, Melsen G, Ellehauge J, Mejer J (2010) Variants in the dihydropyrimidine dehydrogenase, methylenetetrahydrofolate reductase and thymidylate synthase genes predict early toxicity of 5-fluorouracil in colorectal cancer patients. J Int Med Res 38(3):870–883
Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W, Handgretinger R, Downing JR, Evans WE, Relling MV (2009) Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360(26):2730–2741. https://doi.org/10.1056/NEJMoa0900386
Yousef A-M, Zawiah M, Al-Yacoub S, Kadi T, Al-Ramadhani H (2018) The association of polymorphisms in folate-metabolizing genes with response to adjuvant chemotherapy of colorectal cancer. Cancer Chemother Pharmacol 82(2):237–243
National Cancer Institute (2006) Common toxicity criteria version 3. https://www.eortc.be/services/doc/ctc/. Accessed 25 Dec 2017
Zgheib NK, Akra-Ismail M, Aridi C, Mahfouz R, Abboud MR, Solh H, Muwakkit SA (2014) Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharmacogenet Genom 24(8):387–396. https://doi.org/10.1097/fpc.0000000000000069
Haase R, Elsner K, Merkel N, Stiefel M, Mauz-Korholz C, Kramm CM, Korholz D (2012) High dose methotrexate treatment in childhood ALL: pilot study on the impact of the MTHFR 677C> T and 1298A>C polymorphisms on MTX-related toxicity. Klin Padiatr 224(3):156–159. https://doi.org/10.1055/s-0032-1304623
Gaunt TR, Rodriguez S, Zapata C, Day IN (2006) MIDAS: software for analysis and visualisation of interallelic disequilibrium between multiallelic markers. BMC Bioinformatics 7:227. https://doi.org/10.1186/1471-2105-7-227
Seung AH (2013) Adverse effects of chemotherapy and targeted agents. In: Kimble K (ed) Applied therapeutics, 10th edn. Wolters Kluwer Health—Lippincott Williams & Wilkins, Philadelphia, p 2109
El-Khodary NM, El-Haggar SM, Eid MA, Ebeid EN (2012) Study of the pharmacokinetic and pharmacogenetic contribution to the toxicity of high-dose methotrexate in children with acute lymphoblastic leukemia. Med Oncol 29(3):2053–2062. https://doi.org/10.1007/s12032-011-9997-6
Tantawy AA, El-Bostany EA, Adly AA, Abou El Asrar M, El-Ghouroury EA, Abdulghaffar EE (2010) Methylene tetrahydrofolate reductase gene polymorphism in Egyptian children with acute lymphoblastic leukemia. Blood Coagul Fibrinolysis 21(1):28–34. https://doi.org/10.1097/MBC.0b013e32833135e9
Kaluzna E, Strauss E, Zajac-Spychala O, Gowin E, Swiatek-Koscielna B, Nowak J, Fichna M, Mankowski P, Januszkiewicz-Lewandowska D (2015) Functional variants of gene encoding folate metabolizing enzyme and methotrexate-related toxicity in children with acute lymphoblastic leukemia. Eur J Clin Pharmacol 769:93–99. https://doi.org/10.1016/j.ejphar.2015.10.058
D’Angelo V, Ramaglia M, Iannotta A, Crisci S, Indolfi P, Francese M, Affinita MC, Pecoraro G, Napolitano A, Fusco C, Oreste M, Indolfi C, Casale F (2011) Methotrexate toxicity and efficacy during the consolidation phase in paediatric acute lymphoblastic leukaemia and MTHFR polymorphisms as pharmacogenetic determinants. Cancer Chemother Pharmacol 68(5):1339–1346. https://doi.org/10.1007/s00280-011-1665-1
Araoz HV, D’Aloi K, Foncuberta ME, Sanchez La Rosa CG, Alonso CN, Chertkoff L, Felice M (2015) Pharmacogenetic studies in children with acute lymphoblastic leukemia in Argentina. Leuk Lymphoma 56(5):1370–1378. https://doi.org/10.3109/10428194.2014.951844
Giletti A, Vital M, Lorenzo M, Cardozo P, Borelli G, Gabus R, Martinez L, Diaz L, Assar R, Rodriguez MN, Esperon P (2017) Methotrexate pharmacogenetics in Uruguayan adults with hematological malignant diseases. Eur J Pharm Sci 109:480–485. https://doi.org/10.1016/j.ejps.2017.09.006
Costea I, Moghrabi A, Laverdiere C, Graziani A, Krajinovic M (2006) Folate cycle gene variants and chemotherapy toxicity in pediatric patients with acute lymphoblastic leukemia. Haematologica 91(8):1113–1116
Ramirez-Pacheco A, Moreno-Guerrero S, Alamillo I, Medina-Sanson A, Lopez B, Moreno-Galvan M (2016) Mexican childhood acute lymphoblastic leukemia: a pilot study of the MDR1 and MTHFR gene polymorphisms and their associations with clinical outcomes. Genet Test Mol Biomark 20(10):597–602. https://doi.org/10.1089/gtmb.2015.0287
Gervasini G, De Murillo SG, Jiménez M, María D, Vagace JM (2017) Dihydrofolate reductase genetic polymorphisms affect methotrexate dose requirements in pediatric patients with acute lymphoblastic leukemia on maintenance therapy. J Pediatr Hematol Oncol 39(8):589–595
Ongaro A, De Mattei M, Della Porta MG, Rigolin G, Ambrosio C, Di Raimondo F, Pellati A, Masieri FF, Caruso A, Catozzi L, Gemmati D (2009) Gene polymorphisms in folate metabolizing enzymes in adult acute lymphoblastic leukemia: effects on methotrexate-related toxicity and survival. Haematologica 94(10):1391–1398. https://doi.org/10.3324/haematol.2009.008326
de Deus DM, de Lima EL, Seabra Silva RM, Leite EP, Cartaxo Muniz MT (2012) Influence of methylenetetrahydrofolate reductase C677T, A1298C, and G80A polymorphisms on the survival of pediatric patients with acute lymphoblastic leukemia. Leuk Res Treat 2012:292043. https://doi.org/10.1155/2012/292043
Kałużna EM, Strauss E, Świątek-Kościelna B, Zając-Spychała O, Gowin E, Nowak JS, Rembowska J, Januszkiewicz-Lewandowska D (2017) The methylenetetrahydrofolate reductase 677T-1298C haplotype is a risk factor for acute lymphoblastic leukemia in children. Medicine 96(51):e9290
Kaluzna E, Strauss E, Zajac-Spychala O, Gowin E, Swiatek-Koscielna B, Nowak J, Fichna M, Mankowski P, Januszkiewicz-Lewandowska D (2015) Functional variants of gene encoding folate metabolizing enzyme and methotrexate-related toxicity in children with acute lymphoblastic leukemia. Eur J Pharmacol 769:93–99. https://doi.org/10.1016/j.ejphar.2015.10.058
Mosaad YM, Abousamra NK, Elashery R, Fawzy IM, Eldein OA, Sherief DM, El Azab HM (2015) Methylenetetrahydrofolate reductase C677T and A1298C polymorphism and susceptibility to acute lymphoblastic leukemia in a cohort of Egyptian children. Leuk Lymphoma 56(9):2699–2705. https://doi.org/10.3109/10428194.2015.1004170
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The protocol of the study was approved by the institutional review board (IRB) of Royal Medical Services (IRB no. 1762, 14/2/2017), and conducted in concordance with the principles of the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards.
Informed consent
Each patient provided their written informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yousef, AM., Farhad, R., Alshamaseen, D. et al. Folate pathway genetic polymorphisms modulate methotrexate-induced toxicity in childhood acute lymphoblastic leukemia. Cancer Chemother Pharmacol 83, 755–762 (2019). https://doi.org/10.1007/s00280-019-03776-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03776-8