Abstract
Copepods are aquatic invertebrates with a key role at the basis of marine food webs due to their high biomass as well as their elevated fatty acid (FA) content. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are two FA abundant in copepods which have a well demonstrated role in growth and reproduction in marine organisms. While the majority of research has focused on planktonic copepod species, less is known for benthic species despite their high richness, abundance and their role as the main food source for many fish larvae. Temperature is a key driver of organism’s fitness as well as ecosystem functioning and sea surface temperature is expected to rise under all CO2 emission scenarios. Thus, understanding how copepods will respond to such changes is crucial given their role in marine food webs. In this study we expose laboratory reared Tachidius discipes, an intertidal benthic copepod to a temperature gradient (12, 15, 18, 21 and 24 °C) including current seasonal variability as well as future scenarios. Survival, FA, growth rates and nauplii production were measured for each temperature. We found decreased survival, increased growth rates and detrimental effects for nauplii production with temperature increase. While relative EPA and DHA decreased with temperature this was not found on absolute levels of these FA. Changes in benthic copepods’ biomass as well as their FA composition in response to temperature changes could amplify to higher trophic levels with consequences for food web functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperature is the main driver of ecosystem metabolism and as such it is a major determinant of ecosystem functions such as nutrient cycling and productivity (Brown et al. 2004). Sea surface temperature (SST) rise, one of the consequences of climate change, will continue to intensify under all CO2 emission scenarios (Kwiatkowski et al. 2020). When considering climate change effects on marine ecosystem functioning copepods are a key focus group due to their high abundance (Drago et al. 2022), their nutritional value for higher trophic levels (Brett et al. 2009) and their role in biogeochemical cycling (Steinberg and Landry 2017). They represent between 70 and 90% of the mesozooplankton in pelagic ecosystems (Turner 2004) and are amongst the most abundant taxa of the meiofauna in benthic ecosystems together with nematodes (Giere 1993). Changes in phenology, distribution (McGinty et al. 2021) and abundance (Garzke et al. 2016) of copepods in response to climate change have been well documented. These changes are driven largely by thermal performance at the individual level which reflects on life history traits that determine Darwinian fitness and ultimately come to alter ecosystem functioning (Sokolova 2021). Although there has been extensive research on global change effects on planktonic copepods, less is known of their benthic counterparts, harpacticoid copepods (Order Harpacticoida, Copepoda, Crustacea) (Sarmento et al. 2017) despite their high species diversity and their dominant role in benthic food webs (Giere 1993; Wells 2007). Harpacticoids are particularly important in estuaries and coastal areas where they constitute the main food source of juvenile fish, including commercially important species like flat fish and salmonids (Huys and Boxshall 1991).
Climate change driven alterations at lower trophic levels cascade further to higher trophic levels in marine food webs through changes in energy transfer efficiency. Trophic biomarkers such as fatty acids (FA) are good indicators for this energy flow (Dalsgaard et al. 2003). Fatty acid composition is an important biochemical trait at the individual level in metazoans as FA are the building blocks of the energy storage, regulate membrane fluidity, and act as chemical signals among others (Parrish 2009; Couturier et al. 2020). Research has particularly focused on eicosapentaenoic acid (20:5n-3, EPA), docosahexaenoic (22:6n-3, DHA) and to a less extent also arachidonic acid (22:4 n-6) since these FA are needed for survival, growth and reproduction in marine organisms (Sargent et al. 1999; Parrish 2009). These FA are considered essential because most organisms have to obtain them from their diet for optimal health (Parrish 2009). Consequently, EPA and DHA are considered important determinants of food quality and key factors in modulating energy transfer efficiency in marine food webs (Brett and Müller-Navarra 1997a; Müller-Navarra et al. 2000).
FA composition is known to change in response to environmental change in marine organisms. The decrease of polyunsaturated FA (PUFA) relative to saturated FA (SFA) with SST rise is a well characterized response across marine taxa (Tan et al. 2022; Holm et al. 2022). Predictions also estimate a decrease in DHA and EPA in response to SST increase in primary producers decreasing the availability of these essential FA (Hixson and Arts 2016; Colombo et al. 2020). Harpacticoids have the enzymatic pathways to perform biosynthesis of DHA and EPA from shorter precursors (Kabeya et al. 2021). This has been previously indirectly demonstrated for several harpacticoid species (Watanabe et al. 1983; Norsker and Støttrup 1994; Nanton and Castell 1998; Parrish et al. 2012; De Lima et al. 2013; Arndt and Sommer 2014) and more recently directly demonstrated by functional characterization of methyl-end desaturase and elongase enzymes (Kabeya et al. 2021; Boyen et al. 2023). Still, the efficiency with which harpacticoid copepods can do this and how this ability is modified by SST remains largely unclear (Boyen et al. 2020; Sahota et al. 2022). Lower DHA (ng ind−1) in response to warming was described before for the harpacticoid Platychelipus littoralis (Werbrouck et al. 2016; Boyen et al. 2020). A mismatch between supply and demand of EPA and DHA in marine ecosystems can lead to decrease growth, increased stress response and altered behaviour in animals ultimately impacting secondary production and nutrient cycling (Bret and Muller-Navarra, 1997; Twining et al. 2016). Temperature driven changes at lower trophic levels have been described to cascade through the food web altering its structure and dynamics, for example shifting fish communities from lipid rich to lipid poor communities (Litzow et al. 2006).
Addressing energy-relevant compound dynamics like FA together with life history traits of harpacticoid species under a warming scenario is crucial for understanding the trade-offs between processes like maintenance, growth and reproduction and changes in both food quality and quantity in marine benthic food webs. Intertidal invertebrates have a wide acclimation capacity to environmental change which results from exploiting mechanisms such as metabolic depression, anaerobic metabolism and expression of heat shock proteins (Pörtner et al. 2017). These organisms may therefore already be at the extreme of their tolerance range and further environmental stress could negatively impact their fitness and eventually population growth (Harley et al. 2006; Helmuth et al. 2006; Kelly et al. 2012; Pörtner et al. 2017). The majority of research on Harpacticoids has focused on one model genus, Tigriopus (Raisuddin et al. 2007; Sasaki and Dam 2021), which inhabits intertidal rockpools and hence may display very different responses from harpacticoids inhabiting intertidal soft sediments. Therefore, in this study we focus on the copepod T. discipes (Giesbrecht 1881), a dominant species of the intertidal sediments of salt marshes and mudflats in the coasts of Norway, the Baltic Sea and the North Sea. The objective of this study is to analyse survival, FA content, growth rates and reproduction of T. discipes in response to temperature increase. We hypothesized that in response to temperature increase of up to 3 °C above summer mean temperatures predicted for temperate coastal areas: (i) survival is negatively impacted in T. discipes, (ii) FA composition shows differences among temperatures with a decrease of PUFAs relative to saturated FA (SFA) and a decrease in EPA and DHA content and (iii) growth rates increase with temperature while reproduction is negatively affected.
Materials and methods
Experimental design and set-up
Tachidius discipes adults were obtained from lab cultures initiated from samples taken from the Paulina intertidal mudflat (51°21′ 24′′ N, 3° 42′ 51′′E) of the Westerscheldt estuary in The Netherlands. Samples were taken by scraping the top sediment layer and subsequently sieving through 1 mm and 250-µm sieves. Copepods were reared with filtered natural sea water (FNSW 0.3 um, Whatmann, salinity 32) in a temperature controlled room at 15 °C in a 12:12 light–dark cycle (3–11 μmol photons m−2 s−1) and fed diatoms Nitzschia sp. (strain DGC 0421) and Navicula arenaria (strain DGC 1006) in excess concentrations. Diatoms were obtained from the BCCM/ DCG Diatoms Collection (hosted by the Laboratory for Protistology and Aquatic Ecology, Ghent University) and grown in non-axenic cultures under the same temperature and light regime as the copepods. The medium for the diatoms is autoclaved natural seawater supplemented with Guillard’s (F/2) Marine Water Enrichment solution (SigmaAldrich, Overijse, Belgium). Two types of experiments were set up, one to measure the effect of temperature increase on survival and fatty acid composition and a second one where growth was monitored and later reproduction. Five temperatures were chosen (12, 15, 18, 21, 24 °C) based on mean temperatures observed in the field throughout the year (12–21 °C; Supplementary information Fig S1) and the projected temperature increase of 3.47 ± 0.78 °C, according to the high emission shared socioeconomic path (SSP5-8.5) (Kwiatkowski et al. 2020). Temperature was controlled in five incubators (Lovibond TC 140 G, ± 1 °C). For each temperature treatment there were four replicates.
Survival and FA
In August 2022, adult T. discipes individuals were retrieved from the cultures by sieving through a 200-µm mesh. With the objective of measuring survival and fatty acid composition, 220 individuals (including both ovigerous and non-ovigerous) were manually picked with a glass Pasteur pipette under a stereomicroscope and placed in a crystallization glass jar with 100 ml FNSW (salinity of 32) (Fig. 1.A). The benthic diatom Nitzschia sp. was added at ad-libitum food conditions (0.34–0.96 mg carbon L−1). Diatom concentrations were measured with a particle analyser (Beckman Coulter’s Multisizer 3). Water and food were refreshed every 4 days by sieving the copepods, discarding the water together with any new offspring that may have been produced by the ovigerous females. Fresh FNSW and diatoms were added. The total incubation period was 18 days after which the number of surviving individuals were counted (Fig. 1A). For the FA analyses, 100 individuals per replicate were washed with FNSW and left overnight to clean their guts. Afterwards, they were cleaned once more with autoclaved FNSW and once with MilliQ water to discard the salt. They were put in glass vials and frozen at −80 °C. Triplicate samples of 100 individuals were also taken prior to the start of the experiment (day 0) in the same way to serve as a control FA profile of the cultures. Triplicate samples of the food source (Nitzschia sp.) were also taken for FA profiling by centrifuging 25 ml of the algal culture. The obtained pellet was stored at −80 °C.
Experimental set up scheme summary for T. discipes fatty acids and survival measurements (A) and for the growth and reproduction measurements (B). t0 = time the experiment starts, tf = time experiment finishes, F0 = parental generation used to measure FA and survival, F1 = all offspring obtained from one individual F0 female used in the growth and reproduction experiment (B)
Growth rates and reproduction
To measure the effect of temperature on growth rates and reproductive traits, eight ovigerous females were selected per temperature treatment on day 0 and placed individually in small petri dishes (diameter 15 mm) with 3 ml of FNSW and the same food (0.43 mg carbon L−1) and light regime as the adults. Each replicate was checked daily for hatching and the adult female was removed from the petri dish five days after hatching since interaction with the maternal fecal pellets has shown to improve nauplii survival (De Troch et al. 2005). Every 24 h, five to ten pictures were taken from randomly selected growing individuals in each petri dish with a Leica Inverted Microscope (Leica DMi1) with a mounted camera and the magnification used was recorded for later calibration of the scale. Once copepodite stages were reached, individuals were transferred to larger petri dishes (diameter 35 mm) with 10 ml of FNSW. For each replicate the date of hatching, date of appearance of first copepodite, as well as the first adult and first egg sac appearance were recorded (Fig. 1B). Pictures were taken until more than half of the individuals in the petri dish reached the adult stage. Female adults were confirmed by length measurements of the pictures (> 480 um) while male adults were recognized by sexual dimorphism on their first antenna (A1, antennule). Once individuals reached adult stage, replicates from the same temperature treatment level were combined to allow reproduction. When egg sacs appeared, ovigerous females were isolated and placed in 24-well plates. Wells were checked daily and when hatching was observed, the date was noted and nauplii were counted. The time between egg sac appearance and hatching was considered as the embryonic development time. This was recorded for eight to twelve females in each temperature treatment.
Image analysis
Pictures were analysed using Image J software (Schneider et al. 2012). The conversion from pixels to um was done using pictures of a certified scale taken under the inverted microscope under the same magnification used for the copepod pictures. Individual length (rostrum to basis of furca for adults and copepodites and rostrum to last segment in nauplii) was measured using an automated code that fitted an ellipse to the copepod and extracted the value of the major ellipse axis. Values were then inspected and corrected manually when incorrect by using a manual measuring tool in Image J. A total of 5200 length measurements were obtained from this analysis.
FA analysis
FA methyl esters (FAMEs) were prepared from lyophilized frozen samples using a direct transesterification procedure with 2.5% (v: v) sulfuric acid in methanol as described by De Troch et al. (2012). The internal standard (19:0, nonadecanoic acid, Sigma-Aldrich, 1.36 μg) was added prior to the extraction. FAMEs were extracted twice with hexane. FA composition analysis was carried out with a gas chromatograph (GC; HP 7890B, Agilent Technologies, Diegem, Belgium) equipped with a flame ionization detector (FID) and connected to an Agilent 5977A Mass Selective Detector (MSD; Agilent Technologies, Diegem, Belgium). The GC was further equipped with a PTV injector (CIS-4, Gerstel, Mülheim an der Ruhr, Germany). An HP88 fused-silica capillary column (60 m × 0.25 mm × 0.20 μm film thickness, Agilent Technologies) was used at a constant helium flow rate (2 ml min−1). The injected sample (2 μl) was split equally between the MS and FID using an Agilent Capillary Flow Technology Splitter. The oven temperature programme was set as described in Boyen et al. (2020). Mass spectra were recorded at 70 eV ionization voltage over the mass range of 50–550 m/z units. Data analysis was done with MassHunter Quantitative Analysis software (Agilent Technologies). The signal obtained with the FID detector was used to generate quantitative data of all compounds. Peaks were identified based on their retention times, compared with external standards as a reference (Supelco 37 Component FAME Mix, Sigma-Aldrich) and by the mass spectra obtained with the MS detector. FAME quantification was based on the area of the internal standard and on the conversion of peak areas to the weight of the FA by a theoretical response factor for each FA (Ackman and Sipos 1964; Wolff et al. 1995). The FA shorthand notation A:Bn-X is used, where A represents the number of carbon atoms, B the number of double bonds and X the position of the first double bond counting from the terminal methyl group.
Data analysis
All data analyses were performed in R software (R core team 4.1.2). Survival counts were transformed to relative survival data by dividing by the total initial number of individuals. A linear regression model was fitted to relative survival data using least square means estimation. Piecewise cubic splines were fitted with the R package splines to assess the effect of temperature on all FA (> 1% abundance) as well as total FA content, the already mentioned FA classes PUFA, and SFA and monounsaturated FA (MUFA) and 16:1 n-7 which is a well- established diatom marker (Dalsgaard et al. 2003). The ARA/EPA ratio was also investigated since it is an indicator of physiological stress (Calder 2010). Splines are piecewise functions (usually low-order polynomials) that are used to introduce smooth curves in linear regression. In order to obtain more flexible curves, the number of knots (k) or the degree of the polynomial can be increased (Perperoglou et al. 2019). Different models with varying numbers of knots were tested and the model with the lowest Akaike information criterion (AIC) value was chosen.
To test the effect of temperature on growth rates, body length data was plotted against the number of days since hatching, and various curves (linear, log and exponential) were checked to determine the best fitting. Based on the calculated AIC values, the exponential model showed the best fit (Supplementary information Fig S4). β1 of the exponential function \(\mathrm{L }= {\upbeta }_{0}*\mathrm{exp}({\upbeta }_{1}*\mathrm{t})\) was used to calculate the percentage change in length per day in each replicate. In this equation, L represents the body length, β0 the body length at hatching, t the time and β1 coincides with β1 of the log linear model, expressed as \(\mathrm{ln L }=\mathrm{ln }{\upbeta }_{0} + {\upbeta }_{1}\mathrm{t}\). We calculated percentage change in length per day as: exp(β1)-1*100. These percentages were plotted against temperature and again the best fitting model was chosen based on the AIC values. For the effect of temperature on nauplii production and embryo development time, the splines approach was used. Normality of residuals, homogeneity of variance and independence were checked for the survival and growth rates models by inspecting the observed vs. fitted residuals and a Shapiro–Wilk (normality) and Bartlett’s test (homogeneity) (Supplementary information Fig, S5).
Results
Survival
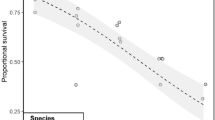
The survival of T. discipes adults after 18 days of incubation decreased significantly, with 2.9% decrease per degree increase in temperature (linear regression, R2 = 0.67, F35,1 = 34.75, p = 2.26·10−5, Fig. 2). The estimated relative survival rate ranged from 78% ± 2% at 12 °C to 43% ± 5% at 24 °C. Absolute survival counts can be found in the Supplementary information (Table S4).
FA composition
A total of 29 FA were identified in T. discipes adults of which 16 FA were present in more than 1% of the total FA content. When analyzing at FA as % of total FA content, 13 out of the 29 showed significant differences with temperature, while this proportion was reduced to 10 out of 29 when looking at absolute values (ng ind−1) (Supplementary information Table S1 and S2). Relative (%) FA composition mirrored that of the diet to a large extent, with PUFA being the most abundant class followed by SFA and MUFA in both the diet and the copepods (Table 1). The detailed relative FA profile of the diatom can be found in Supplementary information (Fig S3). There was no difference in total FA content (ng ind−1) across treatments (Table 2). Total FA content at day 0 (15 °C) did not differ from any of the experimental treatments either (Supplementary information Fig S2). The most abundant FA in all temperature groups were DHA (23.96 to 17.41%), EPA (21.31 to 16.48%), 16:1 n-7 (6.99 to 13.18%), 16:0 (17.04 to 20.32%), 18:0 (4.99 to 9. 76%) and 14:0 (5.16 to 6.74%). Mean relative abundances per FA classes are listed in Table 1 as well as the mean abundance of EPA, DHA, ARA,16:1 n-7 and the ARA/EPA and PUFA/SFA ratios.
A summary of all models is presented in Table 2. Relative content of EPA and DHA decreased significantly with increasing temperature (Fig. 3 a and b and Table 2). The ratio of PUFA to SFA also decreased significantly with temperature (Table 2). Conversely, looking at the absolute content (ng ind−1) a different pattern is observed: DHA and EPA remain constant with increasing temperature (Fig. 3 a and b and Table 2). Relative ARA, 16:1 n-7, and the ARA/EPA ratio all increase significantly with increasing temperature which is also observed in absolute content of these FA (Table 2). In general absolute FA content values present more variability within treatments compared to relative values.
Relationships of relative (yellow) and absolute (grey) abundances of EPA (A) and DHA (B) with temperature in Tachidius discipes, n = 4. The lines were fitted using natural splines (k = 2) and the grey areas represent the 95% confidence intervals. In both A and B the yellow line shows significant decrease (Table 2) with increasing temperature. There is no significant decrease in absolute amounts (ng ind−1) in either EPA or DHA (Table 2)
Growth rates and reproduction
One replicate was lost in treatments 15, 18 and 24 °C, resulting in a total of seven replicates in those treatments. Tachidius discipes relative growth rates increased significantly and exponentially with temperature (exponential regression, R2 = 0.49, F1,35 = 13.92, p = 6.73·10−5, Fig. 4). At 24 °C the first adult was observed after 8 days while at 12 °C it took 22 days until the first adult appeared. Egg sac carrying females appeared after 15 and 31 days at 24 °C and 12 °C, respectively. The number of nauplii produced per female decreased significantly with temperature (R2 = 0.36, F2,49 = 15.51, p = 6.05·10−6, Fig. 5A) with a steeper decrease after 18 °C. Embryo development time also significantly decreased with temperature (R2 = 0.61, F2,49 = 41.3, p = 3.07·10−11, Fig. 5B).
Number of nauplii produced per female of T. discipes (A) and embryo development time (B) across temperatures. Embryo development time was recorded as the number of days from appearance of the egg sac until hatching. The black lines were estimated by fitting natural splines (k = 2) and the grey area represents the 95% confidence intervals
Discussion
The overall thermal performance of ectotherms is a complex interaction of individual traits that act at different levels, ranging from molecular to cellular to organismal (Pörtner et al. 2017; Sokolova 2021). A mismatch or decoupling between these traits can potentially compromise fitness and ultimately population persistence (Sokolova 2021). Identifying thresholds at which trade-offs between traits (i.e. reproduction and growth) are triggered is thus important to understand governing mechanisms and predict changes in populations in response to projected temperature increases (Alcaraz et al. 2014). In the present study we have found decreased PUFA/SFA, EPA and DHA relative content in response to temperature increase while no significant changes for EPA and DHA were found in absolute terms. Survival and reproductive output decreased with increasing temperature in T. discipes while growth rates increased exponentially in response to an increased temperature gradient.
Survival
The observed detrimental effect of temperature on the survival of T. discipes adults after 18 days exposure was contrary to our expectations, since harpacticoid intertidal species are adapted to wide fluctuations in their environmental conditions and thus they are typically resilient to changes in temperature, especially when compared with their planktonic counterparts (Sasaki and Dam 2021; Sahota et al. 2022). The cultures were started from individuals collected in the field in April, when temperatures averaged 10 °C (Supplementary information Fig S1, Table S1) and cultured in the lab at 15 °C for several months. For short generation-time organisms such as copepods, temperature fluctuations throughout the year have been shown to lead to a seasonality effect on thermal tolerance and this effect can be maintained even after several generations (Sasaki and Dam 2020). We hypothesize that populations of T. discipes sampled at different seasons or locations will exhibit a different response to temperature and that for example populations sampled in summer or at lower latitudes will exhibit higher thermal tolerance with higher tipping points. It is important to mention that our temperature exposure at each treatment was kept constant while in intertidal environments the tidal regime creates wide daily temperature fluctuations (Meire et al. 2005), which we did not consider and could affect the results. In vitro studies are useful to establish baselines of organisms for which little is known but caution should be taken when extrapolating to the field considering factors such as latitude, acclimation temperatures and season have an effect on thermal sensitivity.
Fatty acids and life history traits
At higher temperatures, we observed that copepods grew faster. Temperature has been demonstrated to be a main factor driving population dynamics of benthic harpacticoids mainly by producing a decrease in generation times (Heip and Smol 1976; Fleeger 1979). However, in this study we also found lower reproductive output with increasing temperatures. Although faster developing individuals have a lower predation risk until maturity than slower developing individuals, the former individuals have a lower potential fecundity and mature at a smaller size than large adults (Kiørboe and Hirst 2008). In our study an apparent decrease in the mean maximum size was found with increasing temperature but this was only significant between 12 °C–21 °C and 12 °C–24 °C treatments (Supplementary information Table S3). In crustaceans and mussels, it has been reported that under warmer temperatures there is a higher energy allocation in protein synthesis associated with faster growth, and that this results in a negative trade-off where other physiological traits were compromised (Whiteley and Faulkner 2005; Pan et al. 2021). We hypothesize that an energetic trade-off was triggered whereby higher allocation to growth resulted in less energy allocated to reproduction (in terms of nauplii production). More than half of the reproductive potential of harpacticoids is achieved during the spring bloom (Heip and Smol 1976). If the reduced nauplii production effect together with increased mortality, is larger than the decrease in generation time then the spring maximum of T. discipes may be affected under increased temperatures.
Total FA content remained constant across temperature treatments in the exposed adults. This is contrary to our expectations given that temperature increase initiates the stress mechanism response that results in higher energetic costs and requires lipid catabolism reducing total FA content (Sokolova 2021). Although neither feeding rates nor assimilation efficiency were measured in this study, 16:1 n-7, a well-established diatom marker (Dalsgaard et al. 2003) abundant in Nitzschia sp. (Table 1) was found to significantly increase with temperature (Table 2) in both relative and absolute terms. This may indicate increased amounts of ingested algae and/or higher assimilation efficiency rates. Changes in feeding rates and assimilation efficiency in response to temperature have been reported before for calanoid copepods (Isla et al. 2008; Saiz et al. 2022) and harpacticoids (Li et al. 2015). We hypothesize that total FA content can be maintained at constant levels across temperatures through increasing ingestion rates, assimilation efficiency, or both.
The observed decrease of PUFA/SFA ratio with increased temperature is typically associated with the homeoviscous adaptation mechanism, whereby the amount of unsaturated FA in the membrane phospholipids increases at lower temperatures to maintain an appropriate fluidity and function (Sinensky 1974; Hazel 1995). This is a widespread response that has previously been reported for marine ectotherms (Pörtner et al. 2007) and for harpacticoid copepods (Werbrouck et. al, 2016). There are other heat stress induced responses that can also produce changes in FA composition. The amount of ROS (reactive oxygen species) is also known to increase with temperature, and this effect has been reported in copepods (Li et al. 2015; Yoon et al. 2023). ROS have the potential to damage cellular macromolecules such as lipids, proteins and DNA (Abele et al. 2011). Among the different FA classes, PUFA are most prone to lipid peroxidation (Abele et al. 2011) which can also contribute to explain the higher decrease in PUFA relative to the increase in SFA (Table 2).
FA also play an important role as signalling molecules, and changes in their composition can therefore have a severe impact modulating the stress response. More specifically, changes in membrane phospholipid fatty acid composition can influence the function of cells involved in inflammatory responses (Calder 2010). ARA and EPA are precursor of eicosanoids, which are molecules involved in regulating and mediating the inflammatory response (Stanley-Samuelson 1987; Calder 2010). While ARA is involved in the inflammatory response, EPA is involved in the anti-inflammatory response. We found that the ARA/EPA ratio in T. discipes increased along with temperature (Table 2) which is indicative of physiological stress (Calder 2010). This response has also been found in other marine invertebrates in response to environmental stress (Gao et al. 2018; Ericson et al. 2019). Inflammation responses are essential for maintaining health and homeostasis under stress conditions, but an excessive inflammatory response can be detrimental for fitness (Calder 2010).
While relative DHA and EPA were also observed to decrease significantly with temperature no change was observed in these FA when absolute concentrations were considered. Boyen et al. (2020) and Werbrouck et al. (2016) also found no effect of temperature on absolute EPA of Platychelipus littoralis and Sahota et al. (2022) also showed that P.littoralis was able to maintain DHA absolute values at high temperatures. While relative EPA concentration in the copepods resembled its concentration in the food source, DHA concentrations in the copepods were far higher than those in the food source. This points to selective retention of this FA and/or biosynthesis. Regardless of the specific mechanisms, the high levels of DHA in the copepod compared to the food source reflect its importance due to the multiple physiological functions of this FA (Colombo et al. 2020).
Untangling the mechanism by which FA and reproduction are coupled is complex, but both EPA and DHA have been shown to play key roles in copepod reproduction and growth (Müller-Navarra et al. 2000; Peters et al. 2007; Jónasdóttir et al. 2009). Jónasdóttir et al., (2009) found that absolute EPA concentration was highly correlated with egg production rates while absolute DHA was highly correlated with hatching success and nauplii production in the planktonic copepod Temora longicornis. Absolute levels of DHA and EPA were maintained at constant levels at high temperature in the present study. Synthesis of DHA and EPA are energetically costly (Twining et al. 2016), if T. discipes was able to maintain absolute DHA and EPA constant at high temperature through biosynthesis, this could have come at the expense of reproductive rates. This trade-off has been suggested previously by other authors who found decreased reproductive output in the copepod Tisbe when converting DHA and EPA from precursors in a DHA and EPA depleted diet (Arendt and Sommer 2014; Norsker and Støttrup, 1994).
It is important to clarify that in this study FA were measured in adults incubated for 18 days while growth and reproductive rates were measured in individuals that developed at the experimental temperatures. This means that we cannot be certain that the FA response is the same in the individuals exposed for 18 days and in the individuals that developed under the experimental conditions since there may have been ontogenetic acclimation that makes their FA profiles differ. Therefore, there is a possibility that there is some energetic imbalance triggered by temperature that is not related to FA and that explains the reduced reproductive output.
Overall, the exact mechanism by which specific FA increases/decreases with increasing temperature cannot be pinpointed since there are different processes that contribute to the overall FA composition of organisms namely, cellular membrane response, immune response, energetic imbalances, oxidative stress and biosynthesis. In this study we looked to the overall pool of FA hence, we cannot distinguish between membrane related processes from energy related processes. To do this, fractionation into specific lipid classes is needed as looking into membrane FA would allow to asses homeoviscous adaptation mechanisms while addressing FA within TAG (triacyclglycerols) allows to test hypothesis related to energetic costs of coping with higher temperatures. Fractionation into specific lipid classes was not performed due to the high number of individuals required for the analysis. Integrative frameworks that consider life history traits together with FA dynamics are needed to better understand trade-offs and feedback loops between traits at the physiological and whole organism level. We suggest bioenergetic approaches as a good example of such a framework since they have recently been applied successfully for other crustaceans incorporating FA dynamics (Lagos et al. 2022).
Food web relevance
Bret and Muller-Navarra (1997) found that in aquatic food webs, productivity and energy transfer efficiency can be predicted from EPA and DHA. In the present study we did not find an effect of temperature on absolute EPA and DHA. However, we did not expose the food source to temperature increases, ensuring that the diatom food quality was not altered. A model built from an extensive meta-analysis (Hixson and Arts 2016) predicted a decrease of 5.9 and 7.4% in EPA and DHA respectively in diatoms for a 2.5 °C increase in temperature. Given harpacticoids’ ability to convert DHA and EPA from precursors, it remains unknown if temperature increase will decrease essential fatty acid contents when an effect on diet is also considered. Future studies should also incorporate the effect of temperature on the food source’s FA and its transfer to the copepods. Copepods are the main food source of juvenile fish, and hence EPA and DHA obtained from their diet have a key role in fish growth and reproduction (Støttrup 2000, Tocher 2003). Given that copepod survival was found to be compromised at higher temperatures, fewer copepods implies less carbon and EPA and DHA available from this trophic level for higher trophic levels.
Changes in FA content together with changes in size, generation time and survival can alter the energy transfer at the plant–animal interface in marine benthic food webs with significant consequences for their functioning. Considering the multiple dimensions of thermal performance, it is more integrative and therefore more informative to examine species responses to climate change across several life history traits than to only focus on survival (Pan et al. 2021). Due to the important roles of FA involved in energy allocation and reproductive investment, and their demonstrated high sensitivity to thermal stress we strongly recommend FA as an important dimension to be considered in integrative thermal performance studies. Finally, we propose that future work focuses on bioenergetic and transgenerational approaches to understand the acclimation and adaptation responses to increasing temperatures.
Data availability
The datasets generated and analysed during the current study are available in the Open Science Framework repository, https://osf.io/6u7t9/
References
Abele D, Zenteno-Savin T, Vazquez-Medina JP (2011) Oxidative stress in aquatic ecosystems. John Wiley & Sons
Ackman RG, Sipos JC (1964) Application of specific response factors in the gas chromatographic analysis of methyl esters of fatty acids with flame ionization detectors. J Am Oil Chem Soc 41:377–378. https://doi.org/10.1007/BF02654818
Alcaraz M, Felipe J, Grote U, Arashkevich E, Nikishina A (2014) Life in a warming ocean: thermal thresholds and metabolic balance of arctic zooplankton. J Plankton Res 36:3–10. https://doi.org/10.1093/plankt/fbt111
Arndt C, Sommer U (2014) Effect of algal species and concentration on development and fatty acid composition of two harpacticoid copepods, Tisbe sp. and Tachidius discipes, and a discussion about their suitability for marine fish larvae. Aquac Nutr 20:44–59. https://doi.org/10.1111/anu.12051
Boyen J, Fink P, Mensens C, Hablützel PI, De Troch M (2020) Fatty acid bioconversion in harpacticoid copepods in a changing environment: a transcriptomic approach. Philos Trans R Soc B Biol Sci 375:20190645. https://doi.org/10.1098/rstb.2019.0645
Boyen J, Ribes-Navarro A, Kabeya N, Monroig Ó, Rigaux A, Fink P, Hablützel PI, Navarro JC, De Troch M (2023) Functional characterization reveals a diverse array of metazoan fatty acid biosynthesis genes. Mol Ecol 32:970–982. https://doi.org/10.1111/mec.16808
Brett M, Müller-Navarra D (1997) The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw Biol 38:483–499. https://doi.org/10.1046/j.1365-2427.1997.00220.x
Brett MT, Müller-Navarra DC, Persson J (2009) Crustacean zooplankton fatty acid composition. In: Kainz M, Brett MT, Arts MT (eds) Lipids in aquatic ecosystems. Springer, New York, NY, pp 115–146
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2:355–374. https://doi.org/10.3390/nu2030355
Colombo SM, Rodgers TFM, Diamond ML, Bazinet RP, Arts MT (2020) Projected declines in global DHA availability for human consumption as a result of global warming. Ambio 49:865–880. https://doi.org/10.1007/s13280-019-01234-6
Couturier LIE, Michel LN, Amaro T, Budge SM, da Costa E, De Troch M, Di Dato V, Fink P, Giraldo C, Le Grand F, Loaiza I, Mathieu-Resuge M, Nichols PD, Parrish CC, Sardenne F, Vagner M, Pernet F, Soudant P (2020) State of art and best practices for fatty acid analysis in aquatic sciences. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsaa121
Dalsgaard J, John MS, Kattner G, Müller-Navarra D, Hagen W (2003) Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol 46:225–340. https://doi.org/10.1016/S0065-2881(03)46005-7
De Lima LCM, Navarro DMAF, Souza-Santos LP (2013) Effect of diet on the fatty acid composition of the copepod Tisbe biminiensis. J Crustac Biol 33:372–381. https://doi.org/10.1163/1937240X-00002135
De Troch MD, Boeckx P, Cnudde C, Van Gansbeke D, Vanreusel A, Vincx M, Caramujo M (2012) Bioconversion of fatty acids at the basis of marine food webs: insights from a compound-specific stable isotope analysis. Mar Ecol Prog Ser 465:53–67. https://doi.org/10.3354/meps09920
Drago L, Panaïotis T, Irisson J-O, Babin M, Biard T, Carlotti F, Coppola L, Guidi L, Hauss H, Karp-Boss L, Lombard F, McDonnell AMP, Picheral M, Rogge A, Waite AM, Stemmann L, Kiko R (2022) Global distribution of zooplankton biomass estimated by in situ imaging and machine learning. Front Mar Sci 9. https://doi.org/10.3389/fmars.2022.894372
Ericson JA, Hellessey N, Kawaguchi S, Nichols PD, Nicol S, Hoem N, Virtue P (2019) Near-future ocean acidification does not alter the lipid content and fatty acid composition of adult Antarctic krill. Sci Rep 9:12375. https://doi.org/10.1038/s41598-019-48665-5
Fleeger JW (1979) Population dynamics of three estuarine meiobenthic harpacticoids (Copepoda) in South Carolina. Mar Biol 52:147–156. https://doi.org/10.1007/BF00390422
Gao Y, Zheng S, Zheng C, Shi Y, Xie X, Wang K, Liu H (2018) The immune-related fatty acids are responsive to CO2 driven seawater acidification in a crustacean brine shrimp Artemia sinica. Dev Comp Immunol 81:342–347. https://doi.org/10.1016/j.dci.2017.12.022
Garzke J, Hansen T, Ismar SMH, Sommer U (2016) Combined effects of ocean warming and acidification on copepod abundance, body size and fatty acid content. PLoS ONE 11:e0155952. https://doi.org/10.1371/journal.pone.0155952
Giere O (1993) Meiobenthology: the microscopic fauna in aquatic sediments. Springer-Verlag, Berlin, New York
Giesbrecht W (1881) Vorläufige Mitteilung aus einer Arbeit über die freilebenden Copepoden des Kieler Hafens. Zool Anz 4:254–258
Harley CDG, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9:228–241. https://doi.org/10.1111/j.1461-0248.2005.00871.x
Hazel JR (1995) Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol 57:19–42. https://doi.org/10.1146/annurev.ph.57.030195.000315
Heip C, Smol N (1976) Influence of temperature on the reproductive potential of two brackish-water harpacticoids (Crustacea: Copepoda). Mar Biol 35:327–334. https://doi.org/10.1007/BF00386643
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu Rev Ecol Evol Syst 37:373–404. https://doi.org/10.1146/annurev.ecolsys.37.091305.110149
Hixson SM, Arts MT (2016) Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton. Glob Change Biol 22:2744–2755. https://doi.org/10.1111/gcb.13295
Holm HC, Fredricks HF, Bent SM, Lowenstein DP, Ossolinski JE, Becker KW, Johnson WM, Schrage K, Van Mooy BAS (2022) Global ocean lipidomes show a universal relationship between temperature and lipid unsaturation. Science 376:1487–1491. https://doi.org/10.1126/science.abn7455
Huys R, Boxshall GA (1991) Copepod evolution. The Ray Society, London
Isla JA, Lengfellner K, Sommer U (2008) Physiological response of the copepod Pseudocalanus sp. in the Baltic Sea at different thermal scenarios. Glob Change Biol 14:895–906. https://doi.org/10.1111/j.1365-2486.2008.01531.x
Jónasdóttir S, Visser A, Jespersen C (2009) Assessing the role of food quality in the production and hatching of Temora longicornis eggs. Mar Ecol Prog Ser 382:139–150. https://doi.org/10.3354/meps07985
Kabeya N, Ogino M, Ushio H, Haga Y, Satoh S, Navarro JC, Monroig Ó (2021) A complete enzymatic capacity for biosynthesis of docosahexaenoic acid (DHA, 22: 6n–3) exists in the marine Harpacticoida copepod Tigriopus californicus. Open Biol 11:200402. https://doi.org/10.1098/rsob.200402
Kelly MW, Sanford E, Grosberg RK (2012) Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc R Soc B Biol Sci 279:349–356. https://doi.org/10.1098/rspb.2011.0542
Kiørboe T, Hirst AG (2008) Optimal development time in pelagic copepods. Mar Ecol Prog Ser 367:15–22. https://doi.org/10.3354/meps07572
Kwiatkowski L, Torres O, Bopp L, Aumont O, Chamberlain M, Christian JR, Dunne JP, Gehlen M, Ilyina T, John JG, Lenton A, Li H, Lovenduski NS, Orr JC, Palmieri J, Santana-Falcón Y, Schwinger J, Séférian R, Stock CA, Tagliabue A, Takano Y, Tjiputra J, Toyama K, Tsujino H, Watanabe M, Yamamoto A, Yool A, Ziehn T (2020) Twenty-first century ocean warming, acidification, deoxygenation, and upper-ocean nutrient and primary production decline from CMIP6 model projections. Biogeosciences 17:3439–3470. https://doi.org/10.5194/bg-17-3439-2020
Lagos PF, Curtsdotter A, Agüera A, Sabadel AJM, Burrit DJ, Lamare MD (2022) Fast changes in the bioenergetic balance of krill in response to environmental stress. Front Mar Sci 8:782524. https://doi.org/10.3389/fmars.2021.782524
Li W, Han G, Dong Y, Ishimatsu A, Russell BD, Gao K (2015) Combined effects of short-term ocean acidification and heat shock in a benthic copepod Tigriopus japonicus Mori. Mar Biol 162:1901–1912. https://doi.org/10.1007/s00227-015-2722-9
Litzow M, Bailey K, Prahl F, Heintz R (2006) Climate regime shifts and reorganization of fish communities: the essential fatty acid limitation hypothesis. Mar Ecol Prog Ser 315:1–11. https://doi.org/10.3354/meps315001
McGinty N, Barton AD, Record NR, Finkel ZV, Johns DG, Stock CA, Irwin AJ (2021) Anthropogenic climate change impacts on copepod trait biogeography. Glob Change Biol 27:1431–1442. https://doi.org/10.1111/gcb.15499
Meire P, Ysebaert T, Damme SV, den Bergh EV, Maris T, Struyf E (2005) The Scheldt estuary: a description of a changing ecosystem. Hydrobiologia 540:1–11. https://doi.org/10.1007/s10750-005-0896-8
Müller-Navarra DC, Brett MT, Liston AM, Goldman CR (2000) A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403:74–77. https://doi.org/10.1038/47469
Nanton DA, Castell JD (1998) The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod, Tisbe sp., for use as a live food for marine fish larvae. Aquaculture 163:251–261. https://doi.org/10.1016/S0044-8486(98)00236-1
Norsker N-H, Støttrup JG (1994) The importance of dietary HUFAs for fecundity and HUFA content in the harpacticoid, Tisbe holothuriae Humes. Aquaculture 125:155–166. https://doi.org/10.1016/0044-8486(94)90292-5
Pan FTC, Applebaum SL, Manahan DT (2021) Differing thermal sensitivities of physiological processes alter ATP allocation. J Exp Biol. https://doi.org/10.1242/jeb.233379
Parrish CC (2009) Essential fatty acids in aquatic food webs. In: Kainz M, Brett MT, Arts MT (eds) Lipids in aquatic ecosystems. Springer New York, New York, pp 309–326
Parrish CC, French VM, Whiticar MJ (2012) Lipid class and fatty acid composition of copepods (Calanus finmarchicus, C. glacialis, Pseudocalanus sp., Tisbe furcata and Nitokra lacustris) fed various combinations of autotrophic and heterotrophic protists. J Plankton Res 34:356–375. https://doi.org/10.1093/plankt/fbs003
Perperoglou A, Sauerbrei W, Abrahamowicz M, Schmid M (2019) A review of spline function procedures in R. BMC Med Res Methodol 19:46. https://doi.org/10.1186/s12874-019-0666-3
Peters J, Dutz J, Hagen W (2007) Role of essential fatty acids on the reproductive success of the copepod Temora longicornis in the North Sea. Mar Ecol Prog Ser 341:153–163. https://doi.org/10.3354/meps341153
Pörtner HO, Peck L, Somero G (2007) Thermal limits and adaptation in marine Antarctic ectotherms: an integrative view. Philos Trans R Soc B Biol Sci 362:2233–2258. https://doi.org/10.1098/rstb.2006.1947
Pörtner H-O, Bock C, Mark FC (2017) Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J Exp Biol 220:2685–2696. https://doi.org/10.1242/jeb.134585
Raisuddin S, Kwok KWH, Leung KMY, Schlenk D, Lee J-S (2007) The copepod Tigriopus: a promising marine model organism for ecotoxicology and environmental genomics. Aquat Toxicol 83:161–173. https://doi.org/10.1016/j.aquatox.2007.04.005
Sahota R, Boyen J, Semmouri I, Bodé S, De Troch M (2022) An inter-order comparison of copepod fatty acid composition and biosynthesis in response to a long-chain PUFA deficient diet along a temperature gradient. Mar Biol 169:133. https://doi.org/10.1007/s00227-022-04121-z
Saiz E, Griffell K, Olivares M, Solé M, Theodorou I, Calbet A (2022) Reduction in thermal stress of marine copepods after physiological acclimation. J Plankton Res 44:427–442. https://doi.org/10.1093/plankt/fbac017
Sargent J, Bell G, McEvoy L, Tocher D, Estevez A (1999) Recent developments in the essential fatty acid nutrition of fish. Aquaculture 177:191–199. https://doi.org/10.1016/S0044-8486(99)00083-6
Sarmento VC, Parreira Santos PJ, Hale R, Ingels J, Widdicombe S (2017) Effects of elevated CO2 and temperature on an intertidal harpacticoid copepod community. ICES J Mar Sci 74:1159–1169. https://doi.org/10.1093/icesjms/fsw192
Sasaki MC, Dam HG (2020) Genetic differentiation underlies seasonal variation in thermal tolerance, body size, and plasticity in a short-lived copepod. Ecol Evol 10:12200–12210. https://doi.org/10.1002/ece3.6851
Sasaki M, Dam HG (2021) Global patterns in copepod thermal tolerance. J Plankton Res 43:598–609. https://doi.org/10.1093/plankt/fbab044
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Sinensky M (1974) Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci 71:522–525. https://doi.org/10.1073/pnas.71.2.522
Sokolova I (2021) Bioenergetics in environmental adaptation and stress tolerance of aquatic ectotherms: linking physiology and ecology in a multi-stressor landscape. J Exp Biol. https://doi.org/10.1242/jeb.236802
Stanley-Samuelson DW (1987) Physiological roles of prostaglandins and other eicosanoids in invertebrates. Biol Bull 173:92–109. https://doi.org/10.2307/1541865
Steinberg DK, Landry MR (2017) Zooplankton and the ocean carbon cycle. Annu Rev Mar Sci 9:413–444. https://doi.org/10.1146/annurev-marine-010814-015924
Tan K, Zhang H, Zheng H (2022) Climate change and n-3 LC-PUFA availability. Prog Lipid Res 86:101161. https://doi.org/10.1016/j.plipres.2022.101161
Troch MD, Steinarsdóttir MB, Chepurnov V, Ólafsson E (2005) Grazing on diatoms by harpacticoid copepods: species-specific density-dependent uptake and microbial gardening. Aquat Microb Ecol 39:135–144. https://doi.org/10.3354/ame039135
Turner JT (2004) The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool Stud 43(2):255–266
Twining CW, Brenna JT, Hairston NG Jr, Flecker AS (2016) Highly unsaturated fatty acids in nature: what we know and what we need to learn. Oikos 125:749–760. https://doi.org/10.1111/oik.02910
Watanabe T, Kitajima C, Fujita S (1983) Nutritional values of live organisms used in Japan for mass propagation of fish: a review. Aquaculture 34:115–143. https://doi.org/10.1016/0044-8486(83)90296-X
Wells JBJ (2007) <p><strong>An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea)</strong></p>. Zootaxa 1568:1–872. https://doi.org/10.11646/zootaxa.1568.1.1
Werbrouck E, Van Gansbeke D, Vanreusel A, Mensens C, De Troch M (2016) Temperature-induced changes in fatty acid dynamics of the intertidal grazer Platychelipus littoralis (Crustacea, Copepoda, Harpacticoida): insights from a short-term feeding experiment. J Therm Biol 57:44–53. https://doi.org/10.1016/j.jtherbio.2016.02.002
Werbrouck E (2016) Temperature-induced changes in fatty acid dynamics of the intertidal grazer Platychelipus littoralis (Crustacea, Copepoda, Harpacticoida)_ Insights from a short-term feeding experiment. J Therm Biol. https://doi.org/10.1016/j.jtherbio.2016.02.002
Whiteley N, Faulkner LS (2005) Temperature influences whole-animal rates of metabolism but not protein synthesis in a temperate intertidal isopod. Physiol Biochem Zool Ecol Evol Approaches 78:227–238. https://doi.org/10.1086/427054
Wolff RL, Bayard CC, Fabien RJ (1995) Evaluation of sequential methods for the determination of butterfat fatty acid composition with emphasis ontrans-18:1 acids. Application to the study of seasonal variations in french butters. J Am Oil Chem Soc 72:1471–1483. https://doi.org/10.1007/BF02577840
Yoon D-S, Choi H, Sayed AE-DH, Shin K-H, Yim JH, Kim S, Lee M-C, Lee J-S (2023) Effects of temperature and starvation on life history traits and fatty acid profiles of the Antarctic copepod Tigriopus kingsejongensis. Reg Stud Mar Sci 57:102743. https://doi.org/10.1016/j.rsma.2022.102743
Acknowledgements
We thank the anonymous reviewers for their thorough revision of the manuscript which significantly improved the final outcome. We also thank Dr. Bruno Vlaeminck, Annick Van Kenhove and Annelien Rigaux from the Marine Biology Research Group, Ghent University for the help with picking copepods and FA analyses and Jens Boyen, Robyn Sahota and Prof. Ulrike Braeckman for the discussions and insights that nurtured this research.
Funding
The research leading to results presented in this publication was carried out with infrastructure funded by EMBRC Belgium—FWO international research infrastructure I001621N. Part of the results presented in this publication were obtained using infrastructure funded by EMBRC Belgium—FWO project GOH3817N. JV holds a Bijzonder Onderzoeksfonds, Special Research Fund PhD grant—BOF, from Ghent University (BOF.DOC.2020.0033.01). We acknowledge funding from the UGent-BOF GOA project ‘Assessing the biological capacity of ecosystem resilience’ (grant BOFGOA2017000601)’.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The experiment execution was performed by JV. The first draft of the manuscript was written by JV and CV, WB, AD and MDT commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interest to disclose.
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
Additional information
Responsible Editor: Xabier Irigoien.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vigliano Relva, J., Van Colen, C., Barhdadi, W. et al. Temperature increase alters relative fatty acid composition and has negative effects on reproductive output of the benthic copepod Tachidius discipes (copepoda: Harpacticoida). Mar Biol 171, 22 (2024). https://doi.org/10.1007/s00227-023-04343-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04343-9