Abstract
Warming of the world’s oceans is predicted to have many negative effects on organisms as they have optimal thermal windows. In coastal waters, however, both temperatures and pCO2 (pH) exhibit diel variations, and biological performances are likely to be modulated by physical and chemical environmental changes. To understand how coastal zooplankton respond to the combined impacts of heat shock and increased pCO2, the benthic copepod Tigriopus japonicus were treated at temperatures of 24, 28, 32 and 36 °C to simulate natural coastal temperatures experienced in warming events, when acclimated in the short term to either ambient (LC, 390 μatm) or future CO2 (HC, 1000 μatm). HC and heat shock did not induce any mortality of T. japonicus, though respiration increased up to 32 °C before being depressed at 36 °C. Feeding rate peaked at 28 °C but did not differ between CO2 treatments. Expression of heat shock proteins (hsps mRNA) was positively related to temperature, with no significant differences between the CO2 concentrations. Nauplii production was not affected across all treatments. Our results demonstrate that T. japonicus responds more sensitively to heat shocks rather than to seawater acidification; however, ocean acidification may synergistically act with ocean warming to mediate the energy allocation of copepods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing global temperatures are broadly predicted to cause changes to the distributions of species. While virtually all biological processes are affected by temperature, there is recent recognition that the degree to which species are affected will be determined in part by their inherent ability to acclimate and adapt to increased temperatures (Sanford and Kelly 2011). In addition, many habitats demonstrate large variability in environmental conditions, both spatially and temporally, altering predicted biological outcomes (Helmuth et al. 2006; Mislan et al. 2014). Coastal marine environments in particular are usually characterized by large changes in both chemical and physical properties, with diel, seasonal and inter-annual perturbations of temperature, pH and oxygen along with tide cycle, upwelling events and biological activities (Duarte et al. 2013; Marshall et al. 2011; Melzner et al. 2013).
Organisms inhabiting coastal waters experience strong fluctuations of temperature both daily and seasonally (Davison and Pearson 1996). Moreover, sea surface temperature (SST) has been increasing in the last several decades, with shallow coastal waters being strongly impacted (Lima and Wethey 2012), and surface ocean temperature likely to further increase between 0.6 and 2.0 °C by the end of this century (Stocker et al. 2013). Importantly, in addition to this rise in mean temperature, there will be an increase in temperature variation, leading to more extreme heating events (Jentsch et al. 2007). For example, Xiamen Bay (Fujian, China) typically has regular, semidiurnal tides (Hu et al. 1988), and water temperatures in the intertidal pools can have a daily temperature range of 25 °C, reaching over 45 °C during low tide in a summer afternoon (Dong et al. personal communication).
Aquatic organisms have different scopes for temperature adaptation and their fitness, and performance can be mediated by the degree to which environmental temperature (and variation) overlaps with their thermal window (Pörtner and Farrell 2008). Though most intertidal animals have developed effective behavioral and physiological adaptations for surviving in this highly variable and harsh environment (Marshall et al. 2011; Somero 2010; Tomanek and Somero 2000), many are still living near their upper thermal limits (Stillman and Somero 2000), meaning that extreme heating events can have deleterious biological effects (Pörtner 2012).
Along with the ocean warming, increasing dissolution of atmospheric CO2 into seawater is decreasing global average pH in surface oceans, causing ocean acidification (OA), and this acidification is superimposed onto natural fluctuations in pH in coastal waters (Cai et al. 2011; Capone and Hutchins 2013). For example, the surface seawater pH can range from 7.85 to 8.15 in the California upwelling system, and this range will be further lowered to 7.50–7.70 given the continuing and rapid increase in CO2 emission due to anthropogenic activities (Capone and Hutchins 2013; Sabine et al. 2004). Moreover, eutrophication could contribute additional drop of pH in subsurface of coastal waters, causing more stressful acidification of coastal areas (Cai et al. 2011).
While OA is known to have various effects on processes such as calcification (Comeau et al. 2015; Gao et al. 1993; Ries et al. 2009), other physiological processes in zooplankton such as respiration (Li and Gao 2012; Thomsen and Melzner 2010), pH regulation (Pörtner et al. 2010), feeding (Dupont and Thorndyke 2008; Li and Gao 2012), egg production and nauplius development (Kurihara et al. 2004) were well documented. There were also some studies focus on the combined effects of OA with rising temperature (Munday et al. 2009; Parker et al. 2009). For example, the thermal window of fishes may be narrowed under acidified conditions (Pörtner et al. 2010; Pörtner and Farrell 2008), reducing their thermal scope and fitness with respect to warming. However, what is unknown is how marine organisms, particularly intertidal ectotherms, will respond to rapid and extreme heating events when they are exposed to acidified conditions.
In the scenario of climate change, therefore, it is crucial to investigate the combined effects of reduced pH and heat shock on growth, reproduction and related physiological performances of intertidal animals. In the present study, we simulated the temperature change observed in natural coastal waters to test the hypothesis that combined effects of OA and extreme heating events increase the costs for somatic maintenance and allocate less energy for growth and reproduction, and show adverse effects on the physiological processes of a benthic copepod Tigriopus japonicus Mori. This ecologically important species spreads widely in the coastal waters of western Pacific (China, Japan and Korea) (Jung et al. 2006) and is a common species in the tidal pools on rocky shore where environmental conditions such as temperature and pH demonstrate large daily and seasonal variations (Davenport et al. 1997; McAllen et al. 1999). This species has been extensively studied and is seen as a promising model species for ecotoxicology study given the fully sequenced mitochondrial DNA (Machida et al. 2002; Raisuddin et al. 2007) and can be cultured for several years in indoor conditions (Uye 2005). The species could therefore be taken as a model for studying the relationship between physiological response to temperature fluctuation and pH decline in highly variable coastal areas.
Materials and methods
Experimental setup
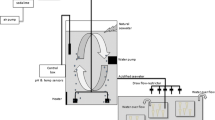
This experiment was designed to test the responses of T. japonicus to combined heat shock, which they naturally experience in tidal pools during low tide, and the reduced pH under a future OA scenario. It has been shown that pH in intertidal zone could be varied by 0.5 unit during day and night and by more than 1 unit seasonally (Wootton et al. 2008). In the present study, the copepod individuals were acclimated to a short period of different CO2 treatments (ambient CO2, LC: 390 µatm, ca. pH 8.15; HC: 1000 µatm, a decrease of ca. 0.4 unit compare with LC) at 20 °C for 24 h, exposed to one of five different temperatures (20, 24, 28, 32 or 36 °C) for 4 h, and returned to 20 °C while being maintained at either LC or HC (see Fig. 1 for experimental design). This heat exposure period was chosen to simulate elevated temperature at low tide in Xiamen Bay. There is a regular semidiurnal tide, which experiences twice of both rising tide and falling tide in 24 h and requires ca. 6 h for a single rising or falling tide, and the surface (1 m layer) seawater temperature of high tide ranges 24.2–27.2 and 12.2–16.4 °C in summer and winter, respectively. Water temperature in intertidal pools during low tides can surpass 45 °C on summer afternoons (Marine Biological Laboratory, Fujian Institute of Oceanology and Division of Marine Biology, Dept. of Biology, Amoy University, 1960). Three different categories of responses to experimental treatments were quantified, physiological (respiration, feeding rate and mortality), molecular (levels of heat shock proteins mRNA) and reproduction (nauplii production).
Draft of the main design and the timing of each measurement of this study. Expressions below or above the dark filled rectangle, circle or up arrows indicate that specific experiment implement. Copepod treated with LC (390 µatm) and HC (1000 µatm) was given gradually increased, constant and gradually decreased temperature (7 h in total), where the respiration rate was measured after the temperature attain the set value (1.5–5.5 h); Tigriopus japonicus acclimated with respective heat shock treatments for 4 h (5.5 h point) were sampled for hsp 70/hsp 90 mRNA expression test; feeding experiment was carried out for 24 h after 7-h heat shock; female production was measured for 48 h after heat shock treatments; mortality of T. japonicus was monitored after 7-h heat shock and the subsequent 72-h culture. Each measurement was an individual experiment, and sample used in one experiment will not be used again
Adult T. japonicus used in this experiment were originally collected from the rocky shore of Xiamen Bay (24°43′N, 118°10′E) and have been cultured in the laboratory in an incubator for 2 years at 20 °C with light intensity of 30 µmol m−2 s−1 (12:12 L:D cycle) (a mixed population of male and female with a density of ca. 500 individuals per liter). To maintain optimal conditions, copepods were cultured with offshore seawater (SEATS station, South China Sea, salinity 33 ppt), filtered (0.22 µm), autoclaved at 105 °C and then bubbled with air until pH stabilized at ~8.15. For the entire culture period, the copepods were only fed on the single diatom Phaeodactylum tricornutum Bohlin with the initial concentration of ~5 × 104 cells ml−1.
CO2 treatments and carbonate chemistry/water conditions
To achieve the different CO2 treatments, seawater was pre-bubbled with air containing either ambient (390 µatm, LC) or elevated (1000 µatm, HC) CO2 concentrations using outdoor air and CO2 chambers (HP1000G-D, Ruihua instrument & equipment Co. Ltd., China), which controls the CO2 concentration with a variation <3 %. To ensure that treatments were maintained throughout the experiment, pH of the water was regularly measured with a pH meter (Mettler Toledo DL15 Titrator, Sweden), which was calibrated with NBS buffer solution (Hanna). Parameters of carbonate system (TA, DIC, HCO3 −, CO3 2− and CO2) were calculated using the program of CO2SYS for Excel (Lewis and Wallace 1998) with the known temperature, salinity (measured with a handheld refractometer, S/Mill-E, Atago, Japan), nutrients level (seawater forms seats station of Southern China Sea; phosphate is undetectable and can be ignored), pH and pCO2. The pCO2 was used for calculation according to the measured pCO2 in CO2 chambers with a portable CO2 detector (Vaisala, CARBOCAP-GM70, Finland). Equilibrium constants for carbonic acid dissociation (K 1 and K 2) were from Roy et al. (1993), and KB for boric acid was after Dickson (1990).
Heat shock treatments
To test the heat shock responses of T. japonicus, a rectangular container (length × width × height = 75 × 30 × 15 cm) made of transparent plastic was divided into five compartments of equal size with plastic dividers. These compartments were used as water baths, with the temperature in each regulated using either hot water or cold water when required to maintain the predetermined temperature. Five target temperatures were set, 20 °C (control), 24, 28, 32 and 36 °C. To simulate natural rates of warming experienced by the copepods on a daily basis, the copepods in bottles with both LC and HC seawater were transferred to 21, 22, 23 and 24 °C in one step, and then, temperature was gradually increased during the next 1.5 h to attain target temperatures. After reaching the target temperature, each water bath was maintained at the designated temperature for 4 h and then gradually decreased back to 20 °C at the same rate as for the heating. The respiration rate was measured after the temperature attains the set value (1.5–5.5 h); T. japonicus acclimated with respective heat shock for 4 h (5.5 h point) were sampled for hsp 70/hsp 90 mRNA expression measurements; feeding experiment was carried out for 24 h after 7-h heat shock; female production was measured for 48 h after heat shock treatments; mortality of T. japonicus was monitored after 7-h heat shock and the subsequent 72-h culture (see Fig. 1 for a detailed diagram of the experimental design). Individual copepods were only used for one experiment (i.e., mortality, respiration, feeding, hsp mRNA quantification and reproduction measurements, below) so as to not confound other measurements.
Respiration rate and Q 10
The respiration rate of adult T. japonicus (randomly collected, mixed sex, no gravid female were used) was measured using an oxygen optode (Fibox3,Germany) in sealed 20-ml PerkinElmer glass bottles, during the heat shock treatment. Prior to heating, copepods were placed into bottles containing either LC or HC water (n = 20 individuals per bottle; n = 3 bottles per treatment) in which an oxygen electrode sensor had been adhered to the inside bottle wall. Oxygen consumption rates were continuously measured over the period that temperature remained stable at the target value (see Fig. 1). No food was added during the respiration trials.
Respiration rates were calculated from the change in oxygen concentration in bottles during the 4-h incubation period:
where “K exp.” and “K control” were the oxygen changes per min (μg l−1 min−1) in experimental and control bottles (bottles without copepod), “V” the bottle volume (l), “N” the copepod number.
The metabolic sensitivity of T. japonicus under different pH treatments can be expressed as the Q 10, reflecting the change in metabolic rate over a 10 °C temperature gradient. The Q 10 was calculated according to Atkin and Tjoelker (2003): Q 10 = 10(slope * 10), where slope indicates the regression slope of log10-transformed respiratory rates versus corresponding temperature points (20, 24, 28, 32 and 36 °C).
Feeding rate
The feeding rate was quantified at both LC and HC following the heat shock treatments. As the effects of OA and heat shock may be sustained following removal of the elevated temperature, and because T. japonicus primarily feed during the dark (Stearns 1986), the energy consumption (metabolism) and acquisition (feeding) may not temporally coincide (Li and Gao 2012). Therefore, feeding assays were run immediately following return of the water temperature to 20 °C, when copepods were transferred to 110-ml polyethylene bottles containing seawater pre-equilibrated with either LC or HC (as appropriate, n = 30 bottles per CO2 and heat shock combination) at a density of 20 individuals per bottle. P. tricornutum were added at a concentration of 5 × 104 cells ml−1. To control for growth of the diatoms over the period of the feeding trials, 20 replicate (each temperature or CO2 had 2 replicates) bottles containing only diatoms were established. All bottles were then transferred into a dark incubator and held at 20 °C for 24 h, following which feeding and filtering rates were calculated according to Frost (1972) with changes in diatom cell concentrations that measured with a coulter counter (Z2, Beckman Coulter, USA):
where “V” the volume (ml) of culture, “N” the copepod number in each replicate, “C 0” the initial cell concentration, “C t ” cell concentration in the control bottle after 24 h (t), “C tf ” cell concentration in the experimental bottle after 24 h.
Reproductive output
Following the heat shock trial, female T. japonicus with egg sacs were randomly selected and transferred into six-well culture plates (n = 1 per well, n = 4–6 replicates per treatment) to test the effects of short-term OA and heat shock on nauplii production. Nauplii production requires a period of up to 2 days (Hildebrandt et al. 2014; Kurihara et al. 2004); thus, we checked the nauplii production in the subsequent 2 days after the heat shock to determine the temperature or/and OA effects. During the test, copepod was fed with P. tricornutum at a concentration of 5 × 104 cells ml−1 for 2 days under both LC and HC condition at 20 °C. Water in the wells was changed daily and the number of nauplii in the wells enumerated.
Mortality
The mortality of copepods was quantified immediately and for the 3 days following the heat shock trial as described above (n = 20 individuals per bottle; n = 3 bottles per treatment). Live animals that were not used for other measurements (i.e., reproductive output and heat shock protein production, below) were held at 20 °C in their respective LC or HC conditions and fed with P. tricornutum at a concentration of 5 × 104 cells ml−1. The culture water was changed daily with water pre-bubbled to the appropriate CO2 conditions.
Hsp 70 and hsp 90 measurements
To quantify the production of heat shock proteins under the different treatments, a subsample of T. japonicus was taken immediately following the heat shock experiment, washed using phosphate buffer (PBS) for 2–3 times and transferred into 1.5-ml centrifuge tubes containing 0.6 ml PBS (pH 7.80). Specimens were frozen with liquid nitrogen and stored at −80 °C for further processing.
Total RNA was isolated from ca. 50 individuals per treatment (n = 3–7) using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The first strand of cDNA was synthesized using total RNA as a template. Reverse transcriptase (RT) reactions were performed using PrimeScript™ RT reagent Kit with cDNA Eraser (TAKARA, Shiga, Japan). A partial sequence of the 18S gene was selected as a reference housekeeping gene to normalize the level of expression. The 18S gene is a reliable housekeeping gene as previous studies described (Hwang et al. 2010). The levels of hsp 70 and hsp 90 expression were quantified using real-time quantitative PCR with primers (Table 1). PCR efficiency of each primer pair was determined by performing standard curves from serial dilutions to ensure that PCR efficiency ranged from 95 to 101 % (R 2 > 0.99). PCR was carried out in an ABI 7500 real-time PCR system (Applied Biosystems, MA, USA) in a 20 μl reaction volume containing 10 μl of 2 × FastStart DNA Universal SYBR Green Master (Roche, Germany), 0.8 μl of each primer (10 nmol/μl), 1 μl of cDNA template and 7.4 μl of RNase-free water. The PCR conditions were as follows: 94 °C/4 min; 35 cycles of 94 °C/30 s, 55 °C/30 s, 72 °C/30 s; and 72 °C/7 min and melt curve confirmation at 95 °C/min, 55 °C/1 min, 80 cycles of 55 °C/10 s with 0.5 °C increase per cycle. All samples were measured in triplicate. Ct (dR) values were analyzed using the ABI 7500 system software (Applied Biosystems, MA, USA). The expression values of hsp 70 and hsp 90 mRNA for the various heat treatments were determined as the relative value of 18S using 2−ΔΔCT method for experimental against control treatment (Pfaffl 2001). These experiments were carried out in three technical replicates.
Statistical analysis
Respiration, feeding and filtering rates, reproductive output and HSP production were analyzed using two-way analysis of variance (ANOVA), with both CO2 concentration (two levels: LC vs. HC) and temperature (five levels: 20, 24, 28, 32 or 36 °C) being fixed and orthogonal (n = 3). Where significant effects were detected, pairwise post hoc comparison of means was made to determine which factors differed. Q 10 rate was calculated for the range of temperature treatments and compared between CO2 treatments using a one-way ANOVA.
Results
Experimental conditions
During the experiment, the carbonate system was significantly different between LC and HC treatments, but stable across all temperature treatments (Table 2). As an example, before and after the respiration experiment the mean pH values in LC and HC were ca. 8.14 and 7.80, respectively (Table 2). HC significantly enhanced the DIC by 9.1–16.2 %, the HCO3 − by 13.6–20.9 % and the CO2 by 152.3 %, but decreased the CO3 2− by 40.4–48.9 % across all temperature treatments (all p < 0.05, Table 2).
Respiration rate, Q10 and mortality
No mortality of T. japonicus was recorded in either LC or HC during heat shock treatments or in the subsequent 3-day post heat shock culture. For both LC and HC treatments, respiration rates increased with temperature up to 32 °C, but declined under HC and remained steady under LC at 36 °C (Fig. 2a). Respiration rate differed significantly among all temperatures, except between 20 and 24 °C and between 32 and 36 °C (F 4,20 = 11.2, p < 0.001, pairwise comparisons). There was also a significant effect of CO2 concentration on respiration rate (Fig. 2 a, b; F 1,20 = 10.7, p < 0.005), with increased respiration of HC animals by 99 % (24 °C) and 60 % (28 °C) in comparison with the LC animals (Fig. 2a). Q 10 decreased by 30.6 % from LC (2.04 ± 0.29) to HC treatments (1.42 ± 0.22) (F 1,4 = 8.8, p < 0.05) (Fig. 2c). There was no significant interactive effect of elevated CO2 and temperature on respiration (F 4, 20 = 1.3, p > 0.05) (Table 3).
Respiration rates (a), the log10 (respiration) (b) and the calculated Q10 (C) of Tigriopus japonicus treated with different temperatures and the respiration represent the calculated oxygen consumption rate during the 4 h under respective stable temperature conditions. Data are the mean ± SD of 3 measurements. The “*” above the symbol indicates significant differences between treatments at p < 0.05 level
Feeding and filtering rates
The feeding experiment was carried out for 24 h under constant temperature of 20 °C after the heat shock treatment as described before. Heat shock, but not CO2, affected both the filtering (temperature, F 4,20 = 6.3, p < 0.004; CO2, F 1,20 = 0.004, p > 0.9) and feeding rates (temperature, F4,20 = 6.9, p < 0.003; CO2, F1,20 = 0.14, p > 0.7) of copepods (Fig. 3 a, b). Both feeding and filtering rates initially showed a positive relationship with temperature (this trend was present from 24 °C for HC group) but reached maximum values at 28 °C, from which they declined to the minimum values at 36 °C (Fig. 3a, b). There was no significant interactive effect of elevated CO2 and temperature on feeding and filtering rates (F 4,20 = 1.3, p > 0.05) (Table 3).
Hsp 70 and hsp 90 mRNA
Hsp 70 and hsp 90 mRNA production showed a positive relationship with temperature rise, but were not affected by elevated CO2 (F 1,32 = 0.45, p > 0.5). Hsp 70 mRNA levels increased from 20 to 24 °C and did not change from 28 to 32 °C, before rapidly increasing to 36 °C (Fig. 4a, F 4,32 = 7.7, p < 0.001, pairwise comparisons). Hsp 90 showed the same pattern as hsp 70, except that the increase with temperature was more consistent in the LC treatment than under HC conditions (Fig. 4b, Temp × CO2 interaction: F 4,32 = 2.9, p < 0.05) (Table 3).
Reproductive output
Neither the heat shock nor CO2 treatments caused any changes in the production rate of nauplii in the 2 days following the heat shock (Table 4; all p > 0.3).
Discussion
Warming of the world’s oceans is already driving species range shifts and changes to community assemblages (Wernberg et al. 2012). Yet, the underlying mechanisms for these changes are often not well understood. In coastal waters in particular, where daily variation can be greater than seasonal change (Helmuth et al. 2006), biological responses are more likely to be a result of short-term extreme events than changes to average conditions (Helmuth et al. 2014). Here, we show that combined heat shock and OA did not affect the mortality of T. japonicus, a copepod which inhabits a highly variable intertidal environment, even though they were cultured under indoor condition for 2 years in this study. The mechanism underlying this resistance is likely to be twofold, according to the present results, the regulation of metabolic rates and the production of protective heat shock proteins.
Below the upper thermal limit, metabolic rates in aquatic invertebrates are predominantly driven by temperature (Perry et al. 2005; Rosenzweig et al. 2008). In addition, temperature variation is known to dramatically mediate physiological processes such as the respiration, enzyme activity, production and development of organisms (Angilletta 2009; Pörtner and Farrell 2008). In face of the extreme temperature, organisms can passively mediate their metabolism, for example, through a reduction in their metabolic rate below the basal level (called dormancy), which is seen as an energy conservation process (Marshall et al. 2011; McAllen et al. 1999). Further, upper thermal tolerance limits differ among species and different populations of the same species from different environments (Stillman and Somero 2000). In particular, populations that inhabit variable environments and are exposed to extremes tend to demonstrate physiological coping mechanisms (Willmer et al. 2005). In the present study, there was no mortality of T. japonicus that long term cultured in indoor condition, even in the most extreme temperature and CO2 treatments, which could be expected as T. japonicus inhabit the nearshore zone and are known to be a tolerant species with strong adaptive ability (Davenport et al. 1997; Kwok and Leung 2005). The used indoor cultured species which can be considered as representative for copepod long term acclimated under constant condition (type of stable condition) and may helpful to clarify the responses to the sudden heat shocks under OA condition.
Both elevated temperature and pCO2 affect the metabolic rate of the copepods. The respiration rate of T. brevicornis is known to have a linear relationship with temperature increases from 5 to 30 °C; however, at temperatures above 35 °C the respiration rate is not stimulated further (McAllen et al. 1999), which is in accordance with our present result. Yet, the effects of CO2 on respiration of copepods are not consistent among taxa; high CO2 elevates respiration in Centropages tenuiremis (Li and Gao 2012), but it only had a significant effect on respiration of Acartia clausi in combination with high temperature (Zervoudaki et al. 2013). In another recent study, elevated temperature but not CO2 significantly stimulated the respiration of female Calanus hyperboreus (Hildebrandt et al. 2014). The different responses of the aerobic performance to OA may be related to differential resistance or energy requirements among species, especially under extreme thermal conditions, at which thermal windows will potentially be narrowed (Pörtner and Farrell 2008).
Increasing metabolic rate under future OA conditions may relate to acid–base regulation in many marine animals, which is an energy-demanding process that can be affected by seawater acidification due to the changed pH gradient between intracellular and extracellular compartments (Pörtner et al. 2000). Therefore, it is not surprising that enhanced metabolic rate or Na/K+ ATPase activities (an indicator of ion regulatory ability) can be evident in moderate hypercapnic conditions (Melzner et al. 2009; Wood et al. 2008). In the present study, short-term exposure to acidification caused elevated respiration in T. japonicus. Yet, Tigriopus spp. are known to have excellent osmoregulation and/or hemolymph regulation ability (Davenport et al. 1997), likely because of the coastal areas they inhabit in always with a large natural range of environmental variations (salinity, pCO2, etc.). It is likely that respiration was stimulated at 24 and 28 °C under OA but not in higher temperatures (32 and 36 °C) because the combined effects caused metabolic down-regulation at a lower temperature (e.g., also demonstrated in Calanus glacialis; Hildebrandt et al. 2014). It is possible, therefore, that the elevated respiration rates seen here are a shock response to the shorter-term exposure to acidified conditions. Indeed, changes to metabolic processes in response to acidification are generally followed by an increase in energy acquisition or metabolic cost (Thor and Oliva 2015; Thomsen and Melzner 2010), a mechanism that has been shown to increase survival in several taxa (Burnell et al. 2013; Tunnicliffe et al. 2009), and the response that is tested could be mediated by food concentration in the same species within different populations (Thor and Oliva 2015).
To avoid mortality as temperature increases, the elevated metabolic demands must be met by increased consumption of food (Burnell et al. 2013). Feeding and filtering rates in T. japonicus increased from 20 to 28 °C, but decreased above 28 °C, with the lowest feeding and filtering rates at 36 °C. In contrast, metabolic rate continued to increase to 32 °C, before being suppressed at 36 °C. Therefore, at the highest temperature the copepods had higher metabolic demands than they were able to service with feeding, meaning that they were drawing on energetic reserves. While there was no mortality from this single extreme heating event, subsequent events or sustained extreme warming (e.g., the 5 weeks of warming observed in Western Australia) (Wernberg et al. 2012) may cause mortality because of cumulative effects and metabolic debt. Indeed, such mismatch between metabolic rates and feeding are becoming increasingly recognized in invertebrates (Lemoine and Burkepile 2012) and may lead to mortality on longer time scales (Mertens et al. in press). Alternatively, the mismatch between energy acquisition (reduced feeding) and consumption (higher respiration) may also be due to the exposure duration to such extreme fluctuations not being sufficient for the copepods to adapt (Li and Gao 2012), but subsequent events combined with the short generation time of these animals may enable them to adapt to these new conditions. For example, acclimated to higher temperatures and OA for longer periods (5 weeks), the intertidal snail Littorina littorea show enhanced feeding compared with individuals acclimated for shorter periods (2 weeks) (Russell et al. 2013). Further, enhanced grazing rate of micro-zooplankton as a consequence of OA and enhanced temperature was also observed based on a ca. 20-day mesocosm study (Kim et al. 2010). In these cases, long-term survival under altered conditions seems to be related to increasing consumption of food to compensate for increased metabolic costs.
Heat shock proteins, a defensive strategy that can play a critical protective role when exposed to extreme temperature stress, were strongly up-regulated in response to warming in T. japonicus (Raisuddin et al. 2007; Rhee et al. 2009). HSPs are important molecular chaperones which help avoid damage of cells by aiding in the repair of nucleoprotein and in preventing coagulation (Bierkens 2000), potentially improving the adaptive ability of organisms against high temperature and other various environmental stresses (Ohtsuka and Hata 2000). Thus, enhanced expression of HSPs under thermal stress is a common defense strategy (Feder and Hofmann 1999; Lee et al. 2008). Again, the 100 % survival of T. japonicus indicates that these copepods are resistant to thermal stress, and it is likely that this is at least partially a result of the protective effects of heat shock protein that evidenced in up-regulated hsp 70 and hsp 90 mRNA. However, the synthesis and function of heat shock proteins are energy-intensive, meaning that more energy must be allocated into somatic maintenance in high temperature (Sokolova et al. 2012). Whether this extra cost contributes to the increased metabolic rate in elevated temperature is unclear, especially as the highest hsp mRNA expression was at 36 °C when metabolic rate was depressed. Regardless, the extra energy that needs to be allocated to metabolic processes and HSP production results in less energy being allocated to growth and reproduction (Sokolova et al. 2012). Thus, under extended or repeated heating events, increased individual survival would come at the expense of population growth through reduced reproductive output (Hofmann and Todgham 2010).
While OA is ongoing with ocean warming, heat shock in intertidal areas or coastal waters could reduce the overall physiological performances (Marshall et al. 2011), thereby strongly influencing population dynamics of rocky intertidal species (Garrabou et al. 2009). Therefore, understanding the combined effects of acidification and warming in the context of natural fluctuation in abiotic conditions will provide insights into the potential influences of global change on species inhabiting coastal environments. For most experimental studies on marine organisms, their growth conditions are usually quite different from that in the sea, since they are often grown in vessels under constant conditions during experimental periods. Therefore, it has to be pointed out that cautions in interpreting the data obtained in enclosures should be taken, especially to reflect ecological or global change impacts. The copepod used in this study was maintained under indoor constant temperature and light for almost 2 years, which may lead to evolutionary changes in its physiological characteristics. Consequently, the data obtained here can only reflect the copepod’s conditional responses to future climate changes. To achieve the goal we aimed, further tests in the laboratory and/or in filed are needed to address the copepod’s responses to multiple stressors. Obviously, multifaceted, but long-term, in situ experiments are needed to shed light on whether species inhabiting marine harsh environments can adapt to, and survive, global changes superimposed on the natural variation in abiotic conditions (Russell et al. 2012).
References
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Bierkens JGEA (2000) Applications and pitfalls of stress-proteins in biomonitoring. Toxicology 153:61–72
Burnell O, Russell B, Irving A, Connell S (2013) Eutrophication offsets increased sea urchin grazing on seagrass caused by ocean warming and acidification. Mar Ecol Prog Ser 485:37–46
Cai W-J et al (2011) Acidification of subsurface coastal waters enhanced by eutrophication. Nat Geosci 4:766–770
Capone DG, Hutchins DA (2013) Microbial biogeochemistry of coastal upwelling regimes in a changing ocean. Nat Geosci 6:711–717
Comeau S, Carpenter R, Lantz C, Edmunds P (2015) Ocean acidification accelerates dissolution of experimental coral reef communities. Biogeosciences 12:365–372
Davenport J, Barnett PRO, McAllen RJ (1997) Environmental tolerances of three species of the harpacticoid copepod genus Tigriopus. J Mar Biol Assoc UK 77:3–16
Davison IR, Pearson GA (1996) Stress tolerance in intertidal seaweeds. J Phycol 32:197–211
Dickson AG (1990) Standard potential of the reaction: AgCl(s) + ½ H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion HSO4 − in synthetic seawater from 273.15 to 318.15 K. J Chem Thermodyn 22:113–127
Duarte CM et al (2013) Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuar Coast 36:221–236
Dupont S, Thorndyke MC (2008) Ocean acidification and its impact on the early life-history stages of marine animals. In: Briand F (ed) Impacts of acidification on biological, chemical and physical systems in the Mediterranean and Black Seas. CIESM Monographs, Monaco, pp 89–97
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Frost BW (1972) Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol Oceanogr 17:805–815
Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M (1993) Calcification in the articulated coralline alga Corallina pilulifera, with special reference to the effect of elevated CO2 concentration. Mar Biol 117:129–132
Garrabou J et al (2009) Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob Change Biol 15:1090–1103
Helmuth B et al (2006) Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monogr 76:461–479
Helmuth B et al (2014) Beyond long-term averages: making biological sense of a rapidly changing world. Clim Change Responses 1:6
Hildebrandt N, Niehoff B, Sartoris FJ (2014) Long-term effects of elevated CO2 and temperature on the Arctic calanoid copepods Calanus glacialis and C. hyperboreus. Mar Pollut Bull 80:59–70
Hofmann GE, Todgham AE (2010) Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu Rev Physiol 72:127–145
Hu J, Fu Z, Wu L (1988) Tidal residual circulation in the regular semidiurnal tide. J Xiamen Univ (Nat Sci) 27:323–327
Hwang D-S et al (2010) Modulation of p53 gene expression in the intertidal copepod Tigriopus japonicus exposed to alkylphenols. Mar Environ Res 69:S77–S80
Jentsch A, Kreyling J, Beierkuhnlein C (2007) A new generation of climate-change experiments: events, not trends. Front Ecol Environ 5:365–374
Jung S-O et al (2006) The complete mitochondrial genome of the intertidal copepod Tigriopus sp. (Copepoda, Harpactidae) from Korea and phylogenetic considerations. J Exp Mar Biol Ecol 333:251–262
Kim J-M, Lee K, Yang EJ, Shin K, Noh JH, K-t Park, Hyun B, Jeong H-J, Kim J-H, Kim KY (2010) Enhanced production of oceanic dimethylsulfide resulting from CO2-induced grazing activity in a high CO2 world. Environ Sci Technol 44:8140–8143
Kurihara H, Shimode S, Shirayama Y (2004) Effects of raised CO2 concentration on the egg production rate and early development of two marine copepods (Acartia steueri and Acartia erythraea). Mar Pollut Bull 49:721–727
Kwok KWH, Leung KMY (2005) Toxicity of antifouling biocides to the intertidal harpacticoid copepod Tigriopus japonicus (Crustacea, Copepoda): effects of temperature and salinity. Mar Pollut Bull 51:830–837
Lee K-W, Raisuddin S, Hwang D-S, Park HG, Dahms H-U, Ahn I-Y, Lee JS (2008) Two-generation toxicity study on the copepod model species Tigriopus japonicus. Chemosphere 72:1359–1365
Lemoine NP, Burkepile DE (2012) Temperature-induced mismatches between consumption and metabolism reduce consumer fitness. Ecology 93:2483–2489
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee
Li W, Gao K (2012) A marine secondary producer respires and feeds more in a high CO2 ocean. Mar Pollut Bull 64:699–703
Lima FP, Wethey DS (2012) Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat Commun 3:704
Machida RJ, Miya MU, Nishida M, Nishida S (2002) Complete mitochondrial DNA sequence of Tigriopus japonicus (Crustacea: Copepoda). Mar Biotechnol 4:406–417
Marine Biological Laboratory, Fujian Institute of Oceanology, Division of Marine Biology, Dept. of Biology, Amoy University (1960) Studies on the littoral ecology of Amoy and its vicinity. J Xiamen Univ (Nat Sci) 3:74–95
Marshall DJ, Dong YW, McQuaid CD, Williams GA (2011) Thermal adaptation in the intertidal snail Echinolittorina malaccana contradicts current theory by revealing the crucial roles of resting metabolism. J Exp Biol 214:3649–3657
McAllen R, Taylor AC, Davenport J (1999) The effects of temperature and oxygen partial pressure on the rate of oxygen consumption of the high-shore rock pool copepod Tigriopus brevicornis. Comp Biochem Physiol A Mol Integr Physiol 123:195–202
Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner H-O, Lucassen M (2009) Swimming performance in Atlantic Cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater PCO2. Aquat Toxicol 92:30–37
Melzner F et al (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160:1875–1888
Mislan K, Helmuth B, Wethey DS (2014) Geographical variation in climatic sensitivity of intertidal mussel zonation. Global Ecol Biogeogr 23:744–756
Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB (2009) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA 106:1848–1852
Ohtsuka K, Hata M (2000) Molecular chaperone function of mammalian Hsp 70 and Hsp 40-a review. Int J Hyperth 16:231–245
Parker LM, Ross PM, O’Connor WA (2009) The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Glob Chang Biol 15:2123–2136
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Pörtner HO (2012) Integrating climate-related stressor effects on marine organisms: unifying principles linking molecule to ecosystem-level changes. Mar Ecol Prog Ser 470:273–290
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Pörtner HO, Bock C, Reipschläger A (2000) Modulation of the cost of pHi regulation during metabolic depression: a 31P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J Exp Biol 203:2417–2428
Pörtner HO, Dupont S, Melzner F, Storch D, Thorndyke M (2010) Studies of metabolic rate and other characters across life stages. In: Riebesell U, Fabry VJ, Hansson L, Gattuso J-P (eds) Guide to best practices ocean acidification and data reporting. Publications Office of the European Union, Luxembourg, pp 137–165
Raisuddin S, Kwok KW, Leung KM, Schlenk D, Lee JS (2007) The copepod Tigriopus: a promising marine model organism for ecotoxicology and environmental genomics. Aquat Toxicol 83:161–173
Rhee J-S et al (2009) Heat shock protein (Hsp) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants. Comp Biochem Physiol C Toxicol Pharmacol 149:104–112
Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–1134
Rosenzweig C et al (2008) Attributing physical and biological impacts to anthropogenic climate change. Nature 453:353–357
Roy RN et al (1993) The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45 C. Mar Chem 44:249–267
Russell BD, Harley CD, Wernberg T, Mieszkowska N, Widdicombe S, Hall-Spencer JM, Connell SD (2012) Predicting ecosystem shifts requires new approaches that integrate the effects of climate change across entire systems. Biol Lett 8:164–166
Russell BD, Connell SD, Findlay HS, Tait K, Widdicombe S, Mieszkowska N (2013) Ocean acidification and rising temperatures may increase biofilm primary productivity but decrease grazer consumption. Philos Trans R Soc Lond B Biol Sci 368:20120438
Sabine CL et al (2004) The oceanic sink for anthropogenic CO2. Science 305:367–371
Sanford E, Kelly MW (2011) Local adaptation in marine invertebrates. Annu Rev Mar Sci 3:509–535
Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA (2012) Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res 79:1–15
Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920
Stearns DE (1986) Copepod grazing behavior in simulated natural light and its relation to nocturnal feeding. Mar Ecol Prog Ser 30:65–76
Stillman JH, Somero GN (2000) A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol Biochem Zool 73:200–208
Stocker TF et al (2013) Climate change 2013: the physical science basis. In: Intergovernmental panel on climate change, working group I contribution to the IPCC Fifth Assessment Report (AR5). Cambridge University Press, New York
Thomsen J, Melzner F (2010) Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Mar Biol 157:2667–2676
Thor P, Oliva EO (2015) Ocean acidification elicits different energetic responses in an Arctic and a boreal population of the copepod Pseudocalanus acuspes. Mar Biol 162:799–807
Tomanek L, Somero GN (2000) Time course and magnitude of synthesis of heat-shock proteins in congeneric marine snails (Genus Tegula) from different tidal heights. Physiol Biochem Zool 73:249–256
Tunnicliffe V, Davies KT, Butterfield DA, Embley RW, Rose JM, Chadwick WW Jr (2009) Survival of mussels in extremely acidic waters on a submarine volcano. Nat Geosci 2:344–348
Uye S (2005) A brief review of mass culture of copepods used for fish food in Japanese mariculture and a proposed plan to use high biomass natural populations of brackish-water copepods. In: Lee C, O’Bryen PJ, Marcus NH (eds) Copepods in aquaculture. Blackwell Publishing, Oxford, pp 75–90
Wernberg T, Smale DA, Thomsen MS (2012) A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob Change Biol 18:1491–1498
Willmer P, Stone G, Johnston I (2005) Environmental physiology of animals. Blackwell, Malden
Wood HL, Spicer JI, Widdicombe S (2008) Ocean acidification may increase calcification rates, but at a cost. Proc R Soc Lond B Biol Sci 275:1767–1773
Wootton JT, Pfister CA, Forester JD (2008) Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc Natl Acad Sci USA 105:18848–18853
Zervoudaki S, Frangoulis C, Giannoudi L, Krasakopoulou E (2013) Effects of low pH and raised temperature on egg production, hatching and metabolic rates of a Mediterranean copepod species (Acartia clausi) under oligotrophic conditions. Mediterr Mar Sci 15:74–83
Acknowledgments
This study was supported by National Natural Science Foundation (Nos. 41430967; 41120164007), Joint project of NSFC and Shandong province (Grant No. U1406403), Strategic Priority Research Program of CAS (Grant No. XDA11020302), SOA (GASI-03-01-02-04) and China–Japan collaboration project from MOST (S2012GR0290). YWD is funded by Program for New Century Excellent Talents of Ministry of Education, China. BDR is funded by an Australian Research Council Discovery Grant. WL and BDR are funded by MEL Visiting Fellowship of State Key Laboratory of Marine Environmental Science, Xiamen University. We would like to thank Dr. Guizhong Wang (Xiamen University, China) for providing Tigriopus japonicus; we also thank Yahe Li and Nana Liu (Xiamen University, China) for their kind assistance during the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard
This study used copepod as material. All procedures performed in studies involving copepod were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by H. Pörtner.
Reviewed by undisclosed experts.
Rights and permissions
About this article
Cite this article
Li, W., Han, G., Dong, Y. et al. Combined effects of short-term ocean acidification and heat shock in a benthic copepod Tigriopus japonicus Mori. Mar Biol 162, 1901–1912 (2015). https://doi.org/10.1007/s00227-015-2722-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2722-9